Plasma Dickkopf-1 Levels Are Associated with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measurement
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| hsCRP | High-sensitivity C-reactive protein |
| CV | Coefficient of variation |
| DKK-1 | Dickkopf-1 |
| DM | Diabetes mellitus |
| eGFR | Estimated glomerular filtration rate |
| MACE | Major adverse cardiac event |
| OR | Odds ratio |
| ROC | Receiver operating characteristic |
| UACR | Urine albumin-to-creatinine ratio |
| CI | Confidence interval |
References
- Guo, J.; Liu, Z.; Wang, P.; Wu, H.; Fan, K.; Jin, J.; Zheng, L.; Liu, Z.; Xie, R.; Li, C. Global, regional, and national burden inequality of chronic kidney disease, 1990–2021: A systematic analysis for the global burden of disease study 2021. Front. Med. 2024, 11, 1501175. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Shao, X.; Qin, H.; Yin, P.; Lin, Y.; Wu, J.; Ren, J.; Zheng, Y. Disease Burden and Epidemiological Trends of Chronic Kidney Disease at the Global, Regional, National Levels from 1990 to 2019. Nephron 2024, 148, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zheng, B.; Zhang, L.; Zhang, A.; Jia, Z.; Ding, G. Wnt/beta-Catenin Signaling and Congenital Abnormalities of Kidney and Urinary Tract. Kidney Dis. 2024, 10, 588–599. [Google Scholar]
- Saxena, S.; Dagar, N.; Shelke, V.; Puri, B.; Gaikwad, A.B. Wnt/beta-catenin modulation: A promising frontier in chronic kidney disease management. Fundam. Clin. Pharmacol. 2024, 38, 1020–1030. [Google Scholar] [CrossRef]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT-beta-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Zhao, B.R.; Hu, X.R.; Wang, W.D.; Zhou, Y. Cardiorenal syndrome: Clinical diagnosis, molecular mechanisms and therapeutic strategies. Acta Pharmacol. Sin. 2025. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Hou, F.F.; Zhou, L.; Liu, Y. Wnt/beta-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int. 2019, 95, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Baetta, R.; Banfi, C. Dkk (Dickkopf) Proteins. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1330–1342. [Google Scholar] [CrossRef]
- Li, Y.H.; Wu, M.H.; Lee, W.J.; Lee, I.T. A Synergistic Effect between Plasma Dickkopf-1 and Obstructive Coronary Artery Disease on the Prediction of Major Adverse Cardiac Events in Patients with Angina: An Observational Study. Biomolecules 2022, 12, 1408. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Ho, C.; Shih, Y.H.; Ni, W.C.; Li, Y.C.; Chang, H.C.; Lin, C.L. Knockout of KLF10 Ameliorated Diabetic Renal Fibrosis via Downregulation of DKK-1. Molecules 2022, 27, 2644. [Google Scholar] [CrossRef]
- He, W.; Dai, C.; Li, Y.; Zeng, G.; Monga, S.P.; Liu, Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009, 20, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yen, L.L.; Chen, S.Y.; Kao, M.D.; Tzeng, M.S.; Huang, P.C.; Pan, W.H. Prevalence of overweight and obesity and its associated factors: Findings from National Nutrition and Health Survey in Taiwan, 1993–1996. Prev. Med. 2003, 37, 233–241. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Huang, X.F.; Yan, Q.R.; Bao, C.D. Aberrant activation of the WNT/beta-catenin signaling pathway in lupus nephritis. PLoS ONE 2014, 9, e84852. [Google Scholar]
- Lin, C.L.; Wang, J.Y.; Ko, J.Y.; Huang, Y.T.; Kuo, Y.H.; Wang, F.S. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J. Am. Soc. Nephrol. 2010, 21, 124–135. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Rusu, E.; Zilisteanu, D.; Necula, L.G.; Anton, G.; Tanase, C. Inflammation-Related Patterns in the Clinical Staging and Severity Assessment of Chronic Kidney Disease. Dis. Markers 2019, 2019, 1814304. [Google Scholar] [CrossRef]
- Behets, G.J.; Viaene, L.; Meijers, B.; Blocki, F.; Brandenburg, V.M.; Verhulst, A.; D’Haese, P.C.; Evenepoel, P. Circulating levels of sclerostin but not DKK1 associate with laboratory parameters of CKD-MBD. PLoS ONE 2017, 12, e0176411. [Google Scholar] [CrossRef]
- Hamada-Ode, K.; Taniguchi, Y.; Shimamura, Y.; Fujimoto, S.; Terada, Y. Serum dickkopf-related protein 1 and sclerostin may predict the progression of chronic kidney disease in Japanese patients. Nephrol. Dial. Transplant. 2019, 34, 1426–1427. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chang, C.C.; Hsieh, C.C.; Huang, Y.T.; Shih, Y.H.; Chang, H.C.; Chang, P.J.; Lin, C.L. Dickkopf-1 Acts as a Profibrotic Mediator in Progressive Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 7679. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.B.; Zhang, W.; Wu, L.D.; Liu, Y.S.; Hu, B.; Bi, C.L.; Chen, Y.F.; Liu, X.X.; Ge, C.; et al. Dickkopf-1 as a novel predictor is associated with risk stratification by GRACE risk scores for predictive value in patients with acute coronary syndrome: A retrospective research. PLoS ONE 2013, 8, e54731. [Google Scholar] [CrossRef]
- Jaschke, N.P.; Pahlig, S.; Sinha, A.; Adolph, T.E.; Colunga, M.L.; Hofmann, M.; Wang, A.; Thiele, S.; Schwarzler, J.; Kleymann, A.; et al. Dickkopf1 fuels inflammatory cytokine responses. Commun. Biol. 2022, 5, 1391. [Google Scholar] [CrossRef]
- Park, M.H.; Shin, J.H.; Bothwell, A.L.M.; Chae, W.J. Dickkopf proteins in pathological inflammatory diseases. J. Leukoc. Biol. 2022, 111, 893–901. [Google Scholar] [CrossRef]
- Toso, A.; Leoncini, M.; Maioli, M.; Villani, S.; Bellandi, F. Biomarkers of residual risk and all-cause mortality after acute coronary syndrome. Am. J. Prev. Cardiol. 2025, 21, 100934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, Z.Y.; Wang, K.; Li, W.; Shi, J.M.; Osoro, E.K.; Ullah, N.; Zhou, Y.; Ji, S.R. C-reactive protein exacerbates epithelial-mesenchymal transition through Wnt/beta-catenin and ERK signaling in streptozocin-induced diabetic nephropathy. FASEB J. 2019, 33, 6551–6563. [Google Scholar] [CrossRef] [PubMed]
- Goliasch, G.; Wiesbauer, F.; Kastl, S.P.; Katsaros, K.M.; Blessberger, H.; Maurer, G.; Schillinger, M.; Huber, K.; Wojta, J.; Speidl, W.S. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis 2012, 222, 251–256. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Xu, X.; Wang, S.; Yang, Y.; Zhou, J.; Zhang, L.; Zheng, F.; Li, X.; Wang, B. Embelin attenuates adipogenesis and lipogenesis through activating canonical Wnt signaling and inhibits high-fat diet-induced obesity. Int. J. Obes. 2017, 41, 729–738. [Google Scholar] [CrossRef]
- Yang, T.; Chou, Y.C.; Chu, C.H.; Lin, S.H.; Hsieh, P.C.; Hsu, C.H.; Bai, C.H.; You, S.L.; Sun, C.A. Metabolic syndrome and C-reactive protein concentration as independent correlates of chronic kidney disease. Endocr. Res. 2014, 39, 94–98. [Google Scholar] [CrossRef]


| CKD (n = 62) | nonCKD (n = 311) | p | |||
|---|---|---|---|---|---|
| Age (year) | 68.5 | ±10.7 | 58.2 | ±10.8 | <0.001 |
| Male, n (%) | 49 | (79.0%) | 238 | (76.5%) | 0.793 |
| Current smoker, n (%) | 17 | (27.4%) | 106 | (34.1%) | 0.384 |
| CAD, n (%) | 37 | (59.7%) | 151 | (48.6%) | 0.144 |
| Waist circumference (cm) | 90.2 | ±8.7 | 91.0 | ±9.7 | 0.578 |
| BMI (kg/m2) | 25.4 | ±3.2 | 26.3 | ±3.9 | 0.078 |
| Systolic BP (mmHg) | 132.7 | ±17.5 | 127.0 | ±17.5 | 0.020 |
| Diastolic BP (mmHg) | 73.2 | ±10.7 | 74.9 | ±10.3 | 0.257 |
| DKK-1 (pg/mL) | 697.2 | ±174.7 | 589.0 | ±193.3 | <0.001 |
| Fasting glucose (mmol/L) | 5.3 | ±1.1 | 5.3 | ±0.7 | 0.847 |
| HbA1c (%) | 5.8 | ±0.6 | 5.8 | ±0.6 | 0.828 |
| Total cholesterol (mmol/L) | 4.5 | ±0.9 | 4.5 | ±1.0 | 0.795 |
| HDL cholesterol (mmol/L) | 1.2 | ±0.3 | 1.3 | ±0.3 | 0.558 |
| Triglycerides (mmol/L) | 1.6 | ±1.2 | 1.5 | ±0.9 | 0.514 |
| eGFR (mL/min/1.73 m2) | 53.7 | ±14.1 | 90.3 | ±23.5 | <0.001 |
| C-reactive protein (mg/L) | 3.5 | ±3.2 | 2.1 | ±2.2 | <0.001 |
| Increased UACR, n (%) | 22 | (35.5%) | 34 | (10.9%) | <0.001 |
| Metabolic syndrome, n (%) | 24 | (38.7%) | 138 | (44.4%) | 0.496 |
| Use of antiplatelet agents, n (%) | 56 | (90.3%) | 295 | (94.9%) | 0.231 |
| Use of statins, n (%) | 31 | (50.0%) | 148 | (47.6%) | 0.835 |
| Hypertension, n (%) | 60 | (96.8%) | 289 | (92.9%) | 0.396 |
| Use of antihypertensive agents, n (%) | 53 | (85.5%) | 269 | (86.5%) | 0.993 |
| ACE inhibitor or ARB, n (%) | 38 | (61.3%) | 159 | (51.1%) | 0.185 |
| α-blocker, n (%) | 5 | (8.1%) | 11 | (3.5%) | 0.159 |
| β-blocker, n (%) | 19 | (30.6%) | 83 | (26.7%) | 0.630 |
| Calcium channel blocker, n (%) | 35 | (56.5%) | 159 | (51.1%) | 0.530 |
| Diuretics, n (%) | 16 | (25.8%) | 36 | (11.6%) | 0.006 |
| Continuous Variable | σ | p |
|---|---|---|
| Age | 0.142 | 0.006 |
| Waist | −0.014 | 0.787 |
| BMI | −0.073 | 0.157 |
| Systolic BP | 0.054 | 0.298 |
| Diastolic BP | 0.005 | 0.920 |
| Fasting glucose | −0.005 | 0.931 |
| HbA1c | 0.069 | 0.184 |
| Total cholesterol | 0.112 | 0.031 |
| HDL cholesterol | −0.095 | 0.066 |
| Triglycerides | 0.192 | <0.001 |
| C-reactive protein | 0.094 | 0.068 |
| UACR | 0.113 | 0.029 |
| Variable | Group | Patient Number | Mean | ±SD | Difference in Mean (95% CI) | p | ||
|---|---|---|---|---|---|---|---|---|
| Age | <60 years | 182 | 589.3 | ±199.7 | −34.6 | (−74.1, 4.9) | 0.086 | |
| ≥60 years | 191 | 623.9 | ±188.1 | |||||
| Sex | Female | 86 | 611.1 | ±184.2 | 5.3 | (−41.8, 52.3) | 0.825 | |
| Male | 287 | 605.8 | ±197.6 | |||||
| Current smoker | No | 250 | 593.2 | ±196.6 | −41.8 | (−83.7, 0.1) | 0.051 | |
| Yes | 123 | 635.0 | ±187.5 | |||||
| CAD | No | 185 | 612.0 | ±191.2 | 9.9 | (−29.8, 49.5) | 0.625 | |
| Yes | 188 | 602.1 | ±197.8 | |||||
| Hypertension | No | 24 | 611.3 | ±229.4 | 4.6 | (−76.2, 85.3) | 0.911 | |
| Yes | 349 | 606.7 | ±192.1 | |||||
| Central obesity * | No | 161 | 608.6 | ±188.6 | 2.8 | (−37.2, 42.8) | 0.890 | |
| Yes | 212 | 605.8 | ±199.0 | |||||
| BMI | <27 kg/m2 | 248 | 614.3 | ±191.0 | 21.6 | (−20.3, 63.6) | 0.310 | |
| ≥27 kg/m2 | 125 | 592.6 | ±200.9 | |||||
| Systolic BP | <130 mmHg | 202 | 595.7 | ±197.8 | −24.7 | (−64.4, 14.9) | 0.221 | |
| ≥130 mmHg | 171 | 620.4 | ±189.9 | |||||
| Diastolic BP | <80 mmHg | 258 | 612.5 | ±201.1 | 17.8 | (−25.1, 60.6) | 0.416 | |
| ≥80 mmHg | 115 | 594.7 | ±178.5 | |||||
| Fasting glucose | <7.2 mmol/L | 52 | 574.8 | ±186.1 | −31.6 | (−90.8, 27.7) | 0.296 | |
| ≥7.2 mmol/L | 255 | 606.3 | ±200.3 | |||||
| HbA1c | <8.5% | 274 | 605.9 | ±194.9 | −4.0 | (−48.8, 40.9) | 0.862 | |
| ≥8.5% | 99 | 609.9 | ±193.7 | |||||
| Total cholesterol | <4.14 mmol/L | 139 | 598.9 | ±212.0 | −13.0 | (−53.9, 28.0) | 0.533 | |
| ≥4.14 mmol/L | 234 | 611.8 | ±183.4 | |||||
| Low HDL cholesterol # | No | 269 | 590.8 | ±188.8 | −58.2 | (−102.0, −14.5) | 0.009 | |
| Yes | 104 | 649.0 | ±203.0 | |||||
| Triglycerides | <1.7 mmol/L | 253 | 592.1 | ±191.2 | −46.2 | (−88.4, −4.1) | 0.032 | |
| ≥1.7 mmol/L | 120 | 638.3 | ±198.1 | |||||
| C-reactive protein | <2 mg/L | 216 | 584.0 | ±181.9 | −54.7 | (−94.4, −15.0) | 0.007 | |
| ≥2 mg/L | 157 | 638.7 | ±206.7 | |||||
| UACR | <30 mg/g | 317 | 601.3 | ±193.1 | −38.1 | (−93.4, 17.3) | 0.177 | |
| ≥30 mg/g | 56 | 639.3 | ±200.0 | |||||
| Metabolic syndrome | No | 211 | 592.5 | ±191.6 | −33.5 | (−73.3, 6.3) | 0.099 | |
| Yes | 162 | 625.9 | ±196.9 | |||||
| Use of statins | No | 194 | 607.9 | ±187.0 | 1.9 | (−37.7, 41.6) | 0.923 | |
| Yes | 179 | 606.0 | ±202.5 | |||||
| Use of antihypertensive drugs | No | 51 | 628.8 | ±214.9 | 25.3 | (−32.4, 82.9) | 0.389 | |
| Yes | 322 | 603.5 | ±191.0 | |||||
| Use of antiplatelet drugs | No | 22 | 584.9 | ±222.4 | −23.5 | (−107.6, 60.6) | 0.583 | |
| Yes | 351 | 608.4 | ±192.7 | |||||
| ACE inhibitor or ARB | No | 176 | 617.6 | ±190.6 | 20.2 | (−19.5, 59.8) | 0.318 | |
| Yes | 197 | 597.5 | ±197.7 | |||||
| α-blocker | No | 357 | 608.5 | ±195.1 | 34.0 | (−63.8, 131.7) | 0.495 | |
| Yes | 16 | 574.5 | ±178.7 | |||||
| β-blocker | No | 271 | 603.7 | ±195.9 | −12.1 | (−56.5, 32.4) | 0.594 | |
| Yes | 102 | 615.8 | ±190.9 | |||||
| Calcium channel blocker | No | 179 | 601.7 | ±194.0 | −10.2 | (−49.8, 29.5) | 0.614 | |
| Yes | 194 | 611.9 | ±195.0 | |||||
| Diuretics | No | 321 | 606.3 | ±194.8 | −5.0 | (−62.2, 52.2) | 0.864 | |
| Yes | 52 | 611.3 | ±193.4 | |||||
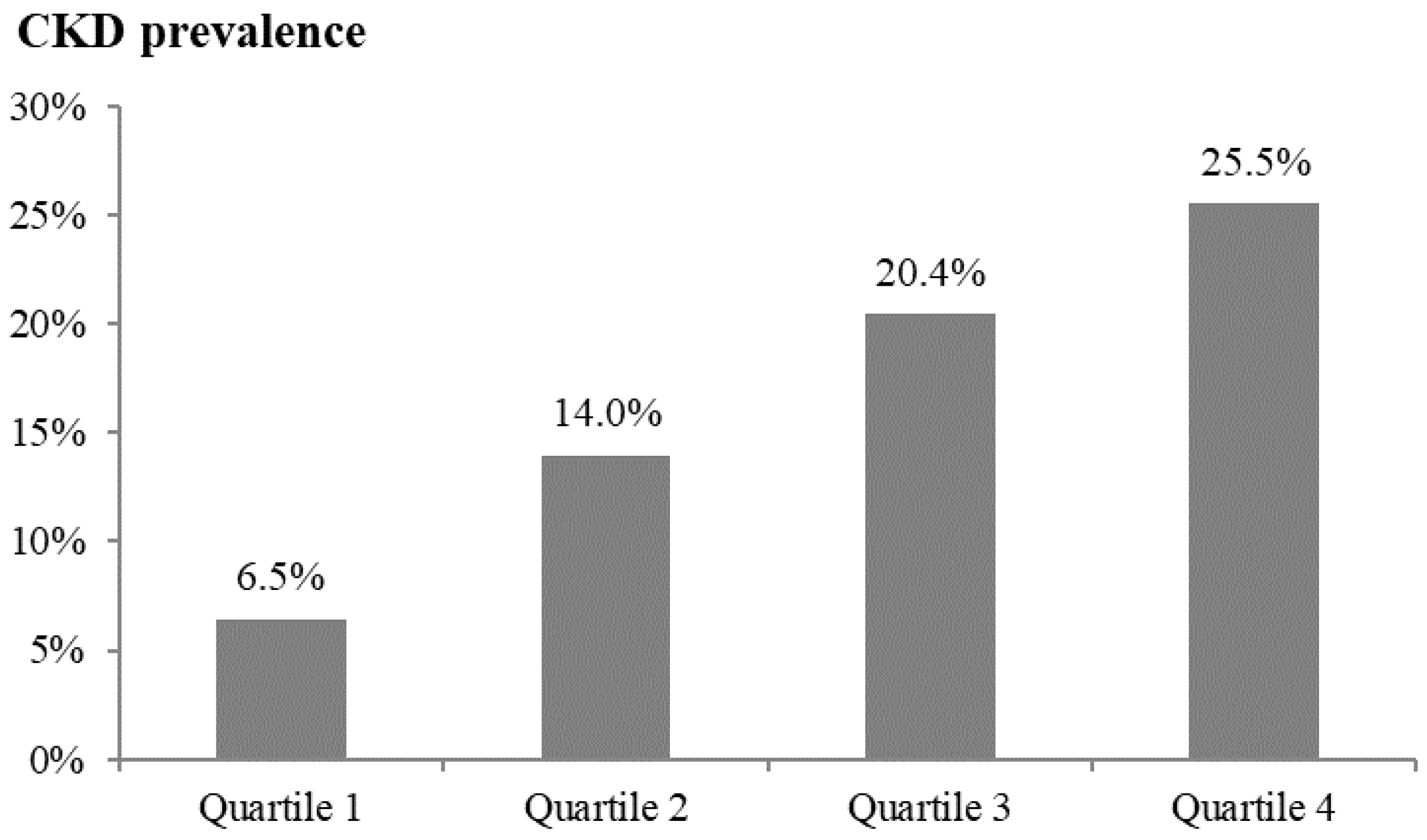
| Quartile 1 n = 93 (180.7–456.7 pg/mL) | Quartile 2 n = 93 (456.8–608.5 pg/mL) | Quartile 3 n = 93 (608.6–747.5 pg/mL) | Quartile 4 n = 94 (747.6–1141.1 pg/mL) | p | |
|---|---|---|---|---|---|
| CKD/nonCKD | 6/87 | 13/80 | 19/74 | 24/70 | |
| Crude | 1.000 (reference) | 2.356 (0.855, 6.494) ** | 3.723 (1.413, 9.809) ** | 4.971 (1.926, 12.833) ** | 0.006 |
| Model 1 | 1.000 (reference) | 2.173 (0.765, 6.173) | 3.769 (1.381, 10.285) ** | 4.437 (1.664, 11.829) ** | 0.014 |
| Model 2 | 1.000 (reference) | 2.193 (0.771, 6.235) | 3.580 (1.307, 9.801) * | 4.188 (1.564, 11.212) ** | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-H.; Cheng, Y.-C.; Wu, J.; Lee, I.-T. Plasma Dickkopf-1 Levels Are Associated with Chronic Kidney Disease. Metabolites 2025, 15, 300. https://doi.org/10.3390/metabo15050300
Li Y-H, Cheng Y-C, Wu J, Lee I-T. Plasma Dickkopf-1 Levels Are Associated with Chronic Kidney Disease. Metabolites. 2025; 15(5):300. https://doi.org/10.3390/metabo15050300
Chicago/Turabian StyleLi, Yu-Hsuan, Yu-Cheng Cheng, Junyi Wu, and I-Te Lee. 2025. "Plasma Dickkopf-1 Levels Are Associated with Chronic Kidney Disease" Metabolites 15, no. 5: 300. https://doi.org/10.3390/metabo15050300
APA StyleLi, Y.-H., Cheng, Y.-C., Wu, J., & Lee, I.-T. (2025). Plasma Dickkopf-1 Levels Are Associated with Chronic Kidney Disease. Metabolites, 15(5), 300. https://doi.org/10.3390/metabo15050300






