From Biomarker Discovery to Clinical Applications of Metabolomics in Glioblastoma
Abstract
1. Introduction
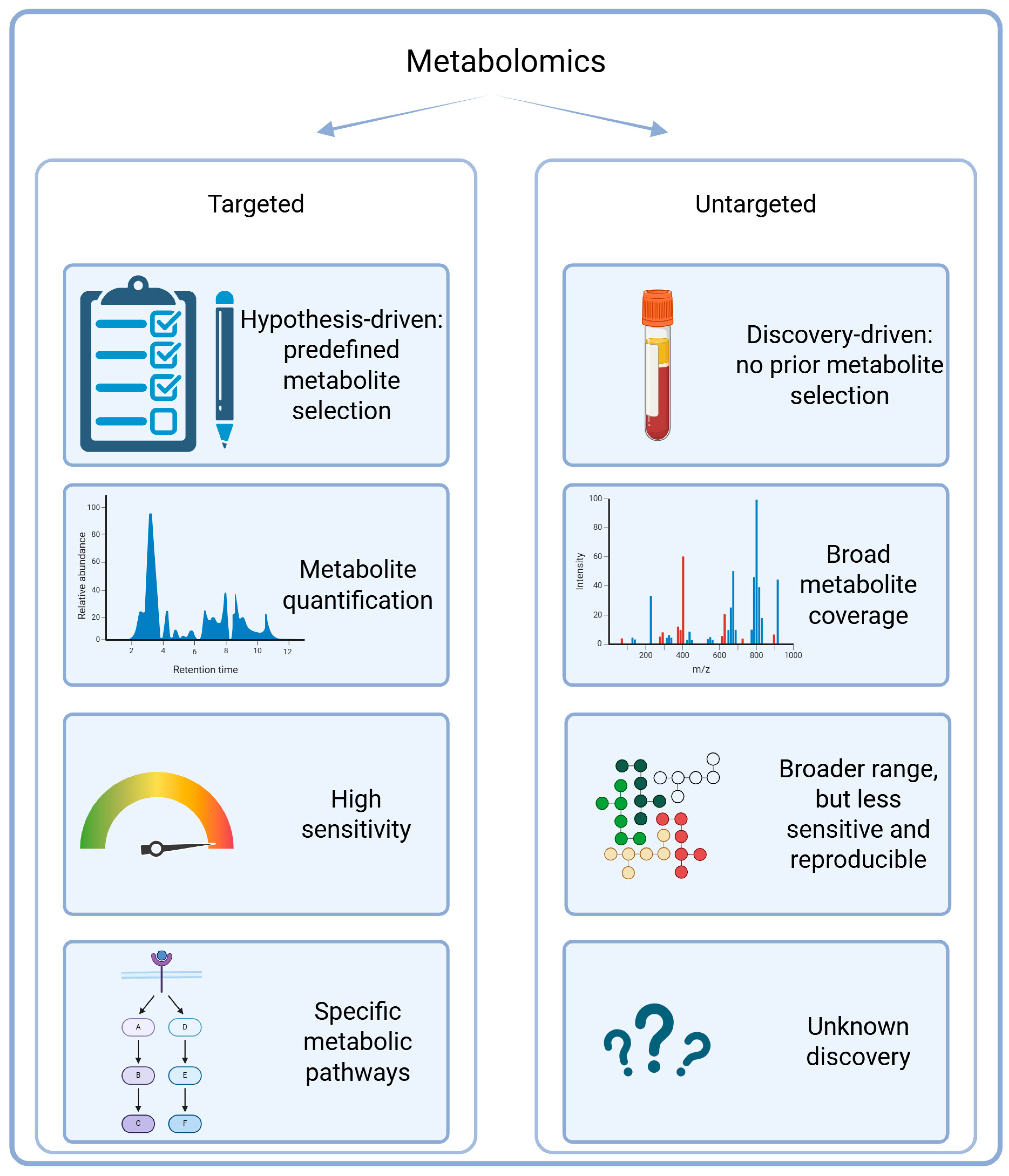
1.1. Metabolomics
1.2. The Metabolic Profile of Glioblastoma
2. Metabolic Reprogramming in Glioblastoma
2.1. Glycolysis, Ketone Bodies, and TCA
| Metabolic Pathway | Metabolites | Sample Type | Identification Method | GBM Specificity | Ref. |
|---|---|---|---|---|---|
| Glycolysis and glucose metabolism | Pyruvate | Plasma CSF | ESI-LTQ-MS H-NMR GC-MS | ↑ IDH-wt GBM vs. IDH-mut astrocytoma ↑ glioma patients vs. healthy individuals | [6,21,23,24] |
| Lactate | CSF Tumor Plasma | MALDI-TOF-MSI H-NMR GC-MS LC-MS/MS-SRM | ↑ TP53-wt vs. TP-mut GBM ↑ GBM vs. peritumoral tissue ↓ Glioma patients vs. healthy individuals ↑ Glioma patients vs. healthy individuals ↓ IDH-wt vs. IDH-mut GBM ↑ associated with poor OS in grade IV gliomas | [3,6,23,24,28] | |
| α/β-glucose | Plasma | H-NMR | ↓ Glioma patients vs. healthy individuals | [23] | |
| D-Fructose | Tumor | GC-MS | ↓ GBM vs. peritumoral tissue | [31] | |
| Glycerol-3-phosphate | Tumor | IMAC–SRM LC/GC-MS | ↑ IDH-mut grade IV astrocytoma vs. IDH-wt GBM ↑ invasive vs. non-invasive regions of GBM | [32,33] | |
| Tricarboxylic acid cycle | Citric acid | CSF | GC-MS | ↑ GBM vs. low-grade glioma ↓ IDH-wt vs. IDH-mut grade IV astrocytoma | [6] |
| Citrate | Tumor Plasma | MALDI-TOF-MSI H-NMR | ↑ GBM vs. peritumoral tissue ↓ glioma patient vs. healthy individual ↓ GBM vs. low-grade gliomas | [3,13,23] | |
| Isocitric acid | CSF | GC-MS | ↑ GBM vs. low-grade gliomas ↓ IDH-wt vs. IDH-mut grade IV astrocytoma | [6] | |
| Succinate | Tumor | LC-MS/MS | ↓ IDH-wt GBM vs. healthy individuals | [13,34] | |
| D-2-hydroxyglutarate | Tumor CSF | IMAC-SRM LC-MS/MS Enzymatic assay MALDI-TOF-MSI HR-NMR | ↑ IDH-mut vs. IDH-wt grade IV astrocytoma | [3,13,29,32,34,35,36,37] | |
| Homocitrate | Tumor | LC-MS/MS | ↓ IDH-wt GBM | [34] | |
| Itaconate | Tumor | LC-MS/MS | ↓ IDH-wt GBM | [34] | |
| Malate | Tumor Plasma | LC-MS/MS GC-MS | ↑ IDH-wt GBM ↑ Glioma patients vs. healthy individuals | [24,34] | |
| Fumarate | Plasma | GC-MS | ↑ Glioma patients vs. healthy individuals | [24] | |
| α-ketoglutaric acid | Tumor | GC-MS | ↑ GBM vs. healthy surrounding brain tissue | [31] | |
| Ketone bodies | 3-hydroxybutanoic acid | Tumor | GC-MS | ↑ GBM vs. healthy surrounding brain tissue | [31] |
| Ketovalerate | Tumor | LC-MS/MS | ↑ IDH-wt GBM | [34] | |
| Carbohydrates and derivatives | Myo-inositol | Tumor Plasma | IMAC-SRM H-NMR HR-MRS GC-MS | ↑ IDH-mut grade IV astrocytoma vs. GBM ↓ Glioma patients vs. healthy individuals ↓ GBM patients vs. healthy individuals ↓ GBM vs. healthy surrounding brain tissue | [23,31,32,36,38,39] |
| Arabinose/arabitol Maltitol Trehalose Pentonic acid | Tumor | GC-MS | ↓ GBM vs. healthy surrounding brain tissue | [31] |
2.2. Amino Acid Metabolism
| Molecules | Metabolites | Sample Type | Identification Method | GBM Specificity | Ref. |
|---|---|---|---|---|---|
| Amino acids | Valine | Plasma Tumor | LC-MS/MS H-NMR GC-MS | ↑ GBM vs. healthy individuals ↑ Glioma patients vs. healthy individuals ↑ GBM vs. surrounding healthy brain tissue | [23,26,27,31] |
| Alanine | Plasma Tumor | H-NMR GC-MS MRS | ↓ Glioma patients vs. healthy individuals ↑ GBM vs. surrounding healthy brain tissue ↑ IDH-wt grade IV astrocytoma ↑ Associated with poor OS | [23,31,39] | |
| Tyrosine | Plasma | H-NMR | ↓ Glioma patients vs. healthy individuals | [23] | |
| Leucine | Plasma | H-NMR LC-MS/MS | ↓ Glioma patients vs. healthy individuals | [23,26] | |
| Isoleucine | Plasma | H-NMR LC-MS/MS | ↓ Glioma patients vs. healthy individuals | [23,26] | |
| Asparagine | Plasma Tumor | LC-MS/MS | ↓ GBM patients vs. healthy individuals ↑ IDH-wt GBM | [26,27,31,34] | |
| Serine | Plasma Tumor | LC-MS/MS GC-MS | ↑ High levels associated with poor OS ↑ GBM vs. surrounding healthy brain tissue | [26,31] | |
| Taurine | Plasma Tumor | LC-MS/MS MALDI-FTICR-MS | ↑ High levels associated with poor OS ↑ GBM patients vs. healthy individuals | [3,26] | |
| Citrulline | Plasma | LC-MS/MS | ↑ associated with GBM progression and poor OS ↑ GBM patients vs. healthy individuals | [26,27] | |
| Glutamine | Plasma Tumor | LC-MS/MS MALDI-TOF-MS | ↑ GBM patients vs. healthy individuals ↑ GBM vs. peritumoral tissue | [3,13,27] | |
| Lysine | Plasma Tumor | LC-MS/MS GC-MS | ↑ GBM patients vs. healthy individuals ↑ GBM vs. healthy surrounding brain tissue | [27,31] | |
| Ornithine | Plasma Tumor | LC-MS/MS GC-MS | ↑ GBM patients vs. healthy individual ↑ GBM vs. healthy surrounding brain tissue | [25,27,31] | |
| Threonine | Plasma | LC-MS/MS | ↓ GBM patients vs. healthy individuals | [27] | |
| Tryptophan | Plasma Tumor | LC-MS/MS GC-MS | ↓ GBM patients vs. healthy individuals ↑ GBM vs. healthy surrounding brain tissue | [27,31] | |
| Methionine | Plasma | LC-MS/MS | ↑ IDH-mut vs. IDH-wt grade IV astrocytoma | [25,45] | |
| Arginine | Plasma | LC-MS/MS | ↓ Associated with better OS ↑ Associated with high grade gliomas | [25,45] | |
| Phenylalanine | Plasma Tumor | H-NMR GC-MS | ↓ Glioma patients vs. healthy individuals ↑ GBM vs. healthy surrounding brain tissue | [23,31] | |
| Aspartate | Tumor | HR-MAS | ↑ Associated with better OS | [36] | |
| Glycine | Tumor | GC-MS | ↑ GBM vs. healthy surrounding brain tissue | [31] | |
| Sarcosine | Plasma | LC-MS/MS | ↑ Associated with IDH-mut GBM | [25] | |
| Amino acid derivatives | N-Acetylaspartate | Tumor | MALDI-TOF-MSI HR-MAS | ↓ GBM vs. peritumoral tissue ↓ GBM vs. low grade astrocytoma | [3,36] |
| Glutamate | Plasma Tumor | LC-MS/MS H-NMR | ↑ GBM patients vs. healthy individuals ↑ associated with poor OS ↓ Glioma patients vs. healthy individuals ↓ IDH-mut vs. IDH-wt grade IV astrocytoma | [23,26,42] | |
| Cystathionine | Tumor | LC-MS/MS GC-MS | ↑ Invasive vs. non-invasive regions of GBM | [33] | |
| S-Methyl-L-cysteine | Tumor | GC-MS | ↑ GBM vs. healthy surrounding brain tissue | [31] | |
| 4-Hydroxyglutamate | Tumor | LC-MS/MS | ↑ Associated with IDH-wt GBM | [34] | |
| Methylhistidine | Plasma | H-NMR | ↓ Glioma patients vs. healthy individuals | [23] | |
| Allothreonine | Tumor | GC-MS | ↑ GBM vs. healthy surrounding brain tissue | [31] | |
| Kynurenate | Plasma | LC-MS/MS | ↑ High values associated with low OS | [45] | |
| 3-Cyanoalanine | Tumor | GC-MS | ↑ GBM vs. healthy surrounding brain tissue | [31] | |
| Arginyl-proline | Plasma | ESI-LTQ-MS | ↑ Associated with IDH-wt GBM | [21] | |
| Pyroglutamic acid | Plasma | LC-MS/MS GC-MS | ↓ Glioma patients vs. healthy individuals ↑ Associated with high grade gliomas | [24,25] | |
| Aminoadipate | Tumor | LC-MS/MS | ↑ IDH-wt GBM | [34] | |
| 4-Hydroxyphenylpyruvate | Tumor | LC-MS/MS | ↑ IDH-wt GBM | [34] | |
| Cis-4-Hydroxyproline | Plasma | LC-MS/MS MALDI-TOF | ↑ GBM patients vs. healthy individuals | [27] | |
| Trans-4-Hydroxyproline | Plasma | LC-MS/MS MALDI-TOF | ↓ GBM patients vs. healthy individuals | [27] | |
| Neurotransmitter-related amino acid derivatives | GABA | CSF | MRS | ↑ TP53-wt vs. TP53-mut GBM ↑ PTEN-mut vs. PTEN-wt GBM | [42] |
| 5-Methoxytryptamine | Tumor | LC-MS/MS GC-MS | ↑ Invasive vs. non-invasive regions of GBM | [33] | |
| Aminobutanal | CSF | LC-MS/MS-SRM | ↑ Associated with poor OS | [42] | |
| Acetylcholine | CSF | LC-MS/MS-SRM | ↑ Associated with poor OS | [28] | |
| Polyamines | Putrescine | Plasma | LC-MS/MS MALDI-TOF | ↑ GBM patients vs. healthy individuals | [27] |
| Spermidine | Plasma Tumor | LC-MS/MS | ↑ GBM patients vs. healthy individuals ↑ Invasive vs. core regions of GBM | [26,33] | |
| Spermine | Plasma | LC-MS/MS | ↓ GBM patients vs. healthy individuals | [27] | |
| N-acetylputrescine | CSF Plasma | LC-MS/MS-SRM LC-MS | ↑ GBM pre- vs. post-treatment ↑ Associated with IDH-wt GBM | [25,42] | |
| Creatine related | Guanidoacetic acid | Plasma | LC-MS/MS | ↑ Associated with IDH-wt GBM | [25] |
| Creatinine | Tumor Plasma | LC-MS/MS H-NMR HR-MAS LC-MS | ↓ Associated with IDH-wt GBM ↑ Associated with IDH-wt GBM ↑ Associated with invasive GBM borders ↓ Glioma patients vs. healthy individuals ↓ Associated with grade IV astrocytoma | [23,25,33,34,36] |
2.3. Lipid Metabolism
| Molecules | Metabolites | Sample Type | Identification Method | GBM Specificity | Ref. |
|---|---|---|---|---|---|
| Fatty acids | Arachidonic acid | Tumor | MALDI-FTICR-MS GC/LC-MS/MS | ↓ GBM vs. peritumoral tissue ↑ Higher in mesenchymal-like GBM subtype | [3,42] |
| Adrenic acid | Tumor | MALDI-FTICR-MS | ↓ GBM vs. peritumoral tissue | [3] | |
| Docosahexaenoic acid (22:6) | Tumor | GC/LC-MS/MS | ↑ Higher in proneural-like GBM subtype | [42] | |
| 3-oxodecanoyl-CoA | Plasma | ESI-LTQ-MS | ↑ Associated with IDH-wt GBM vs. IDH-mut GBM | [21] | |
| α-hydroxyisovalerate | Plasma | GC-MS | ↑ Glioma patients vs. healthy individuals | [24] | |
| Methyl hexadecanoic acid | Plasma | GC-MS | ↓ Glioma patients vs. healthy individuals | [24] | |
| Acylcarnitines | Carnitine | CSF Tumor Plasma | LC-MS/MS-SRM LC-MS/MS LC-MS/MS | ↑ GBM pre- vs. post-treatment ↑ IDH-wt GBM ↑ P53-wt vs. P53-mut GBM | [25,27,28,34] |
| Propionylcarnitine 2-methylbutyrylcarnitine Isobutyryl-L-carnitine Deoxycarnitine L-palmitoylcarnitine | CSF | LC-MS/MS-SRM | ↑ GBM pre- vs. post-treatment ↑ P53-wt vs. P53-mut GBM | [28] | |
| Pymeloylcarnitine | Plasma | FIA-MS | ↑ GBM patients vs. healthy individuals | [26] | |
| Hydroxyhexadecenoylcarnitine Hydroxyhexadecadienylcarnitine | Plasma | LC-MS/MS | ↑ Associated with better OS | [26] | |
| Octanylcarnitine | Plasma | LC-MS/MS | ↑ Associated with poor OS | [26] | |
| Stearoylcarnitine | Tumor | LC-MS/MS | ↑ GBM patients vs. healthy individuals | [27] | |
| Cholesterol and isoprenoids | Cholesterol | Tumor Plasma | LC-MS/MS | ↑ Associated with EGFR activation in GBM ↑ GBM patients vs. healthy individuals ↑ Invasive vs. core GBM regions | [27,33,52] |
| Farnesyl diphosphate | CSF | LC-MS/MS-SRM | ↑ GBM pre- vs. post-treatment | [28] | |
| Phospholipids | Phosphatidylserine (38:9) | Plasma | ESI-LTQ-MS | ↑ Associated with IDH-wt GBM | [21] |
| Phosphatidylcholine | Tumor | LC-MS/MS HR-MAS | ↑ Associated with poor OS ↑ Associated with GBM | [34,36] | |
| Lyso PC a C18:0 | Plasma | FIA-MS | ↑ GBM patients vs. healthy individuals ↑ Associated with poor OS | [26] | |
| Lyso PC a C16:0 Lyso PC a C18:1 Lyso PC a C20:3 PC aa C38:5 PC ae C42:5 | Plasma | FIA-MS | ↑ Associated with poor OS | [26] | |
| PC aa C14:2 | Tumor | LC-MS/MS | ↓ GBM patients vs. healthy individuals | [27] | |
| PC ae C40:3 | Plasma | FIA-MS | ↑ GBM patients vs. healthy individuals | [26] | |
| PC ae C40:6 | Tumor | LC-MS/MS | ↑ GBM patients vs. healthy individuals | [27] | |
| PC aa C36:5 | Plasma | FIA-MS | ↓ GBM patients vs. healthy individuals | [26] | |
| PC aa C36:4 | Plasma | FIA-MS | ↑ Associated with better OS | [26] | |
| PC aa C38:6 PC aa C34:1 | Plasma | LC-MS/MS | ↑ GBM patients vs. healthy individuals | [27] | |
| PC aa C32:1 | Plasma Tumor | FIA-MS MALDI-TOF | ↓ GBM patients vs. healthy individuals ↑ GBM patients vs. healthy individuals | [26,27] | |
| O-phosphoethanolamine | Plasma | GC-MS | ↓ Glioma patients vs. healthy individuals | [24] | |
| Triglycerids | Triglycerol [48:1, 48:2, 50:2, 50:3, 52:2, 52:3, 52:4, 52:5, 54:3, 54:4, 54:5, 54:6] | Plasma | LC-MS/MS MALDI-TOF | ↑ GBM patients vs. healthy individuals | [27] |
| Sphingolipids | 3-O-sulfogalactosylceramide | Plasma | ESI-LTQ-MS | ↓ Associated with IDH-wt GBM | [21] |
| Sphingomyelin (33:1) | Tumor | MALDI-TOF | ↑ GBM patients vs. healthy individuals | [27] |
2.4. Metabolism of Nucleotides, Vitamins, and Hormones
| Molecules | Metabolites | Sample Type | Identification Method | GBM Specificity | Ref. |
|---|---|---|---|---|---|
| Nucleotide and nucleic acid metabolism | Adenine | Tumor | LC-MS/MS | ↑ Associated with poor OS | [34] |
| Uracil | Tumor Plasma | LC-MS/MS | ↑ Associated with IDH-wt GBM ↑ Associated with high-grade gliomas | [25,34] | |
| Thymine | Tumor | LC-MS/MS | ↑ Associated with IDH-wt GBM | [34] | |
| Uridine | Tumor CSF Plasma | LC-MS/MS MSI LC-MS/MS-SRM LC-MS/MS | ↑ Associated with IDH-wt GBM ↑ GBM vs. peritumoral tissue ↑ GBM pre- vs. post-treatment | [3,25,28,34] | |
| Deoxyinosine | Tumor | LC-MS/MS | ↑ Associated with IDH-wt GBM | [34] | |
| Guanosine | Tumor | LC-MS/MS | ↓ Associated with IDH-wt GBM | [34] | |
| AMP | Tumor | LC-MS/MS MSI | ↑ Associated with poor OS ↑ GBM vs. peritumoral tissue | [3,34] | |
| ADP UMP UDP | Tumor | MSI | ↑ GBM vs. peritumoral tissue | [3] | |
| dGMP | Tumor | LC-MS/MS | ↓ Associated with poor OS | [34] | |
| dCMP Nicotinamide mononucleotide | Tumor | LC-MS/MS | ↓ Associated with IDH-wt GBM | [34] | |
| 5-hydroxymethyluracil | Plasma | ESI-LTQ-MS | ↑ Associated with IDH-wt GBM | [21] | |
| Vitamins, hormones, redox metabolism | Ascorbic acid Glutathione | Tumor | MALDI-FTICR-MS | ↑ GBM core vs. peritumoral tissue | [3] |
| Thiamine | Tumor | LC-MS/MS | ↑ Associated with IDH-wt GBM | [34] | |
| Pyridoxal phosphate | Tumor | LC-MS/MS | ↓ Associated with IDH-wt GBM | [34] | |
| N-acylphosphatidylethanolamine | Plasma | ESI-LTQ-MS | ↑ Associated with IDH-wt GBM | [21] | |
| Choline | CSF Plasma Tumor | LC-MS/MS-SRM H-NMR HR-MAS | ↑ TP53-wt vs. TP53-mut GBM ↑ PTEN-mut vs. PTEN-wt GBM ↓ Glioma patients vs. healthy individuals ↑ Associated with GBM | [23,28,36] | |
| Other | Shikimate | CSF | LC-MS/MS-SRM | ↑ GBM pre- vs. post-treatment | [28] |
| Trimethylamine-N-oxide | Plasma | LC-MS/MS | ↑ Associated with IDH-wt gliomas | [25] |
3. Metabolomics Approaches in Glioblastoma
3.1. Targeted and Untargeted Approaches
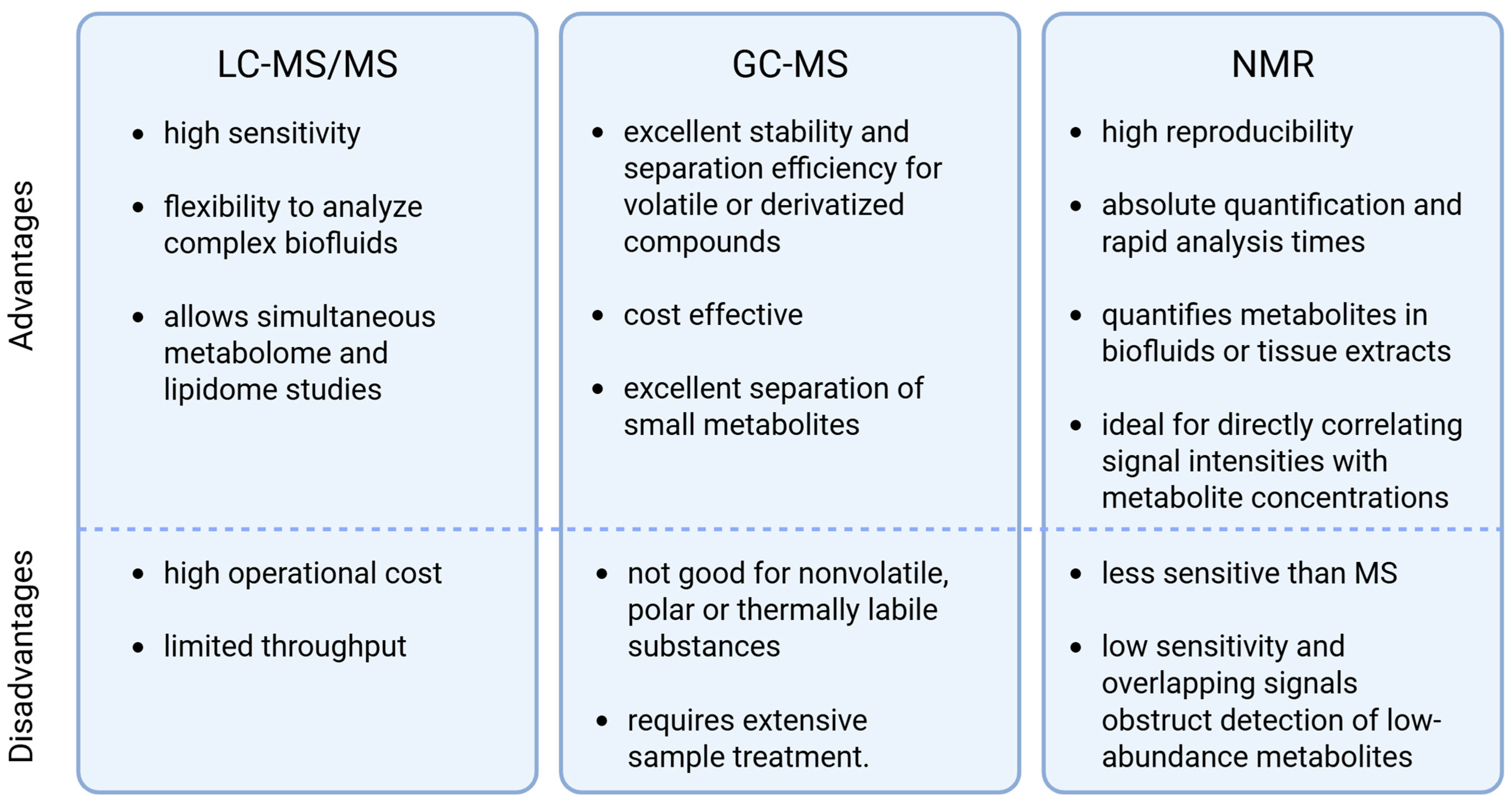
3.1.1. Overview of Targeted Approaches
3.1.2. Overview of Untargeted Approaches
3.1.3. Data Processing and Analysis
3.2. Multiomics Integration
4. Clinical Translation of Metabolomics
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandher, R.; Ducruix, C.; Eccles, S.A.; Raynaud, F.I. Cross-platform Q-TOF validation of global exo-metabolomic analysis: Application to human glioblastoma cells treated with the standard PI 3-Kinase inhibitor LY294002. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- St-Coeur, P.D.; Touaibia, M.; Cuperlovic-Culf, M.; Morin, P., Jr. Leveraging metabolomics to assess the next generation of temozolomide-based therapeutic approaches for glioblastomas. Genom. Proteom. Bioinform. 2013, 11, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kampa, J.M.; Kellner, U.; Marsching, C.; Ramallo Guevara, C.; Knappe, U.J.; Sahin, M.; Giampà, M.; Niehaus, K.; Bednarz, H. Glioblastoma multiforme: Metabolic differences to peritumoral tissue and IDH-mutated gliomas revealed by mass spectrometry imaging. Neuropathology 2020, 40, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Dang, C.V. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006, 66, 8927–8930. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Nakamizo, S.; Sasayama, T.; Shinohara, M.; Irino, Y.; Nishiumi, S.; Nishihara, M.; Tanaka, H.; Tanaka, K.; Mizukawa, K.; Itoh, T.; et al. GC/MS-based metabolomic analysis of cerebrospinal fluid (CSF) from glioma patients. J. Neuro-Oncol. 2013, 113, 65–74. [Google Scholar] [CrossRef]
- Zarzuela, L.; Duran, R.V.; Tome, M. Metabolism and signaling crosstalk in glioblastoma progression and therapy resistance. Mol. Oncol. 2025, 19, 592–613. [Google Scholar] [CrossRef]
- Yoshida, M.; Hatano, N.; Nishiumi, S.; Irino, Y.; Izumi, Y.; Takenawa, T.; Azuma, T. Diagnosis of gastroenterological diseases by metabolome analysis using gas chromatography-mass spectrometry. J. Gastroenterol. 2012, 47, 9–20. [Google Scholar] [CrossRef]
- Hirayama, A.; Kami, K.; Sugimoto, M.; Sugawara, M.; Toki, N.; Onozuka, H.; Kinoshita, T.; Saito, N.; Ochiai, A.; Tomita, M.; et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009, 69, 4918–4925. [Google Scholar] [CrossRef]
- Masui, K.; Cavenee, W.K.; Mischel, P.S.; Shibata, N. The metabolomic landscape plays a critical role in glioma oncogenesis. Cancer Sci. 2022, 113, 1555–1563. [Google Scholar] [CrossRef]
- Righi, V.; Cavallini, N.; Valentini, A.; Pinna, G.; Pavesi, G.; Rossi, M.C.; Puzzolante, A.; Mucci, A.; Cocchi, M. A metabolomic data fusion approach to support gliomas grading. NMR Biomed. 2020, 33, e4234. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, P.; Kensicki, E.; Bloom, G.; Prabhu, A.; Sarcar, B.; Kahali, S.; Eschrich, S.; Qu, X.; Forsyth, P.; Gillies, R. The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer Res. 2012, 72, 5878–5888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, X.; Gao, P.; Han, X.; Zhao, P.; Xie, F.; Liu, M. Advancing glioblastoma treatment by targeting metabolism. Neoplasia 2024, 51, 100985. [Google Scholar] [CrossRef]
- Sperry, J.; Condro, M.C.; Guo, L.; Braas, D.; Vanderveer-Harris, N.; Kim, K.K.O.; Pope, W.B.; Divakaruni, A.S.; Lai, A.; Christofk, H.; et al. Glioblastoma Utilizes Fatty Acids and Ketone Bodies for Growth Allowing Progression during Ketogenic Diet Therapy. iScience 2020, 23, 101453. [Google Scholar] [CrossRef]
- Wood, J.; Smith, S.J.; Castellanos-Uribe, M.; Lourdusamy, A.; May, S.T.; Barrett, D.A.; Grundy, R.G.; Kim, D.H.; Rahman, R. Metabolomic characterisation of the glioblastoma invasive margin reveals a region-specific signature. Heliyon 2025, 11, e41309. [Google Scholar] [CrossRef]
- He, W.; Edney, M.K.; Paine, S.M.L.; Griffiths, R.L.; Scurr, D.J.; Rahman, R.; Kim, D.H. Untargeted Metabolomic Characterization of Glioblastoma Intra-Tumor Heterogeneity Using OrbiSIMS. Anal. Chem. 2023, 95, 5994–6001. [Google Scholar] [CrossRef]
- Locasale, J.W.; Melman, T.; Song, S.; Yang, X.; Swanson, K.D.; Cantley, L.C.; Wong, E.T.; Asara, J.M. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol. Cell. Proteom. MCP 2012, 11, M111–014688. [Google Scholar] [CrossRef]
- Borodovsky, A.; Seltzer, M.J.; Riggins, G.J. Altered cancer cell metabolism in gliomas with mutant IDH1 or IDH2. Curr. Opin. Oncol. 2012, 24, 83–89. [Google Scholar] [CrossRef]
- Cuperlovic-Culf, M.; Khieu, N.H.; Surendra, A.; Hewitt, M.; Charlebois, C.; Sandhu, J.K. Analysis and Simulation of Glioblastoma Cell Lines-Derived Extracellular Vesicles Metabolome. Metabolites 2020, 10, 88. [Google Scholar] [CrossRef]
- Ferrasi, A.C.; Puttini, R.; Galvani, A.F.; Hamamoto Filho, P.T.; Delafiori, J.; Argente, V.D.; de Oliveira, A.N.; Dias-Audibert, F.L.; Catharino, R.R.; Silva, O.C.; et al. Metabolomics Approach Reveals Important Glioblastoma Plasma Biomarkers for Tumor Biology. Int. J. Mol. Sci. 2023, 24, 8813. [Google Scholar] [CrossRef] [PubMed]
- Bobeff, E.J.; Szczesna, D.; Bienkowski, M.; Janczar, K.; Chmielewska-Kassassir, M.; Wisniewski, K.; Papierz, W.; Wozniak, L.A.; Jaskolski, D.J. Plasma amino acids indicate glioblastoma with ATRX loss. Amino Acids 2021, 53, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Kelimu, A.; Xie, R.; Zhang, K.; Zhuang, Z.; Mamtimin, B.; Sheyhidin, I. Metabonomic signature analysis in plasma samples of glioma patients based on (1)H-nuclear magnetic resonance spectroscopy. Neurol. India 2016, 64, 246–251. [Google Scholar] [CrossRef]
- Löding, S.; Antti, H.; Sjöberg, R.L.; Melin, B.; Björkblom, B. Blood based metabolic markers of glioma from pre-diagnosis to surgery. Sci. Rep. 2024, 14, 20680. [Google Scholar] [CrossRef]
- Zhao, H.; Heimberger, A.; Lu, Z.; Wu, X.; Hodges, T.; Song, R.; Shen, J. Metabolomics profiling in plasma samples from glioma patients correlates with tumor phenotypes. Oncotarget 2016, 7, 20486. [Google Scholar] [CrossRef]
- Fontanilles, M.; Heisbourg, J.D.; Daban, A.; Di Fiore, F.; Pépin, L.F.; Marguet, F.; Langlois, O.; Alexandru, C.; Tennevet, I.; Ducatez, F.; et al. Metabolic remodeling in glioblastoma: A longitudinal multi-omics study. Acta Neuropathol. Commun. 2024, 12, 162. [Google Scholar] [CrossRef]
- Gilard, V.; Ferey, J.; Marguet, F.; Fontanilles, M.; Ducatez, F.; Pilon, C.; Lesueur, C.; Pereira, T.; Basset, C.; Schmitz-Afonso, I.; et al. Integrative Metabolomics Reveals Deep Tissue and Systemic Metabolic Remodeling in Glioblastoma. Cancers 2021, 13, 5157. [Google Scholar] [CrossRef]
- Wang, D.H.; Fujita, Y.; Dono, A.; Rodriguez Armendariz, A.G.; Shah, M.; Putluri, N.; Pichardo-Rojas, P.S.; Patel, C.B.; Zhu, J.J.; Huse, J.T.; et al. The genomic alterations in glioblastoma influence the levels of CSF metabolites. Acta Neuropathol. Commun. 2024, 12, 13. [Google Scholar] [CrossRef]
- Fujita, Y.; Nunez-Rubiano, L.; Dono, A.; Bellman, A.; Shah, M.; Rodriguez, J.C.; Putluri, V.; Kamal, A.H.M.; Putluri, N.; Riascos, R.F.; et al. IDH1 p.R132H ctDNA and D-2-hydroxyglutarate as CSF biomarkers in patients with IDH-mutant gliomas. J. Neuro-Oncol. 2022, 159, 261–270. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Wibom, C.; Surowiec, I.; Mörén, L.; Bergström, P.; Johansson, M.; Antti, H.; Bergenheim, A.T. Metabolomic patterns in glioblastoma and changes during radiotherapy: A clinical microdialysis study. J. Proteome Res. 2010, 9, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, S.; Imbach, K.J.; Gritsenko, M.A.; Lih, T.-S.M.; Kyle, J.E.; Yaron-Barir, T.M.; Binder, Z.A.; Li, Y.; Strunilin, I.; et al. Multi-scale signaling and tumor evolution in high-grade gliomas. Cancer Cell 2024, 42, 1217–1238.e19. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.H.; Akins, E.A.; Jain, S.; Wolf, K.J.; Zhang, J.; Choudhary, N.; Lad, M.; Shukla, P.; Rios, J.; Seo, K. Multiomic screening of invasive GBM cells reveals targetable transsulfuration pathway alterations. J. Clin. Investig. 2024, 134, e170397. [Google Scholar] [CrossRef]
- Scott, A.J.; Correa, L.O.; Edwards, D.M.; Sun, Y.; Ravikumar, V.; Andren, A.C.; Zhang, L.; Srinivasan, S.; Jairath, N.; Verbal, K.; et al. Metabolomic Profiles of Human Glioma Inform Patient Survival. Antioxid. Redox Signal. 2023, 39, 942–956. [Google Scholar] [CrossRef]
- Kalinina, J.; Carroll, A.; Wang, L.; Yu, Q.; Mancheno, D.E.; Wu, S.; Liu, F.; Ahn, J.; He, M.; Mao, H.; et al. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J. Mol. Med. 2012, 90, 1161–1171. [Google Scholar] [CrossRef]
- Louw, D.F.; Bose, R.; Sima, A.A.; Sutherland, G.R. Evidence for a high free radical state in low-grade astrocytomas. Neurosurgery 1997, 41, 1146–1150; discussion 1151. [Google Scholar] [CrossRef]
- Chou, F.J.; Liu, Y.; Lang, F.; Yang, C. D-2-Hydroxyglutarate in Glioma Biology. Cells 2021, 10, 2345. [Google Scholar] [CrossRef]
- Castillo, M.; Smith, J.K.; Kwock, L. Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am. J. Neuroradiol. 2000, 21, 1645–1649. [Google Scholar]
- Alcicek, S.; Pilatus, U.; Manzhurtsev, A.; Weber, K.J.; Ronellenfitsch, M.W.; Steinbach, J.P.; Hattingen, E.; Wenger, K.J. Amino acid metabolism in glioma: In vivo MR-spectroscopic detection of alanine as a potential biomarker of poor survival in glioma patients. J. Neuro-Oncol. 2024, 170, 451–461. [Google Scholar] [CrossRef]
- Dal Bello, S.; Valdemarin, F.; Martinuzzi, D.; Filippi, F.; Gigli, G.L.; Valente, M. Ketogenic Diet in the Treatment of Gliomas and Glioblastomas. Nutrients 2022, 14, 3851. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-B.; Karpova, A.; Gritsenko, M.A.; Kyle, J.E.; Cao, S.; Li, Y.; Rykunov, D.; Colaprico, A.; Rothstein, J.H.; Hong, R.; et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 2021, 39, 509–528.e20. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- Ghannad-Zadeh, K.; Das, S. One-Carbon Metabolism Associated Vulnerabilities in Glioblastoma: A Review. Cancers 2021, 13, 3067. [Google Scholar] [CrossRef]
- Shen, J.; Song, R.; Hodges, T.; Heimberger, A.; Zhao, H. Identification of metabolites in plasma for predicting survival in glioblastoma. Mol. Carcinog. 2018, 57, 1078–1084. [Google Scholar] [CrossRef]
- Sowers, M.L.; Sowers, L.C. Glioblastoma and Methionine Addiction. Int. J. Mol. Sci. 2022, 23, 7156. [Google Scholar] [CrossRef]
- Kou, Y.; Geng, F.; Guo, D. Lipid Metabolism in Glioblastoma: From De Novo Synthesis to Storage. Biomedicines 2022, 10, 1943. [Google Scholar] [CrossRef]
- Miska, J.; Chandel, N.S. Targeting fatty acid metabolism in glioblastoma. J. Clin. Investig. 2023, 133, e163448. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Bogusiewicz, J.; Burlikowska, K.; Jaroch, K.; Gorynska, P.Z.; Gorynski, K.; Birski, M.; Furtak, J.; Paczkowski, D.; Harat, M.; Bojko, B. Profiling of Carnitine Shuttle System Intermediates in Gliomas Using Solid-Phase Microextraction (SPME). Molecules 2021, 26, 6112. [Google Scholar] [CrossRef]
- Kao, T.J.; Lin, C.L.; Yang, W.B.; Li, H.Y.; Hsu, T.I. Dysregulated lipid metabolism in TMZ-resistant glioblastoma: Pathways, proteins, metabolites and therapeutic opportunities. Lipids Health Dis. 2023, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhao, J.; Li, G.; Yang, C.; Yang, S.; Zhan, Q.; Zhou, J.; Wang, Y.; Xiao, M.; Hong, B.; et al. Blockage of EGFR/AKT and mevalonate pathways synergize the antitumor effect of temozolomide by reprogramming energy metabolism in glioblastoma. Cancer Commun. (Lond. Engl.) 2023, 43, 1326–1353. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Kesarwani, P.; Prabhu, A.; Graham, S.F.; Buelow, K.L.; Nakano, I.; Chinnaiyan, P. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death Dis. 2020, 11, 253. [Google Scholar] [CrossRef]
- Thomas, D.; Wu, M.; Nakauchi, Y.; Zheng, M.; Thompson-Peach, C.A.L.; Lim, K.; Landberg, N.; Köhnke, T.; Robinson, N.; Kaur, S.; et al. Dysregulated Lipid Synthesis by Oncogenic IDH1 Mutation Is a Targetable Synthetic Lethal Vulnerability. Cancer Discov. 2023, 13, 496–515. [Google Scholar] [CrossRef]
- Zhou, W.; Yao, Y.; Scott, A.J.; Wilder-Romans, K.; Dresser, J.J.; Werner, C.K.; Sun, H.; Pratt, D.; Sajjakulnukit, P.; Zhao, S.G.; et al. Purine metabolism regulates DNA repair and therapy resistance in glioblastoma. Nat. Commun. 2020, 11, 3811. [Google Scholar] [CrossRef]
- Lo, C.S.-C.; Kiang, K.M.-Y.; Leung, G.K.-K. Anti-tumor effects of vitamin D in glioblastoma: Mechanism and therapeutic implications. Lab. Investig. 2022, 102, 118–125. [Google Scholar] [CrossRef]
- Osman, D.E.; Phon, B.W.S.; Kamarudin, M.N.A.; Ponnampalam, S.N.; Radhakrishnan, A.K.; Bhuvanendran, S. Biomarkers Regulated by Lipid-Soluble Vitamins in Glioblastoma. Nutrients 2022, 14, 2873. [Google Scholar] [CrossRef]
- Despotović, A.; Mirčić, A.; Misirlić-Denčić, S.; Harhaji-Trajković, L.; Trajković, V.; Zogović, N.; Tovilović-Kovačević, G. Combination of Ascorbic Acid and Menadione Induces Cytotoxic Autophagy in Human Glioblastoma Cells. Oxidative Med. Cell. Longev. 2022, 2022, 2998132. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H.P. Targeted metabolomics for biomarker discovery. Angew. Chem. (Int. Ed. Engl.) 2010, 49, 5426–5445. [Google Scholar] [CrossRef]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Song, Z.; Hong, Y.; Yang, Z.; Song, Y.; Chen, Z.; Chen, Z.; Cai, Z. Large-scale targeted metabolomics method for metabolite profiling of human samples. Anal. Chim. Acta 2020, 1125, 144–151. [Google Scholar] [CrossRef]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef]
- Ahmed, M.; Semreen, A.M.; El-Huneidi, W.; Bustanji, Y.; Abu-Gharbieh, E.; Alqudah, M.A.Y.; Alhusban, A.; Shara, M.; Abuhelwa, A.Y.; Soares, N.C.; et al. Preclinical and Clinical Applications of Metabolomics and Proteomics in Glioblastoma Research. Int. J. Mol. Sci. 2022, 24, 348. [Google Scholar] [CrossRef]
- Camunas-Alberca, S.M.; Moran-Garrido, M.; Sáiz, J.; Gil-de-la-Fuente, A.; Barbas, C.; Gradillas, A. Integrating the potential of ion mobility spectrometry-mass spectrometry in the separation and structural characterisation of lipid isomers. Front. Mol. Biosci. 2023, 10, 1112521. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Cho, K.; Uritboonthai, W.; Zhu, Z.; Patti, G.J.; Siuzdak, G. An accelerated workflow for untargeted metabolomics using the METLIN database. Nat. Biotechnol. 2012, 30, 826–828. [Google Scholar] [CrossRef]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef]
- Migliozzi, S.; Oh, Y.T.; Hasanain, M.; Garofano, L.; D’Angelo, F.; Najac, R.D.; Picca, A.; Bielle, F.; Di Stefano, A.L.; Lerond, J.; et al. Integrative multi-omics networks identify PKCδ and DNA-PK as master kinases of glioblastoma subtypes and guide targeted cancer therapy. Nat. Cancer 2023, 4, 181–202. [Google Scholar] [CrossRef]
- Hendriks, T.F.E.; Krestensen, K.K.; Mohren, R.; Vandenbosch, M.; De Vleeschouwer, S.; Heeren, R.M.A.; Cuypers, E. MALDI-MSI-LC-MS/MS Workflow for Single-Section Single Step Combined Proteomics and Quantitative Lipidomics. Anal. Chem. 2024, 96, 4266–4274. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Behrooz, A.; Latifi-Navid, H.; da Silva Rosa, S.C.; Swiat, M.; Wiechec, E.; Vitorino, C.; Vitorino, R.; Jamalpoor, Z.; Ghavami, S. Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach. Cancers 2023, 15, 3158. [Google Scholar] [CrossRef] [PubMed]
- Semreen, A.M.; Alsoud, L.O.; Semreen, M.H.; Ahmed, M.; Al-Hroub, H.M.; El-Awady, R.; Ramadan, W.S.; Abuhelwa, A.; Bustanji, Y.; Soares, N.C.; et al. Multi-omics analysis revealed significant metabolic changes in brain cancer cells treated with paclitaxel and/or topotecan. Heliyon 2024, 10, e39420. [Google Scholar] [CrossRef]
- Gaca-Tabaszewska, M.; Bogusiewicz, J.; Bojko, B. Metabolomic and Lipidomic Profiling of Gliomas-A New Direction in Personalized Therapies. Cancers 2022, 14, 5041. [Google Scholar] [CrossRef]
- Dias, D.A.; Koal, T. Progress in Metabolomics Standardisation and its Significance in Future Clinical Laboratory Medicine. Ejifcc 2016, 27, 331–343. [Google Scholar]
- Jaroch, K.; Modrakowska, P.; Bojko, B. Glioblastoma Metabolomics-In Vitro Studies. Metabolites 2021, 11, 315. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C. Oncometabolites in Cancer: Current Understanding and Challenges. Cancer Res. 2021, 81, 2820–2823. [Google Scholar] [CrossRef]
- Fink, M.A.; Paland, H.; Herzog, S.; Grube, M.; Vogelgesang, S.; Weitmann, K.; Bialke, A.; Hoffmann, W.; Rauch, B.H.; Schroeder, H.W.S.; et al. L-Carnitine-Mediated Tumor Cell Protection and Poor Patient Survival Associated with OCTN2 Overexpression in Glioblastoma Multiforme. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2874–2886. [Google Scholar] [CrossRef]
- Farahzadi, R.; Hejazi, M.S.; Molavi, O.; Pishgahzadeh, E.; Montazersaheb, S.; Jafari, S. Clinical Significance of Carnitine in the Treatment of Cancer: From Traffic to the Regulation. Oxidative Med. Cell. Longev. 2023, 2023, 9328344. [Google Scholar] [CrossRef]
- Pérez-Tomás, R.; Pérez-Guillén, I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers 2020, 12, 3244. [Google Scholar] [CrossRef]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad Spectrum Anticancer Activity of Myo-Inositol and Inositol Hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef]
- Pan, S.; Yin, L.; Liu, J.; Tong, J.; Wang, Z.; Zhao, J.; Liu, X.; Chen, Y.; Miao, J.; Zhou, Y.; et al. Metabolomics-driven approaches for identifying therapeutic targets in drug discovery. MedComm 2024, 5, e792. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šamec, N.; Krapež, G.; Skubic, C.; Jovčevska, I.; Videtič Paska, A. From Biomarker Discovery to Clinical Applications of Metabolomics in Glioblastoma. Metabolites 2025, 15, 295. https://doi.org/10.3390/metabo15050295
Šamec N, Krapež G, Skubic C, Jovčevska I, Videtič Paska A. From Biomarker Discovery to Clinical Applications of Metabolomics in Glioblastoma. Metabolites. 2025; 15(5):295. https://doi.org/10.3390/metabo15050295
Chicago/Turabian StyleŠamec, Neja, Gloria Krapež, Cene Skubic, Ivana Jovčevska, and Alja Videtič Paska. 2025. "From Biomarker Discovery to Clinical Applications of Metabolomics in Glioblastoma" Metabolites 15, no. 5: 295. https://doi.org/10.3390/metabo15050295
APA StyleŠamec, N., Krapež, G., Skubic, C., Jovčevska, I., & Videtič Paska, A. (2025). From Biomarker Discovery to Clinical Applications of Metabolomics in Glioblastoma. Metabolites, 15(5), 295. https://doi.org/10.3390/metabo15050295








