A Scoring Model Using Multi-Metabolites Based on Untargeted Metabolomics for Assessing Dyslipidemia in Korean Individuals with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plasma Samples and Study Design
2.2. Biochemical Analysis
2.3. Untargeted Metabolomics
2.4. Establishment of MRS and Its Validation
2.4.1. Major and Key Metabolites Related to Dyslipidemia in the Discovery Set
2.4.2. MRS Establishment in the Discovery Set
2.4.3. MRS Validation in Both Discovery and Replication Set
2.5. Statistical Analysis
3. Results
3.1. General Clinical/Biochemical Markers Between the Groups in the Discovery Set
3.2. UPLC-MS/MS Analysis
3.2.1. Metabolic Characteristics Between the Healthy and Dyslipidemia Groups in the Discovery Set
3.2.2. Major Metabolite Screening and Key Metabolite Selection
3.3. Establishment of MRS and Its Assessment Ability for Dyslipidemia
3.4. Discrimination Performance of the MRS in the Re-Grouped Replication Set
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | acylcarnitine |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BCAA | branched-chain amino acids |
| BMI | body mass index |
| BP | blood pressure |
| DPA | docosapentaenoic acid |
| EPA | eicosapentaenoic acid |
| ESI | electrospray ionization |
| FDR | false discovery rate |
| FFA | free fatty acid |
| HDL-C | high-density lipoprotein-cholesterol |
| IFN | interferon |
| IL | interleukin |
| ISTD | internal standard |
| KCD | Korean Standard Classification of Diseases |
| LDL-C | low-density lipoprotein-cholesterol |
| LPC | lysophosphatidylcholine |
| MRS | metabolite risk score |
| OPLS-DA | orthogonal partial least squares-discriminant analysis |
| ox- | oxidized |
| PUFA | polyunsaturated fatty acid |
| ROC | receiver operating characteristic |
| SFA | saturated fatty acid |
| TC | total cholesterol |
| TG | triglyceride |
| TNF | tumor necrosis factor |
| UFA | unsaturated fatty acid |
| UPLC-MS/MS | ultra-performance liquid chromatography-tandem mass spectrometry |
| VIP | variable importance in projection |
| γ-GTP | γ-glutamyltransferase |
References
- Jin, E.S.; Shim, J.S.; Kim, S.E.; Bae, J.H.; Kang, S.; Won, J.C.; Shin, M.J.; Jin, H.Y.; Moon, J.; Lee, H.; et al. Dyslipidemia Fact Sheet in South Korea, 2022. Diabetes Metab. J. 2023, 47, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Berberich, A.J.; Hegele, R.A. A modern approach to dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Surma, S.; Toth, P.P.; endorsed by the International Lipid Expert Panel (ILEP). 2023: The year in cardiovascular disease–the year of new and prospective lipid lowering therapies. Can we render dyslipidemia a rare disease by 2024? Arch. Med. Sci. 2023, 19, 1602–1615. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Du, H.; Rao, Y.; Liu, R.; Deng, K.; Guan, Y.; Luo, D.; Mao, Q.; Yu, J.; Bo, T.; Fan, Z.; et al. Proteomics and metabolomics analyses reveal the full spectrum of inflammatory and lipid metabolic abnormalities in dyslipidemia. Biomed. Chromatogr. 2021, 35, e5183. [Google Scholar] [CrossRef]
- Ke, C.; Zhu, X.; Zhang, Y.; Shen, Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics 2018, 14, 117. [Google Scholar] [CrossRef]
- Wang, N.; Ru, Y.; Yang, Z.; Sun, C.; Li, S.; Min, Y.; Zhao, X.; Lu, Y.; Hsing, A.W.; Zhu, S. Metabolomic profiles of plasma retinol-associated dyslipidemia in men and women. Front. Nutr. 2021, 8, 740435. [Google Scholar] [CrossRef]
- Geidenstam, N.; Hsu, Y.H.; Astley, C.M.; Mercader, J.M.; Ridderstråle, M.; Gonzalez, M.E.; Gonzalez, C.; Hirschhorn, J.N.; Salem, R.M. Using metabolite profiling to construct and validate a metabolite risk score for predicting future weight gain. PLoS ONE 2019, 14, e0222445. [Google Scholar] [CrossRef]
- Gadgil, M.D.; Cheng, J.; Herrington, D.M.; Kandula, N.R.; Kanaya, A.M. Adipose tissue-derived metabolite risk scores and risk for type 2 diabetes in South Asians. Int. J. Obes. 2024, 48, 668–673. [Google Scholar] [CrossRef]
- He, S.; Granot-Hershkovitz, E.; Zhang, Y.; Bressler, J.; Tarraf, W.; Yu, B.; Huang, T.; Zeng, D.; Wassertheil-Smoller, S.; Lamar, M.; et al. Blood metabolites predicting mild cognitive impairment in the study of Latinos-investigation of neurocognitive aging (HCHS/SOL). Alzheimers Dement. 2022, 14, e12259. [Google Scholar] [CrossRef]
- Ström, M.; Wheelock, Å.M. Permutation analysis prior to variable selection greatly enhances robustness of OPLS analysis in small cohorts. bioRxiv 2024. [Google Scholar] [CrossRef]
- The Human Metabolome Database (HMDB). N-(3-Acetamidopropyl)pyrrolidin-2-One. Available online: https://hmdb.ca/metabolites/HMDB0061384 (accessed on 2 November 2023).
- Xu, H.; Liu, R.; He, B.; Bi, C.W.; Bi, K.; Li, Q. Polyamine metabolites profiling for characterization of lung and liver cancer using an LC-Tandem MS method with multiple statistical data mining strategies: Discovering potential cancer biomarkers in human plasma and urine. Molecules 2016, 21, 1040. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Spermidine/spermine-N1-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Wang, Y.; Stewart, T.M.; Devereux, W.; Hacker, A.; Wang, Y.; Smith, R.; Woster, P.M. The role of polyamine catabolism in anti-tumour drug response. Biochem. Soc. Trans. 2003, 31, 361–365. [Google Scholar] [CrossRef]
- Seiler, N. Catabolism of polyamines. Amino Acids 2004, 26, 217–233. [Google Scholar] [CrossRef]
- Fitzgerald, B.L.; Mahapatra, S.; Farmer, D.K.; McNeil, M.R.; Casero, R.A., Jr.; Belisle, J.T. Elucidating the structure of N1-acetylisoputreanine: A novel polyamine catabolite in human urine. ACS Omega 2017, 2, 3921–3930. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, A.; Johansson, H.E.; Orjalo, A.V.; Park, M.H. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2169–2174. [Google Scholar] [CrossRef]
- Puetz, A.; Artati, A.; Adamski, J.; Schuett, K.; Romeo, F.; Stoehr, R.; Marx, N.; Federici, M.; Lehrke, M.; Kappel, B.A. Non-targeted metabolomics identify polyamine metabolite acisoga as novel biomarker for reduced left ventricular function. ESC Heart Fail. 2022, 9, 564–573. [Google Scholar] [CrossRef]
- Alonso, A.; Yu, B.; Sun, Y.V.; Chen, L.Y.; Loehr, L.R.; O’Neal, W.T.; Soliman, E.Z.; Boerwinkle, E. Serum metabolomics and incidence of atrial fibrillation (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2019, 123, 1955–1961. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Li, J.; Wu, K.H.; Lu, M. Diagnostic metabolomic profiling of Parkinson’s disease biospecimens. Neurobiol. Dis. 2023, 177, 105962. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Irajizad, E.; Kobayashi, M.; Vykoukal, J.; Dennison, J.B.; Murage, E.; Wu, R.; Long, J.P.; Do, K.A.; Celestino, J.; et al. A MYC-driven plasma polyamine signature for early detection of ovarian cancer. Cancers 2021, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Jakhwal, P.; Kumar Biswas, J.; Tiwari, A.; Kwon, E.E.; Bhatnagar, A. Genetic and non-genetic tailoring of microalgae for the enhanced production of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Bioresour. Technol. 2022, 344, 126250. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Zhou, N.; Shen, Y.; Li, B.; Chen, B.E.; Li, X. Association between omega-3 fatty acid intake and dyslipidemia: A continuous dose-response meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2023, 12, e029512. [Google Scholar] [CrossRef]
- Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; O’Neal, D.N.; Best, J.D.; Beilin, L.J. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 2000, 71, 1085–1094. [Google Scholar] [CrossRef]
- Grimsgaard, S.; Bonaa, K.H.; Hansen, J.B.; Nordøy, A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am. J. Clin. Nutr. 1997, 66, 649–659. [Google Scholar] [CrossRef]
- Mori, T.A.; Woodman, R.J. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 95–104. [Google Scholar] [CrossRef]
- Satoh, N.; Shimatsu, A.; Kotani, K.; Sakane, N.; Yamada, K.; Suganami, T.; Kuzuya, H.; Ogawa, Y. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care 2007, 30, 144–146. [Google Scholar] [CrossRef]
- Tani, S.; Nagao, K.; Matsumoto, M.; Hirayama, A. Highly purified eicosapentaenoic acid may increase low-density lipoprotein particle size by improving triglyceride metabolism in patients with hypertriglyceridemia. Circ. J. 2013, 77, 2349–2357. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma acylcarnitines and risk of cardiovascular disease: Effect of Mediterranean diet interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar] [CrossRef]
- Deda, O.; Panteris, E.; Meikopoulos, T.; Begou, O.; Mouskeftara, T.; Karagiannidis, E.; Papazoglou, A.S.; Sianos, G.; Theodoridis, G.; Gika, H. Correlation of serum acylcarnitines with clinical presentation and severity of coronary artery disease. Biomolecules 2022, 12, 354. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Ruiz-Canela, M.; Li, J.; Zheng, Y.; Bulló, M.; Wang, D.D.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Plasma acylcarnitines and risk of type 2 diabetes in a Mediterranean population at high cardiovascular risk. J. Clin. Endocrinol. Metab. 2019, 104, 1508–1519. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; Römisch-Margl, W.; Kastenmüller, G.; Prehn, C.; Petersen, A.K.; Illig, T.; Gieger, C.; Wang-Sattler, R.; Meisinger, C.; Peters, A.; et al. Increased amino acids levels and the risk of developing of hypertriglyceridemia in a 7-year follow-up. J. Endocrinol. Investig. 2014, 37, 369–374. [Google Scholar] [CrossRef]
- Fukushima, K.; Harada, S.; Takeuchi, A.; Kurihara, A.; Iida, M.; Fukai, K.; Kuwabara, K.; Kato, S.; Matsumoto, M.; Hirata, A.; et al. Association between dyslipidemia and plasma levels of branched-chain amino acids in the Japanese population without diabetes mellitus. J. Clin. Lipidol. 2019, 13, 932–939.e2. [Google Scholar] [CrossRef]
- Yang, P.; Hu, W.; Fu, Z.; Sun, L.; Zhou, Y.; Gong, Y.; Yang, T.; Zhou, H. The positive association of branched-chain amino acids and metabolic dyslipidemia in Chinese Han population. Lipids Health Dis. 2016, 15, 120. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Yoo, H.J. Application of a metabolite risk score model for dyslipidemia in Koreans (poster). In 2024 ICOMES Integrating Cutting-Edge Insights in Obesity Management, Proceedings of the 2024 ICOMES (International Congress on Obesity and Metabolic Syndrome), Seoul, Republic of Korea, 5–7th September 2024; Korean Society for the Study of Obesity: Seoul, Republic of Korea, 2024; Poster No. PE 06-01. [Google Scholar]

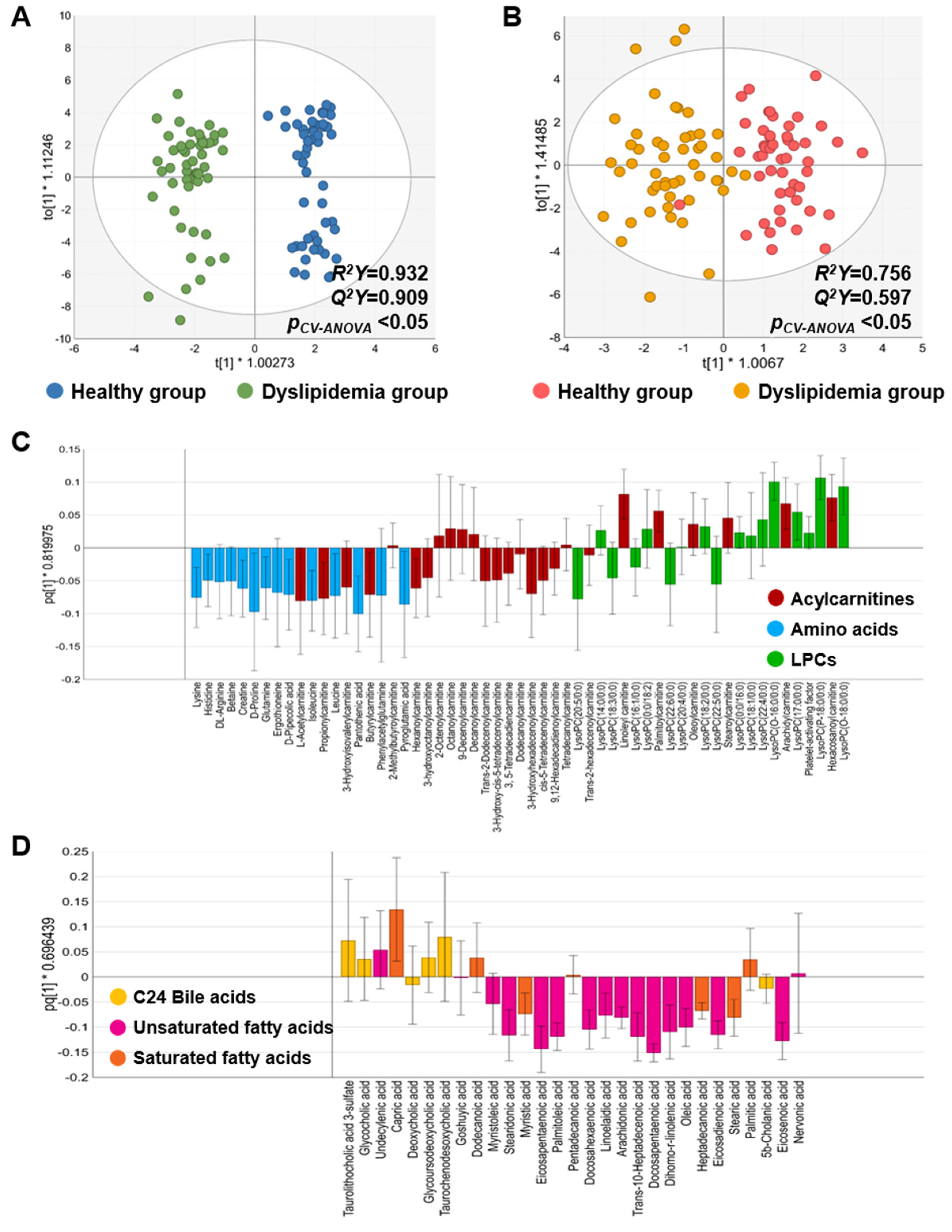
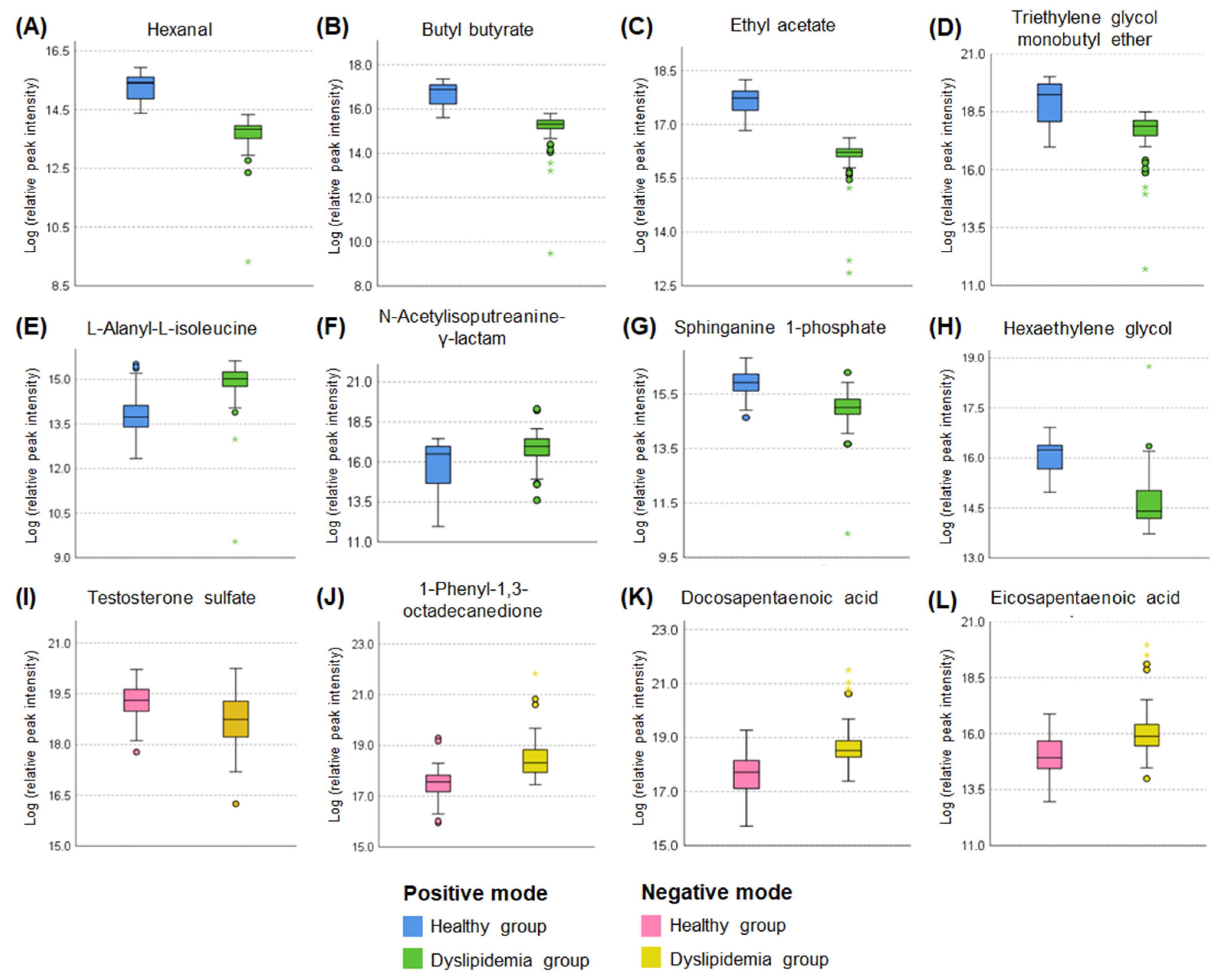
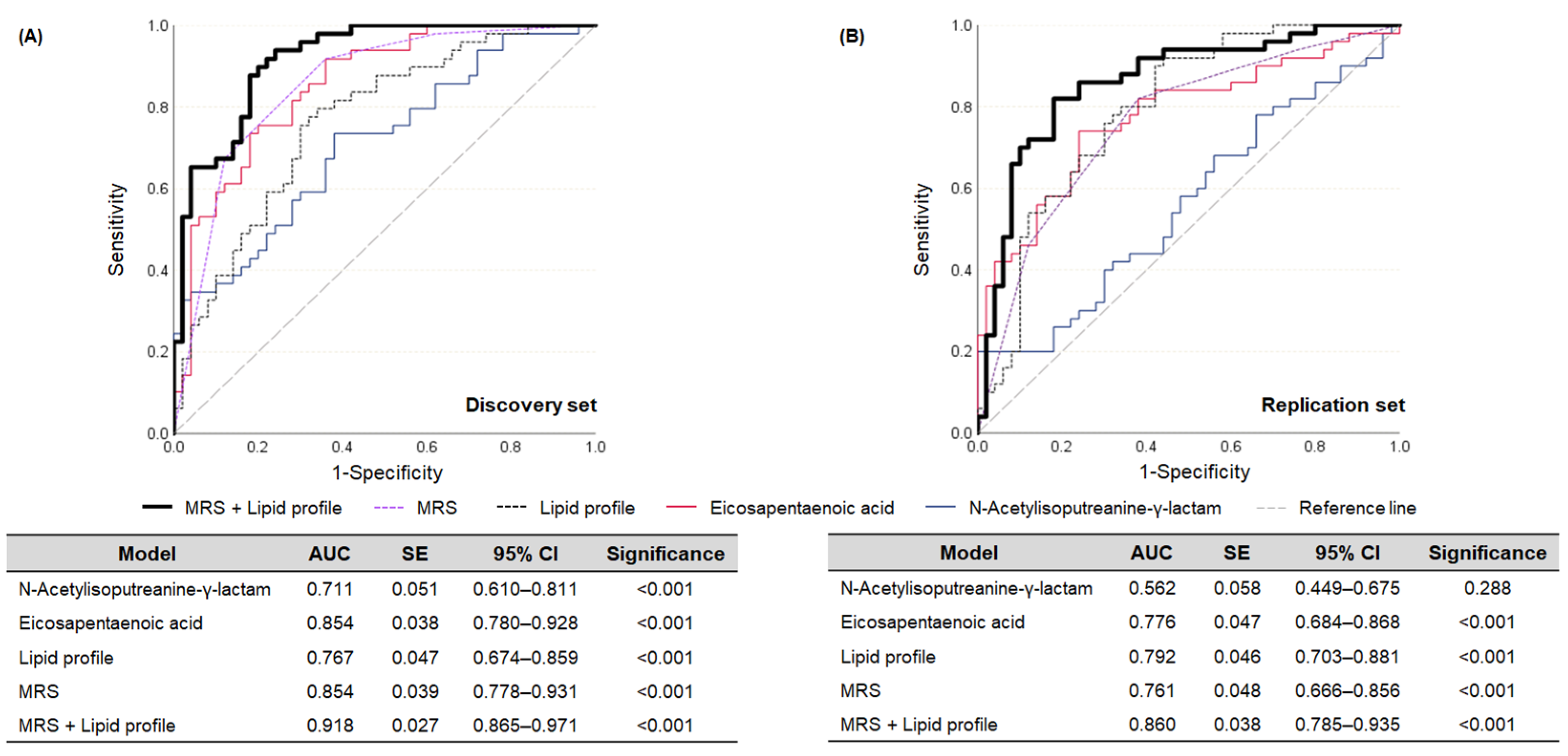

| Total (n = 99) | pa | pb | ||||
|---|---|---|---|---|---|---|
| Healthy (n = 50) | Dyslipidemia (n = 49) | |||||
| Age (year) † | 44.8 | ±0.73 | 52.4 | ±0.61 | <0.001 | - |
| Male/Female n, (%) | 33 (66.0)/17 (34.0) | 36 (73.5)/13 (26.5) | 0.654 | |||
| Body weight (kg) † | 68.9 | ±1.53 | 76.0 | ±1.56 | <0.001 | - |
| BMI (kg/m2) † | 24.5 | ±0.43 | 27.8 | ±0.45 | <0.001 | - |
| Systolic BP (mmHg) † | 122.8 | ±1.57 | 133.1 | ±2.54 | 0.001 | 0.001 |
| Diastolic BP (mmHg) † | 76.2 | ±1.40 | 80.0 | ±1.97 | 0.167 | 0.018 |
| Glucose (mg/dL) † | 96.1 | ±1.38 | 125.4 | ±5.25 | <0.001 | 0.001 |
| TG (mg/dL) † | 140.3 | ±11.0 | 175.4 | ±16.1 | 0.015 | 0.783 |
| HDL-C (mg/dL) | 49.0 | ±1.43 | 46.0 | ±2.03 | 0.240 | 0.625 |
| LDL-C (mg/dL) | 107.0 | ±5.84 | 102.2 | ±6.21 | 0.573 | 0.319 |
| TC (mg/dL) | 201.1 | ±4.66 | 174.6 | ±7.59 | 0.004 | 0.077 |
| AST (U/L) † | 23.7 | ±1.57 | 37.9 | ±4.72 | <0.001 | 0.351 |
| ALT (U/L) † | 23.9 | ±2.00 | 39.3 | ±4.60 | <0.001 | 0.060 |
| TNF-α (pg/mL) † | 11.0 | ±0.69 | 25.1 | ±2.81 | <0.001 | <0.001 |
| IL-1β (pg/mL) † | 4.31 | ±0.31 | 5.59 | ±0.43 | 0.008 | 0.103 |
| IL-6 (pg/mL) † | 2.49 | ±5.75 | 6.41 | ±2.83 | 0.002 | 0.064 |
| IFN-γ (pg/mL) † | 15.8 | ±1.28 | 13.4 | ±1.21 | 0.121 | 0.464 |
| Ox-LDL (U/L) | 49.6 | ±1.96 | 42.7 | ±2.07 | 0.017 | 0.515 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-G.; Yoo, H.J. A Scoring Model Using Multi-Metabolites Based on Untargeted Metabolomics for Assessing Dyslipidemia in Korean Individuals with Obesity. Metabolites 2025, 15, 279. https://doi.org/10.3390/metabo15040279
Yang S-G, Yoo HJ. A Scoring Model Using Multi-Metabolites Based on Untargeted Metabolomics for Assessing Dyslipidemia in Korean Individuals with Obesity. Metabolites. 2025; 15(4):279. https://doi.org/10.3390/metabo15040279
Chicago/Turabian StyleYang, Su-Geun, and Hye Jin Yoo. 2025. "A Scoring Model Using Multi-Metabolites Based on Untargeted Metabolomics for Assessing Dyslipidemia in Korean Individuals with Obesity" Metabolites 15, no. 4: 279. https://doi.org/10.3390/metabo15040279
APA StyleYang, S.-G., & Yoo, H. J. (2025). A Scoring Model Using Multi-Metabolites Based on Untargeted Metabolomics for Assessing Dyslipidemia in Korean Individuals with Obesity. Metabolites, 15(4), 279. https://doi.org/10.3390/metabo15040279






