Circulating Amino Acid Changes Three Years After Bariatric Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Amino Acid Measurements
2.3. Dietary Amino Acids
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
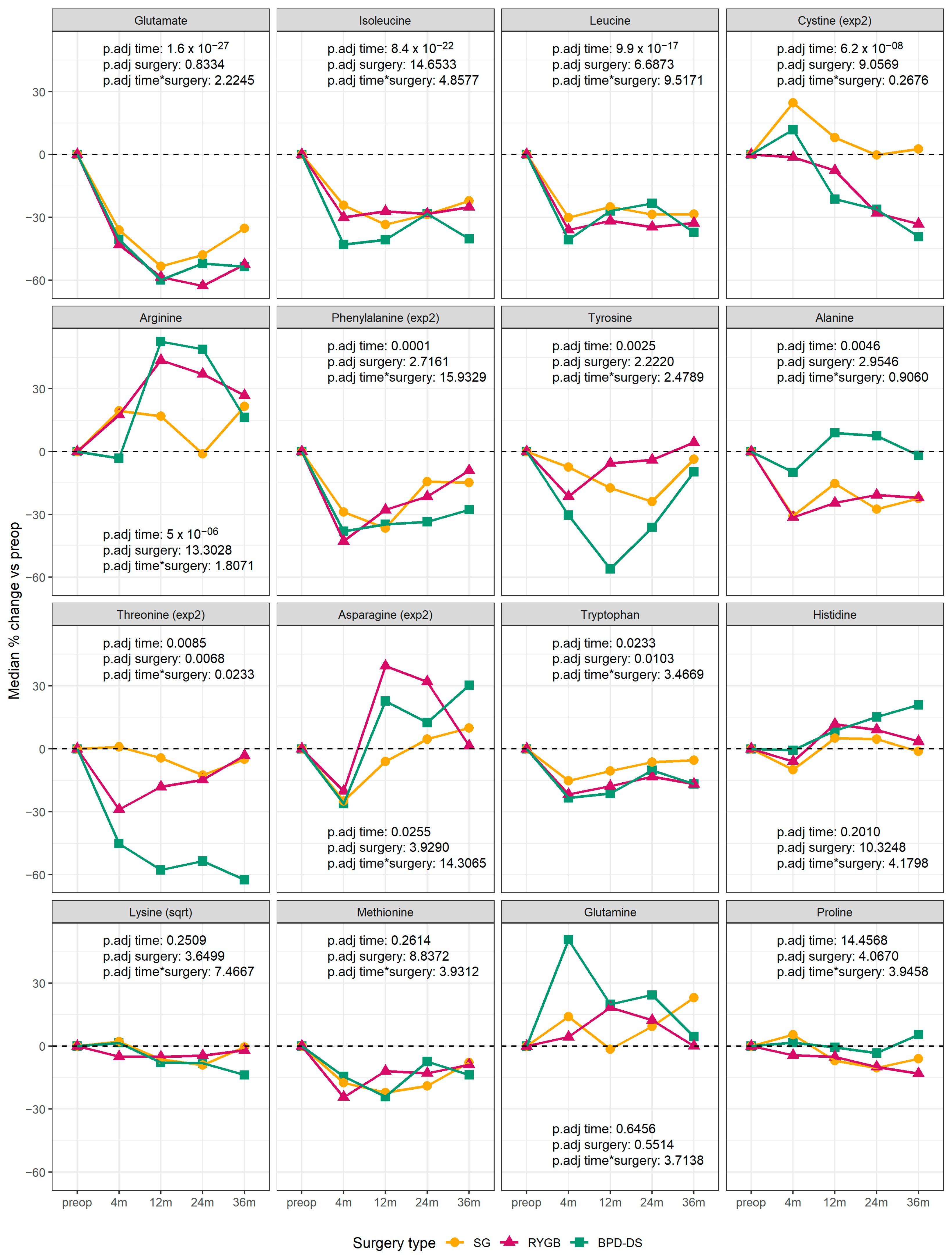
3.2. Changes in Circulating Amino Acids
3.3. Association Between Changes in Amino Acid Levels and Changes in Adiposity/Metabolic Variables
3.4. Association Between Circulating Amino Acids and Weight Regain
3.5. Association Between Circulating Concentrations and Dietary Intakes of Amino Acids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Maltais-Payette, I.; Boulet, M.M.; Prehn, C.; Adamski, J.; Tchernof, A. Circulating glutamate concentration as a biomarker of visceral obesity and associated metabolic alterations. Nutr. Metab. 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Maltais-Payette, I.; Allam-Ndoul, B.; Perusse, L.; Vohl, M.C.; Tchernof, A. Circulating glutamate level as a potential biomarker for abdominal obesity and metabolic risk. Nutr. Metab. Cardiovasc. Dis. NMCD 2019, 29, 1353–1360. [Google Scholar] [CrossRef]
- Maltais-Payette, I.; Vijay, J.; Simon, M.M.; Corbeil, J.; Brière, F.; Grundberg, E.; Tchernof, A. Large-scale analysis of circulating glutamate and adipose gene expression in relation to abdominal obesity. Amino Acids 2022, 54, 1287–1294. [Google Scholar] [CrossRef]
- Kimberly, W.T.; O’Sullivan, J.F.; Nath, A.K.; Keyes, M.; Shi, X.; Larson, M.G.; Yang, Q.; Long, M.T.; Vasan, R.; Peterson, R.T.; et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight 2017, 2, e92989. [Google Scholar] [CrossRef]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256.e245. [Google Scholar] [CrossRef]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps-a Scoping Review. Obes. Surg. 2021, 31, 1755–1766. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2023, 33, 3–14. [Google Scholar] [CrossRef]
- Evers, S.S.; Sandoval, D.A.; Seeley, R.J. The Physiology and Molecular Underpinnings of the Effects of Bariatric Surgery on Obesity and Diabetes. Annu. Rev. Physiol. 2017, 79, 313–334. [Google Scholar] [CrossRef]
- Vaz, M.; Pereira, S.S.; Monteiro, M.P. Metabolomic signatures after bariatric surgery—A systematic review. Rev. Endocr. Metab. Disord. 2021, 23, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Faramia, J.; Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Miard, S.; Carreau, A.M.; Nadeau, M.; Frisch, F.; Baraboi, E.D.; Grenier-Larouche, T.; et al. IGFBP-2 partly mediates the early metabolic improvements caused by bariatric surgery. Cell reports. Med. 2021, 2, 100248. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Maltais-Payette, I.; Tchernof, A. Circulating Glutamate as a Potential Biomarker of Central Fat Accumulation and Concomitant Cardiometabolic Alterations. In Biomarkers in Nutrition; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2022; pp. 955–976. [Google Scholar]
- Gobeil, É.; Maltais-Payette, I.; Taba, N.; Brière, F.; Ghodsian, N.; Abner, E.; Bourgault, J.; Gagnon, E.; Manikpurage, H.D.; Couture, C.; et al. Mendelian Randomization Analysis Identifies Blood Tyrosine Levels as a Biomarker of Non-Alcoholic Fatty Liver Disease. Metabolites 2022, 12, 440. [Google Scholar] [CrossRef]
- Labonté, M.; Cyr, A.; Baril-Gravel, L.; Royer, M.M.; Lamarche, B. Validity and reproducibility of a web-based, self-administered food frequency questionnaire. Eur. J. Clin. Nutr. 2012, 66, 166–173. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef]
- Welch, A.A.; Luben, R.; Khaw, K.T.; Bingham, S.A. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2005, 18, 99–116. [Google Scholar] [CrossRef]
- Aasheim, E.T.; Elshorbagy, A.K.; Diep, L.M.; Sovik, T.T.; Mala, T.; Valdivia-Garcia, M.; Olbers, T.; Bohmer, T.; Birkeland, K.I.; Refsum, H. Effect of bariatric surgery on sulphur amino acids and glutamate. Br. J. Nutr. 2011, 106, 432–440. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Morandi Junqueira-Franco, M.V.; dos Santos, J.E.; Marchini, J.S.; Salgado, W., Jr.; Nonino, C.B. Protein and amino acid status before and after bariatric surgery: A 12-month follow-up study. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2013, 9, 1008–1012. [Google Scholar] [CrossRef]
- Galsgaard, K.D. The Vicious Circle of Hepatic Glucagon Resistance in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2020, 9, 4049. [Google Scholar] [CrossRef] [PubMed]
- Suppli, M.P.; Bagger, J.I.; Lund, A.; Demant, M.; van Hall, G.; Strandberg, C.; Kønig, M.J.; Rigbolt, K.; Langhoff, J.L.; Wewer Albrechtsen, N.J.; et al. Glucagon Resistance at the Level of Amino Acid Turnover in Obese Subjects With Hepatic Steatosis. Diabetes 2020, 69, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e1295. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol.—Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef]
- Laferrère, B.; Reilly, D.; Arias, S.; Swerdlow, N.; Gorroochurn, P.; Bawa, B.; Bose, M.; Teixeira, J.; Stevens, R.D.; Wenner, B.R.; et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med. 2011, 3, 80re82. [Google Scholar] [CrossRef]
- Al-Najim, W.; Docherty, N.G.; le Roux, C.W. Food Intake and Eating Behavior After Bariatric Surgery. Physiol. Rev. 2018, 98, 1113–1141. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Drewnowski, A.; Friedman, M.I. Is there a relationship between dietary MSG and [corrected] obesity in animals or humans? Amino Acids 2014, 46, 2075–2087. [Google Scholar] [CrossRef]
- Lotta, L.A.; Scott, R.A.; Sharp, S.J.; Burgess, S.; Luan, J.; Tillin, T.; Schmidt, A.F.; Imamura, F.; Stewart, I.D.; Perry, J.R.; et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med. 2016, 13, e1002179. [Google Scholar] [CrossRef]
- Krumpochova, P.; Bruyneel, B.; Molenaar, D.; Koukou, A.; Wuhrer, M.; Niessen, W.M.; Giera, M. Amino acid analysis using chromatography-mass spectrometry: An inter platform comparison study. J. Pharm. Biomed. Anal. 2015, 114, 398–407. [Google Scholar] [CrossRef]
- Violi, J.P.; Bishop, D.P.; Padula, M.P.; Steele, J.R.; Rodgers, K.J. Considerations for amino acid analysis by liquid chromatography-tandem mass spectrometry: A tutorial review. TrAC Trends Anal. Chem. 2020, 131, 116018. [Google Scholar] [CrossRef]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Ramos, A.; Shikora, S.; Kow, L. Bariatric Surgery Survey 2018: Similarities and Disparities Among the 5 IFSO Chapters. Obes. Surg. 2021, 31, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lai, Y.; Liu, C.-W.; Ru, H. Towards Mass Spectrometry-Based Chemical Exposome: Current Approaches, Challenges, and Future Directions. Toxics 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Lau, D.C.W.; Douketis, J.D.; Morrison, K.M.; Hramiak, I.M.; Sharma, A.M.; Ur, E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. Can. Med. Assoc. J. 2007, 176, S1. [Google Scholar] [CrossRef] [PubMed]
- MCC. Clinical Laboratory Tests and Adult Normal Values. Available online: https://mcc.ca/examinations-assessments/resources-to-help-with-exam-prep/normal-lab-values/ (accessed on 20 December 2024).
- Rabi, D.M.; McBrien, K.A.; Sapir-Pichhadze, R.; Nakhla, M.; Ahmed, S.B.; Dumanski, S.M.; Butalia, S.; Leung, A.A.; Harris, K.C.; Cloutier, L.; et al. Hypertension Canada’s 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children. Can. J. Cardiol. 2020, 36, 596–624. [Google Scholar] [CrossRef]



| Variable (Unit) | n= | SG | RYBP | BPD-DS | p-Value |
|---|---|---|---|---|---|
| Age (years) | 63 | 51.7 (44.8–55.3) | 51.5 (44.0–54.8) | 51.0 (43.9–55.7) | 0.9471 |
| Sex (female/male) | 63 | 14/7 | 14/7 | 14/7 | 1.0000 |
| Body mass index (kg/m2) | 62 | 44.2 (41.8–48.2) | 40.8 (38.4–43.0) | 49.3 (45.0–50.6) | 3.6 × 10−6 |
| Waist circumference (cm) | 62 | 132 (122–141) | 128 (125–137) | 141 (135–151) | 0.0126 |
| Neck circumference (cm) | 62 | 44.0 (41.0–46.0) | 42.0 (39.0–47.0) | 44.0 (42.5–48.0) | 0.4337 |
| ALT (U/L) | 63 | 27.0 (17.0–36.0) | 32.0 (23.0–44.0) | 29.0 (22.0–36.0) | 0.3783 |
| GGT (U/L) | 63 | 27.0 (21.0–45.0) | 36.0 (22.0–56.0) | 36.0 (24.0–42.0) | 0.6208 |
| HDL-C (mmol/L) | 63 | 1.11 (0.86–1.22) | 1.07 (0.88–1.20) | 1.05 (0.95–1.39) | 0.7518 |
| LDL-C (mmol/L) | 61 | 1.98 (1.68–2.38) | 1.98 (1.51–2.38) | 1.77 (1.51–2.19) | 0.3488 |
| Triglycerides (mmol/L) | 63 | 1.86 (1.20–2.37) | 1.52 (1.33–2.07) | 1.59 (1.16–2.61) | 0.8992 |
| HbA1c (%) | 63 | 6.40 (5.90–7.00) | 7.10 (6.70–7.30) | 6.90 (6.40–7.50) | 0.0537 |
| HOMA-IR | 62 | 13.94 (11.44–17.61) | 11.97 (7.67–16.75) | 14.00 (7.88–26.79) | 0.7092 |
| SBP (mmHg) | 63 | 138 (137–147) | 144 (131–148) | 145 (138–154) | 0.1700 |
| DBP (mmHg) | 63 | 78.0 (71.0–90.0) | 82.0 (74.0–95.0) | 83.0 (77.0–86.0) | 0.5935 |
| Steatosis grade (0/1/2/3) | 57 | 0/13/5/2 | 0/9/5/4 | 0/15/2/2 | 0.4079 |
| NASH (no/yes) | 51 | 5/13 | 5/10 | 6/12 | 0.9199 |
| Fibrosis grade (0/1/2/3/4) | 57 | 5/7/4/4/0 | 5/9/3/1/0 | 3/10/3/3/0 | 0.8084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltais-Payette, I.; Lajeunesse-Trempe, F.; Nadeau, M.; Bouvet-Bouchard, L.; Hould, F.S.; Biertho, L.; Tchernof, A. Circulating Amino Acid Changes Three Years After Bariatric Surgery. Metabolites 2025, 15, 297. https://doi.org/10.3390/metabo15050297
Maltais-Payette I, Lajeunesse-Trempe F, Nadeau M, Bouvet-Bouchard L, Hould FS, Biertho L, Tchernof A. Circulating Amino Acid Changes Three Years After Bariatric Surgery. Metabolites. 2025; 15(5):297. https://doi.org/10.3390/metabo15050297
Chicago/Turabian StyleMaltais-Payette, Ina, Fannie Lajeunesse-Trempe, Mélanie Nadeau, Léonie Bouvet-Bouchard, Frédéric Simon Hould, Laurent Biertho, and André Tchernof. 2025. "Circulating Amino Acid Changes Three Years After Bariatric Surgery" Metabolites 15, no. 5: 297. https://doi.org/10.3390/metabo15050297
APA StyleMaltais-Payette, I., Lajeunesse-Trempe, F., Nadeau, M., Bouvet-Bouchard, L., Hould, F. S., Biertho, L., & Tchernof, A. (2025). Circulating Amino Acid Changes Three Years After Bariatric Surgery. Metabolites, 15(5), 297. https://doi.org/10.3390/metabo15050297








