Brown Algae from San Andres Island, Southwest Caribbean: A Nuclear Magnetic Resonance Spectroscopy–Metabolomic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Biological Material
2.2. General Experimental Procedures
2.3. Comparison of the Metabolic Profile Between Species and Non-Targeted Metabolomic Experiment
2.4. Extraction and Isolation Elucidation of Compounds (1) and (2)
3. Results
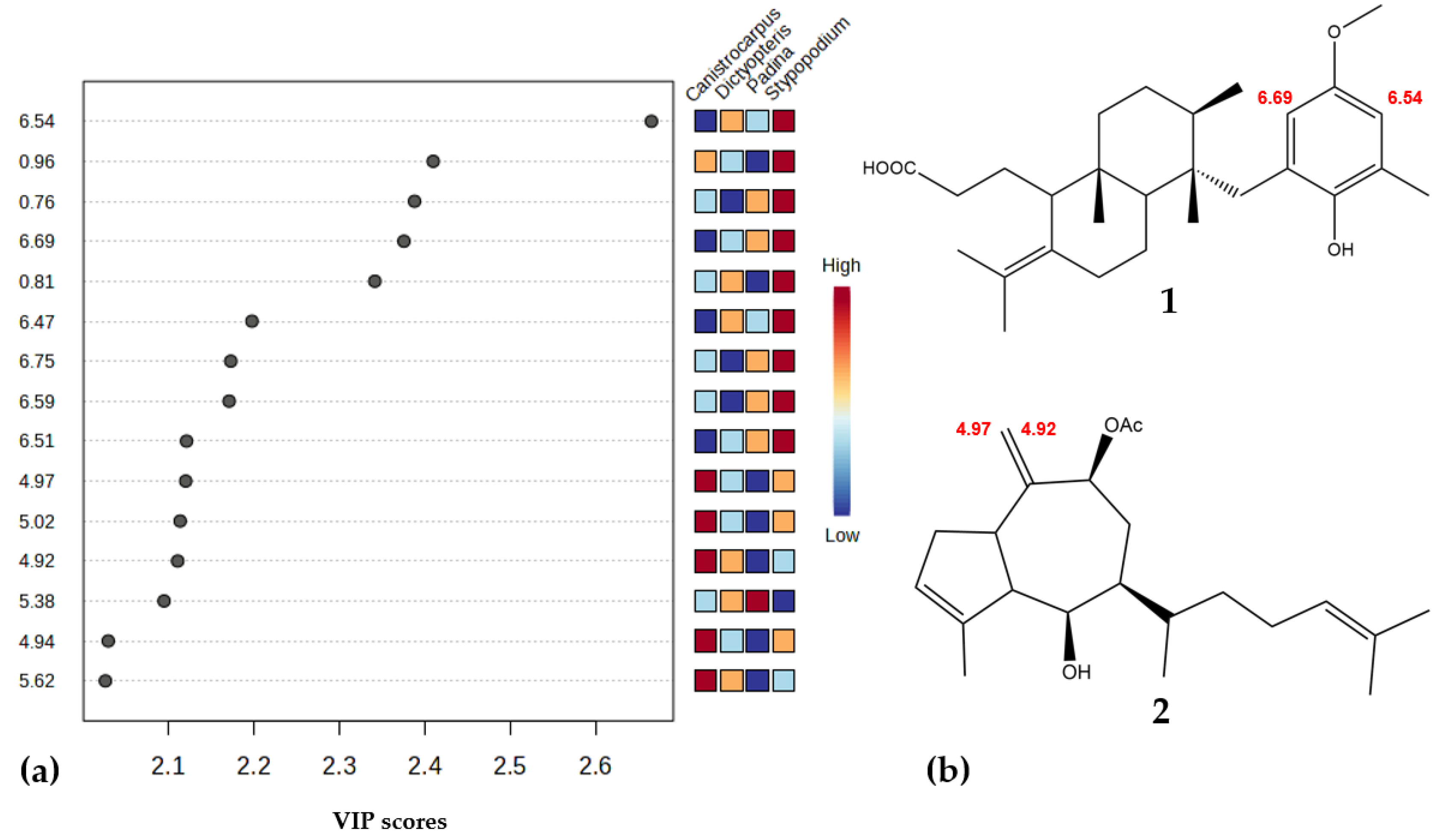
3.1. Metabolic Profiling of Brown Algae Samples of the Order Dictyotales
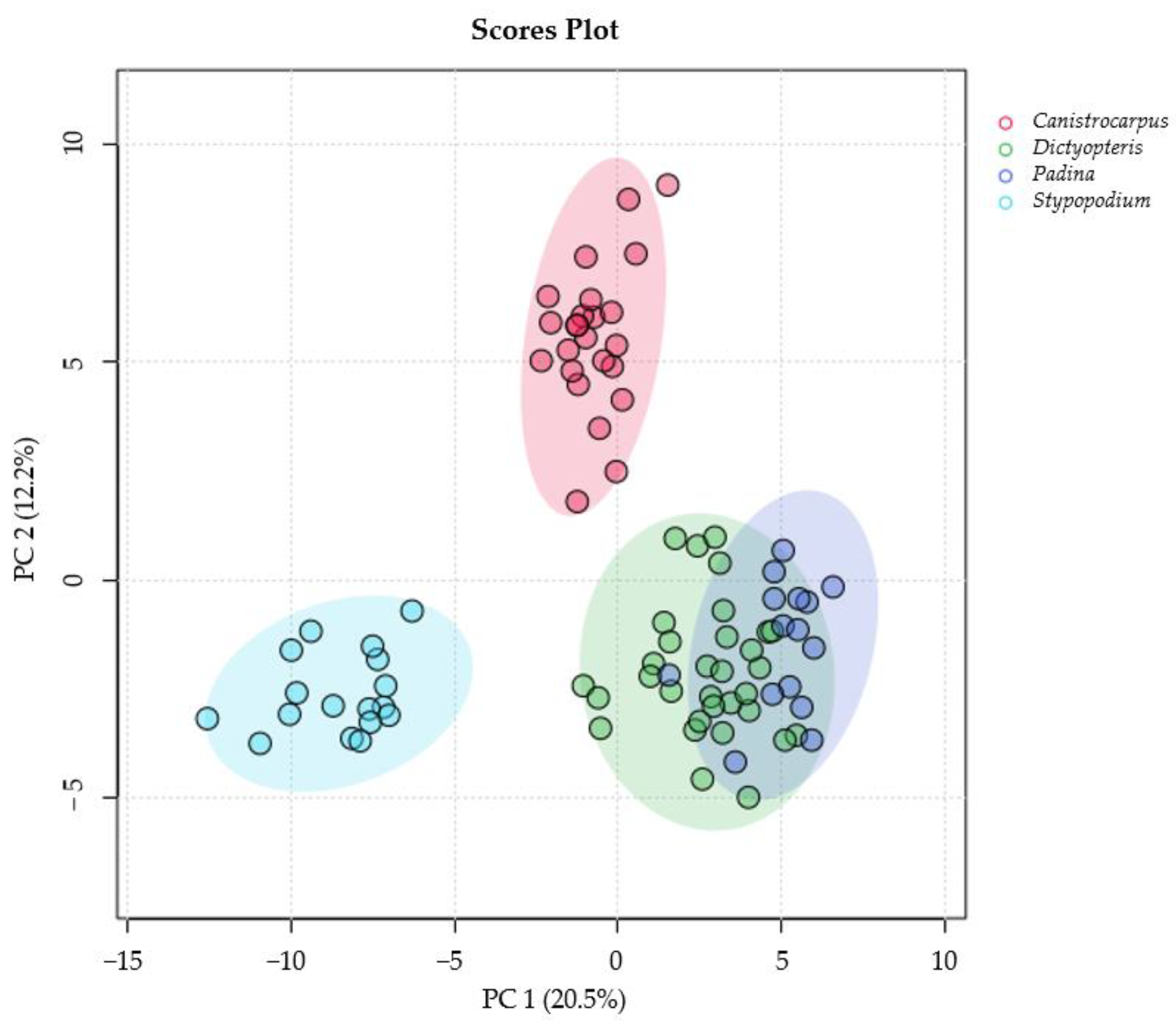
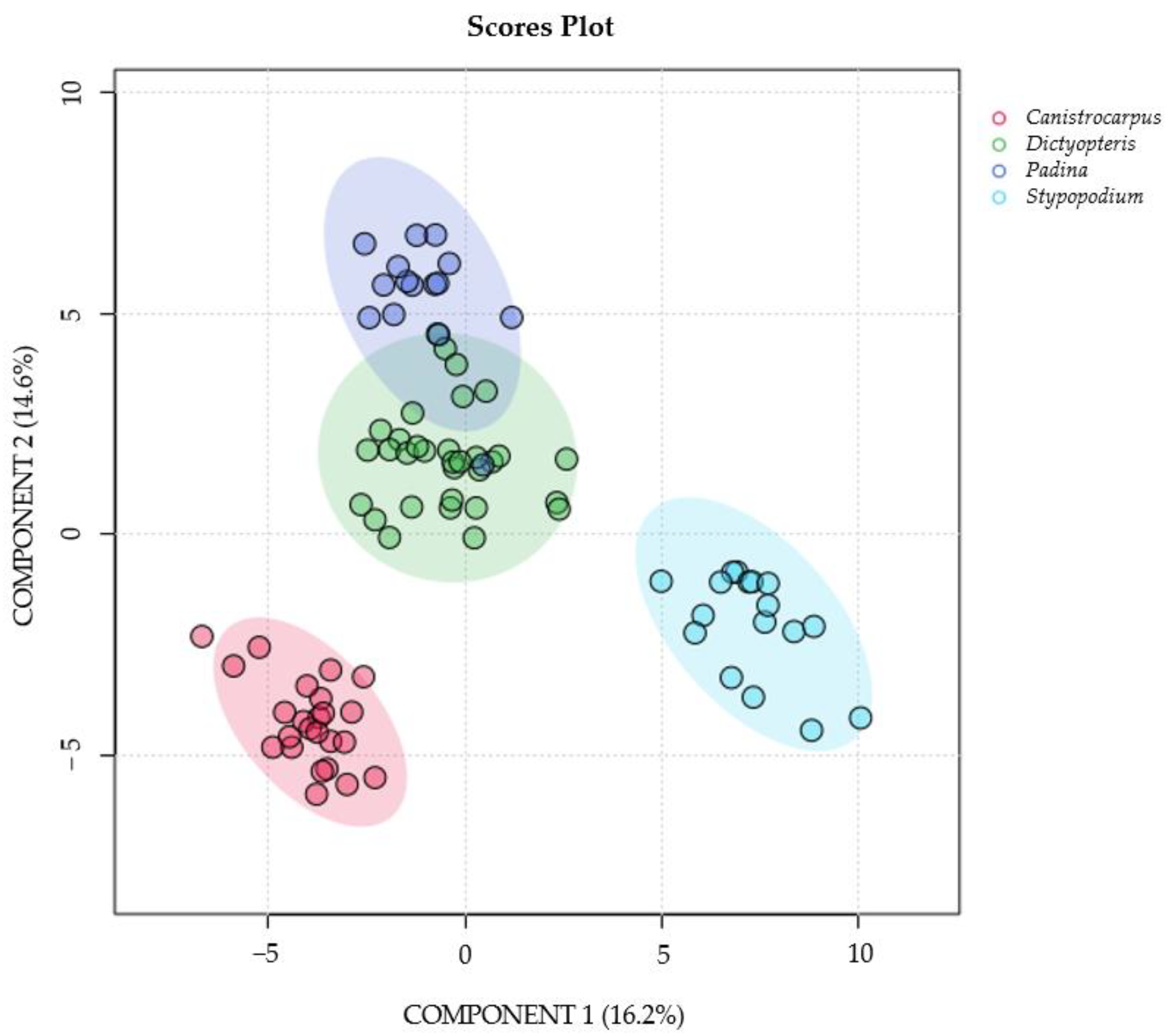
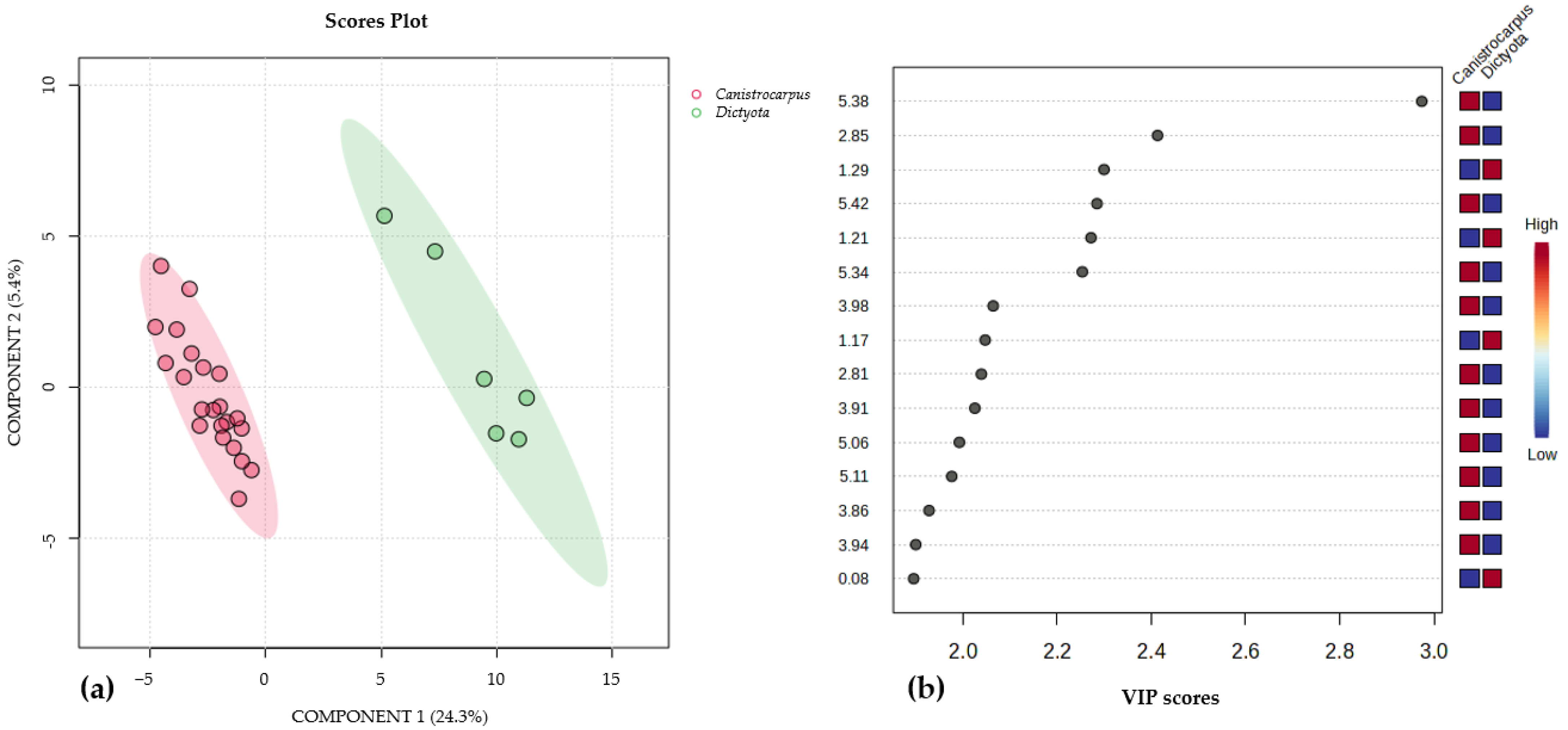
3.2. 1H-NMR Species Comparison Metabolomic Experiment
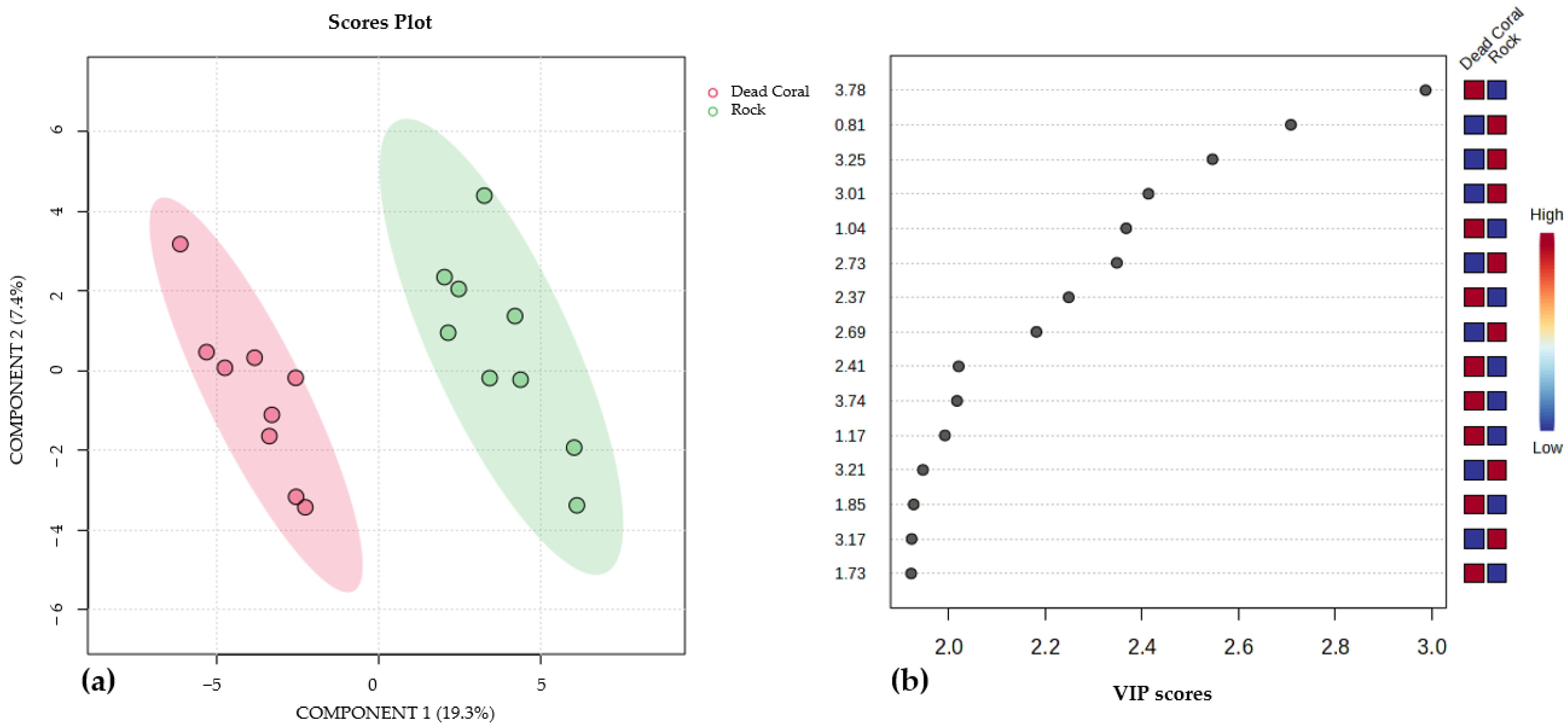
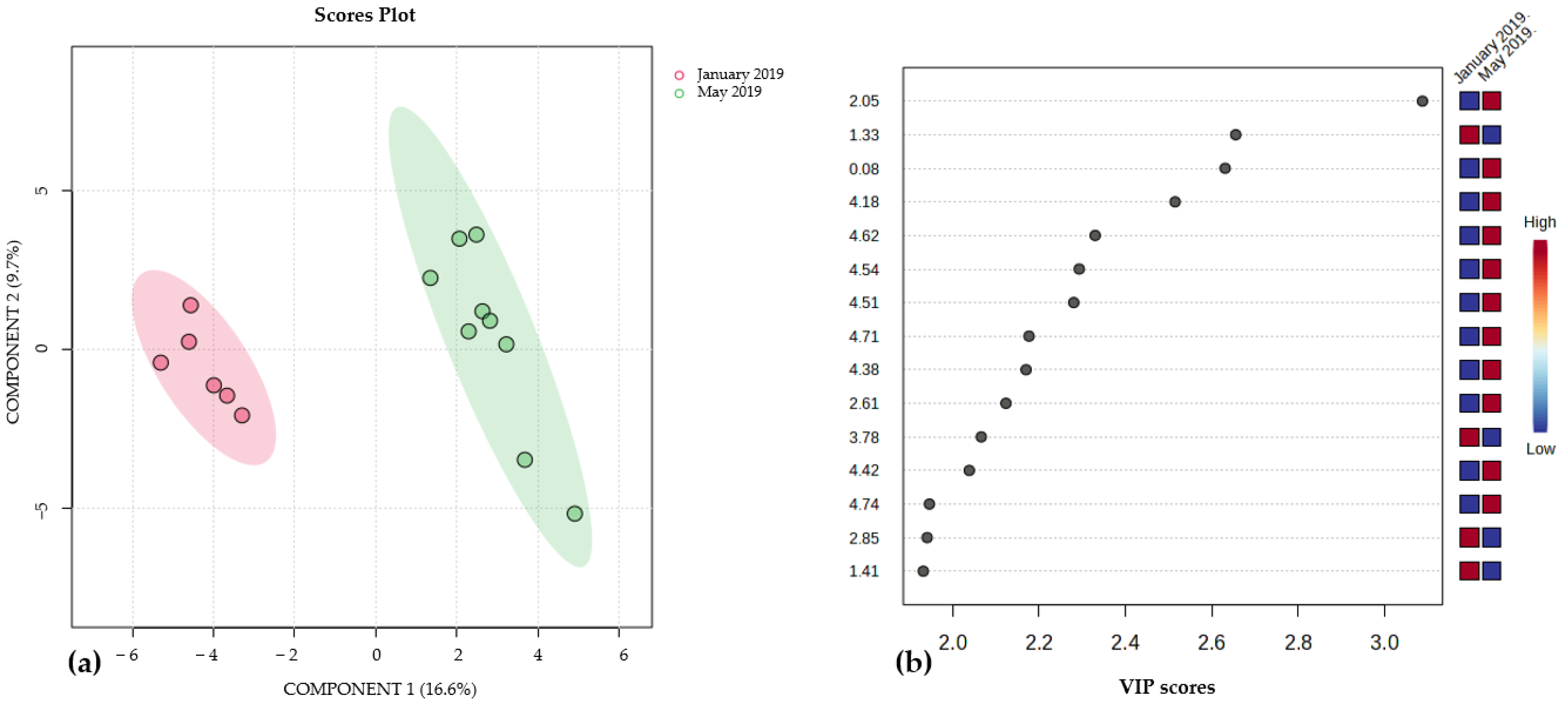
3.3. Effect of Collection Date and Growth Substrate on the Metabolic Profiles of Brown Algae Species
3.4. Juvenile Dictyota spp. Metabolic Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bringloe, T.T.; Starko, S.; Wade, R.M.; Vieira, C.; Kawai, H.; De Clerck, O.; Cock, J.M.; Coelho, S.M.; Destombe, C.; Valero, M.; et al. Phylogeny and Evolution of the Brown Algae. Crit. Rev. Plant Sci. 2020, 39, 281–321. [Google Scholar] [CrossRef]
- Plass-Johnson, J.G.; Ferse, S.C.A.; Jompa, J.; Wild, C.; Teichberg, M. Fish Herbivory as Key Ecological Function in a Heavily Degraded Coral Reef System. Limnol. Oceanogr. 2015, 60, 1382–1391. [Google Scholar] [CrossRef]
- Briones-Fourzán, P.; Lozano-Álvarez, E. The Importance of Lobophora variegata (Phaeophyta: Dictyotales) as a Habitat for Small Juveniles of Panulirus argus (Decapoda: Palinuridae) in a Tropical Reef Lagoon. Bull. Mar. Sci. 2001, 68, 207–2019. [Google Scholar]
- Yñiguez, A.; McManus, J.; Collado-Vides, L. Capturing the Dynamics in Benthic Structures: Environmental Effects on Morphology in the Macroalgal Genera Halimeda and Dictyota. Mar. Ecol. Prog. Ser. 2010, 411, 17–32. [Google Scholar] [CrossRef]
- Paine, E.R.; Schmid, M.; Boyd, P.W.; Diaz-Pulido, G.; Hurd, C.L. Rate and Fate of Dissolved Organic Carbon Release by Seaweeds: A Missing Link in the Coastal Ocean Carbon Cycle. J. Phycol. 2021, 57, 1375–1391. [Google Scholar] [CrossRef]
- Cramer, K.L.; Donovan, M.K.; Jackson, J.B.C.; Greenstein, B.J.; Korpanty, C.A.; Cook, G.M.; Pandolfi, J.M. The Transformation of Caribbean Coral Communities since Humans. Ecol. Evol. 2021, 11, 10098–10118. [Google Scholar] [CrossRef]
- Puyana, M. The Fate of Corals: Will They Overcome Competition with Algae and Cyanobacteria in a Changing Environment? In Corals in a Changing World; Beltran, C.D., Camacho, E.T., Eds.; InTech: Milton, QLD, Australian, 2018. [Google Scholar] [CrossRef]
- Peres, J.C.F.; Carvalho, L.R.D.; Gonçalez, E.; Berian, L.O.S.; Felicio, J.D. Evaluation of Antifungal Activity of Seaweed Extracts. Ciênc. Agrotec. 2012, 36, 294–299. [Google Scholar] [CrossRef]
- Febles, C.I.; Arias, A.; Hardisson, A.; López, A.S.; Gil-Rodríguez, M.C. Antimicrobial Activity of Extracts from Some Canary Species of Phaeophyta and Chlorophyta. Phytother. Res. 1995, 9, 385–387. [Google Scholar] [CrossRef]
- Rubiano-Buitrago, P.; Duque, F.; Puyana, M.; Ramos, F.A.; Castellanos, L. Bacterial Biofilm Inhibitor Diterpenes from Dictyota pinnatifida Collected from the Colombian Caribbean. Phytochem. Lett. 2019, 30, 74–80. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; Barros, C.D.S.; Gomes, M.W.L.; Gomes, R.; Cavalcanti, D.N.; Obando, J.M.C.; Ramos, C.J.B.; Villaça, R.C.; Teixeira, V.L.; Paixão, I.C.N.D.P. In Vitro Antiviral Activity Against Zika Virus From a Natural Product of the Brazilian Brown Seaweed Dictyota menstrualis. Nat. Prod. Commun. 2019, 14, 1934578X19859128. [Google Scholar] [CrossRef]
- Pardo-Vargas, A.; De Barcelos Oliveira, I.; Stephens, P.; Cirne-Santos, C.; De Palmer Paixão, I.; Ramos, F.; Jiménez, C.; Rodríguez, J.; Resende, J.; Teixeira, V.; et al. Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii. Mar. Drugs 2014, 12, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.O.; Maioral, M.F.; Silva, L.O.; Speer, D.B.; Campbell, S.C.; Gallimore, W.; Falkenberg, M.B.; Santos-Silva, M.C. Involvement of the NF-κB and PI3K/Akt/mTOR Pathways in Cell Death Triggered by Stypoldione, an O-quinone Isolated from the Brown Algae Stypopodium zonale. Environ. Toxicol. 2022, 37, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Budzałek, G.; Śliwińska-Wilczewska, S.; Wiśniewska, K.; Wochna, A.; Bubak, I.; Latała, A.; Wiktor, J.M. Macroalgal Defense against Competitors and Herbivores. Int. J. Mol. Sci. 2021, 22, 7865. [Google Scholar] [CrossRef] [PubMed]
- Da Gama, B.A.P.; Pereira, R.C.; Carvalho, A.G.V.; Coutinho, R.; Yoneshigue-Valentin, Y. The Effects of Seaweed Secondary Metabolites on Biofouling. Biofouling 2002, 18, 13–20. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhao, Z.; Xia, X.; Li, B.; Zhang, J.; Yan, X. Diterpenes from the Marine Algae of the Genus Dictyota. Mar. Drugs 2018, 16, 159. [Google Scholar] [CrossRef]
- De Paula, J.C.; Vallim, M.A.; Teixeira, V.L. What Are and Where Are the Bioactive Terpenoids Metabolites from Dictyotaceae (Phaeophyceae). Rev. Bras. Farmacogn. 2011, 21, 216–228. [Google Scholar] [CrossRef]
- Gaubert, J.; Greff, S.; Thomas, O.P.; Payri, C.E. Metabolomic Variability of Four Macroalgal Species of the Genus Lobophora Using Diverse Approaches. Phytochemistry 2019, 162, 165–172. [Google Scholar] [CrossRef]
- Gaubert, J.; Payri, C.E.; Vieira, C.; Solanki, H.; Thomas, O.P. High Metabolic Variation for Seaweeds in Response to Environmental Changes: A Case Study of the Brown Algae Lobophora in Coral Reefs. Sci. Rep. 2019, 9, 993. [Google Scholar] [CrossRef]
- Ivanišević, J.; Thomas, O.P.; Lejeusne, C.; Chevaldonné, P.; Pérez, T. Metabolic Fingerprinting as an Indicator of Biodiversity: Towards Understanding Inter-Specific Relationships among Homoscleromorpha Sponges. Metabolomics 2011, 7, 289–304. [Google Scholar] [CrossRef]
- Jaramillo, K.B.; Reverter, M.; Guillen, P.O.; McCormack, G.; Rodriguez, J.; Sinniger, F.; Thomas, O.P. Assessing the Zoantharian Diversity of the Tropical Eastern Pacific through an Integrative Approach. Sci. Rep. 2018, 8, 7138. [Google Scholar] [CrossRef]
- Mooney, B.D.; Nichols, P.D.; De Salas, M.F.; Hallegraeff, G.M. Lipid, Fatty acid, and Sterol Composition of Eight Species of Kareniaceae (Dinophyta): Chemotaxonomy and Putative Lipid Phycotoxins. J. Phycol. 2007, 43, 101–111. [Google Scholar] [CrossRef]
- Molina, S.L.; Forero, A.M.; Ayala, F.I.; Puyana, M.; Zea, S.; Castellanos, L.; Muñoz, D.; Arboleda, G.; Sandoval-Hernández, A.G.; Ramos, F.A. Metabolic Profiling of the Soft Coral Erythropodium Caribaeorum (Alcyonacea: Anthothelidae) from the Colombian Caribbean Reveals Different Chemotypes. Mar. Drugs 2019, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, D.S.; Shawky, E.; Ibrahim, R.S.; Mohyeldin, M.M. Comprehensive Metabolomics Unveil the Discriminatory Metabolites of Some Mediterranean Sea Marine Algae in Relation to Their Cytotoxic Activities. Sci. Rep. 2022, 12, 8094. [Google Scholar] [CrossRef]
- Dorta, E.; Díaz-Marrero, A.R.; Cueto, M.; Darias, J. On the Relative Stereochemistry of Atomaric Acid and Related Compounds. Tetrahedron 2003, 59, 2059–2062. [Google Scholar] [CrossRef]
- Caamal-Fuentes, E.; Moo-Puc, R.; Freile-Pelegrín, Y.; Robledo, D. Cytotoxic and Antiproliferative Constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm. Biol. 2014, 52, 1244–1248. [Google Scholar] [CrossRef]
- Freitas, O.D.S.P.; De Oliveira, A.S.; De-Paula, J.C.; Pereira, R.C.; Cavalcanti, D.N.; Teixeira, V.L. Chemical Variation in the Diterpenes from the Brazilian Brown Alga Dictyota mertensii (Dictyotaceae, Phaeophyta). Nat. Prod. Commun. 2007, 2, 1934578X0700200104. [Google Scholar] [CrossRef]
- Nunes Pinheiro, A.D.; Pereira Lopes-Filho, E.A.; De-Paula, J.C.; Pereira Netto, A.D.; Teixeira, V.L. Diterpenes from the Brown Alga Dictyota mertensii. Biochem. Syst. Ecol. 2019, 86, 103926. [Google Scholar] [CrossRef]
- Vides, M.; Alonso, D.; Castro, E. Biodiversidad Del Mar de Los Siete Colores; Serie de Publicaciones Generales del INVEMAR; Instituto de Investigaciones Marinas y Costeras—INVEMAR y Corporación para el Desarrollo Sostenible del Archipiélago de San Andrés, Providencia y Santa Catalina—CORALINA: Santa Marta, Colombia, 2016. [Google Scholar]
- Bogaert, K.A.; Delva, S.; De Clerck, O. Concise Review of the Genus Dictyota J.V. Lamouroux. J. Appl. Phycol. 2020, 32, 1521–1543. [Google Scholar] [CrossRef]
- Obando, J.M.C.; Dos Santos, T.C.; Bernardes, M.; Nascimento, N.; Villaça, R.C.; Teixeira, V.L.; Barbarino, E.; Cavalcanti, D.N. Chemical Variation and Analysis of Diterpenes from Seaweed Dictyota Menstrualis under Controlled Conditions. Algal Res. 2022, 62, 102637. [Google Scholar] [CrossRef]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty acids, Amino Acids, Mineral Contents, and Proximate Composition of some Brown Seaweeds. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef]
- Martins, A.P.; Zambotti-Villela, L.; Yokoya, N.S.; Colepicolo, P. Biotechnological Potential of Benthic Marine Algae Collected along the Brazilian Coast. Algal Res. 2018, 33, 316–327. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The Oxylipin Chemistry of Attraction and Defense in Brown Algae and Diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Cronin, G.; Hay, M.E. Within-Plant Variation in Seaweed Palatability and Chemical Defenses: Optimal Defense Theory versus the Growth-Differentiation Balance Hypothesis. Oecologia 1996, 105, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W. Chemical Variation in the Tropical Seaweed Stypopodium zonale (Dictyotaceae). Phytochemistry 1985, 24, 1279–1283. [Google Scholar] [CrossRef]
- Pereira, R.C.; Soares, A.R.; Teixeira, V.L.; Villaça, R.; da Gama, B.A.P. Variation in Chemical Defenses against Herbivory in Southwestern Atlantic Stypopodium zonale (Phaeophyta). Bot. Mar. 2004, 47, 202–208. [Google Scholar] [CrossRef]
- Soares, A.R.; Duarte, H.M.; Tinnoco, L.W.; Pereira, R.C.; Teixeira, V.L. Intraspecific Variation of Meroditerpenoids in the Brown Alga Stypopodium Zonale Guiding the Isolation of New Compounds. Rev. bras. farmacogn. 2015, 25, 627–633. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Fenical, W. Ichthyotoxic and Cytotoxic Metabolites of the Tropical Brown Alga Stypopodium zonale (Lamouroux) Papenfuss. J. Org. Chem. 1981, 46, 22–27. [Google Scholar] [CrossRef]
- De Paula, J.C.; Cassano, V.; Yoneshigue-Valentin, Y.; Teixeira, V.L. Diterpenes from the Brazilian Brown Alga Dictyota crispata (Dictyotaceae, Phaeophyta). Nat. Prod. Commun. 2007, 2, 1934578X0700200205. [Google Scholar] [CrossRef]
- De Rosa, S.; De Stefano, S.; Zavodnik, N. Hydroazulenoid Diterpenes from the Brown Alga Dictyota dichotoma var. Implexa. Phytochemistry 1986, 25, 2179–2181. [Google Scholar] [CrossRef]
- Simas, D.L.R.; Kaiser, C.R.; Gestinari, L.M.; Duarte, H.M.; de Paula, J.C.; Soares, A.R. Diterpenes from the Brown Seaweed Dictyota caribaea (Dictyotaceae, Phaeophyceae): The Ecological and Taxonomic Significance. Biochem. Syst. Ecol. 2014, 52, 33–37. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Attia, E.Z.; Saber, H.; Saber, A.A.; Bringmann, G.; Abdelmohsen, U.R. The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives. Molecules 2022, 27, 672. [Google Scholar] [CrossRef] [PubMed]
- Georgii, A.D.N.P.; Teixeira, V.L. Dictyota and Canistrocarpus Brazilian Brown Algae and Their Bioactive Diterpenes—A Review. Mar. Drugs 2023, 21, 484. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Cavalcanti, D.; Teixeira, V. Effects of Secondary Metabolites from the Tropical Brazilian Brown Alga Dictyota menstrualis on the Amphipod Parhyale hawaiensis. Mar. Ecol. Prog. Ser. 2000, 205, 95–100. [Google Scholar] [CrossRef]
- Ternon, E.; Paix, B.; Thomas, O.P.; Briand, J.-F.; Culioli, G. Exploring the Role of Macroalgal Surface Metabolites on the Settlement of the Benthic Dinoflagellate ostreopsis Cf. Ovata. Front. Mar. Sci. 2020, 7, 683. [Google Scholar] [CrossRef]

| Species | Collection Months | Substrate(s) |
|---|---|---|
| Canistrocarpus crispatus | January 2019, May 2019 | Coralline rock, Dead Coral |
| Stypopodium zonale | May 2018, January 2019, May 2019 | Coralline rock, Dead Coral |
| Padina gymnospora | January 2019, May 2019 | Coralline rock, Sand |
| Dictyopteris delicatula | May 2018, January 2019, May 2019 | Coralline rock, Dead Coral |
| Dictyota spp. | October 2019 | Coralline rock |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Roche, F.; Abril, S.P.; Sepulveda, L.J.; Piza, A.; Castellanos, L.; Rincón, N.; Puyana, M.; Ramos, F.A. Brown Algae from San Andres Island, Southwest Caribbean: A Nuclear Magnetic Resonance Spectroscopy–Metabolomic Study. Metabolites 2025, 15, 305. https://doi.org/10.3390/metabo15050305
de la Roche F, Abril SP, Sepulveda LJ, Piza A, Castellanos L, Rincón N, Puyana M, Ramos FA. Brown Algae from San Andres Island, Southwest Caribbean: A Nuclear Magnetic Resonance Spectroscopy–Metabolomic Study. Metabolites. 2025; 15(5):305. https://doi.org/10.3390/metabo15050305
Chicago/Turabian Stylede la Roche, Felipe, Sara P. Abril, Lady J. Sepulveda, Anderson Piza, Leonardo Castellanos, Natalia Rincón, Mónica Puyana, and Freddy A. Ramos. 2025. "Brown Algae from San Andres Island, Southwest Caribbean: A Nuclear Magnetic Resonance Spectroscopy–Metabolomic Study" Metabolites 15, no. 5: 305. https://doi.org/10.3390/metabo15050305
APA Stylede la Roche, F., Abril, S. P., Sepulveda, L. J., Piza, A., Castellanos, L., Rincón, N., Puyana, M., & Ramos, F. A. (2025). Brown Algae from San Andres Island, Southwest Caribbean: A Nuclear Magnetic Resonance Spectroscopy–Metabolomic Study. Metabolites, 15(5), 305. https://doi.org/10.3390/metabo15050305








