Enhancing Plant Resistance to Sri Lankan Cassava Mosaic Virus Using Salicylic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, SA Treatment, and Collection of Leaf Samples
2.2. Disease Severity
2.3. DNA Extraction
2.4. Quantification of SLCMV Particles
2.5. RNA Extraction and cDNA Synthesis
2.6. RT-qPCR
2.7. Metabolomic Analysis
2.8. Statistical Analyses
3. Results and Discussion
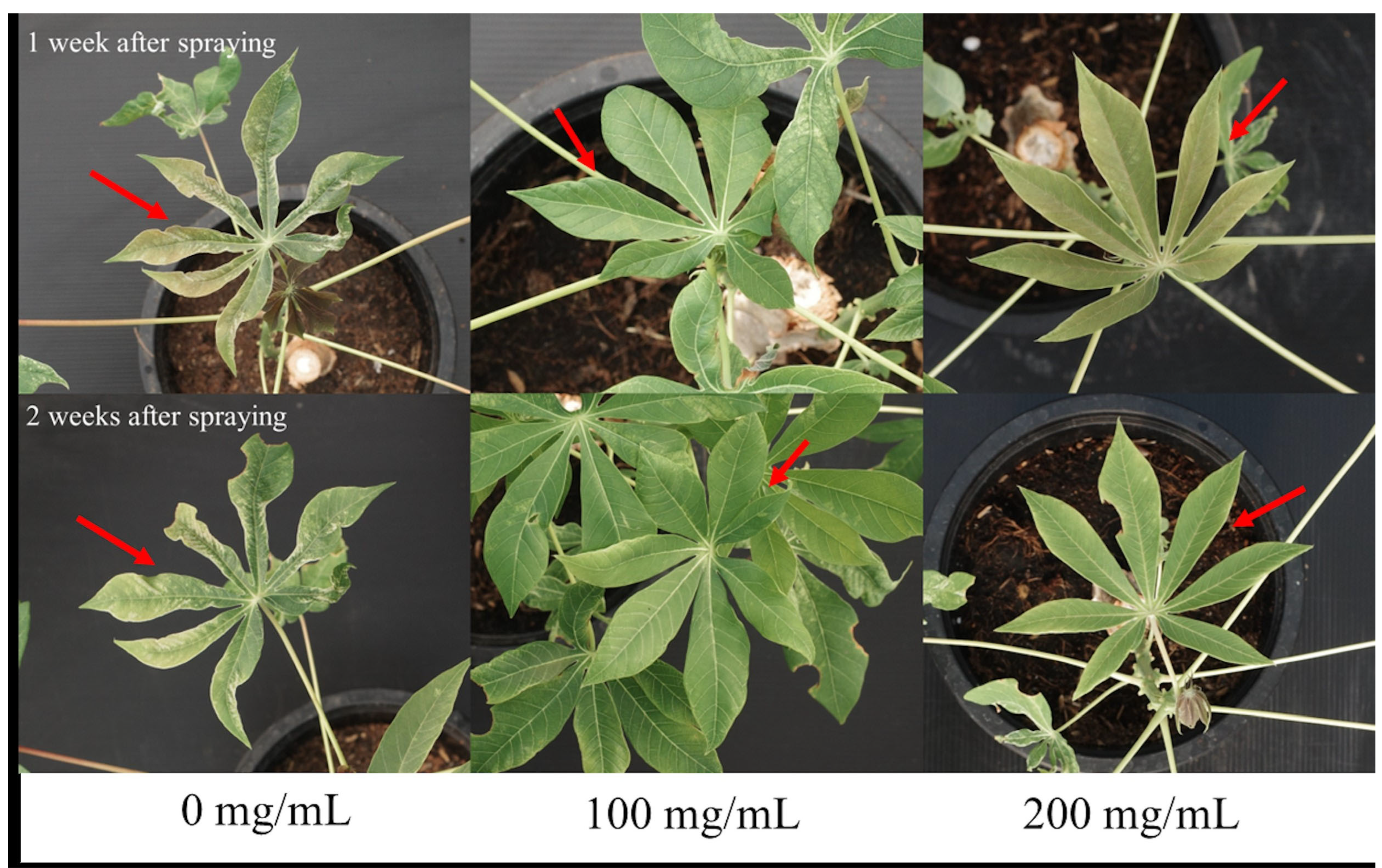
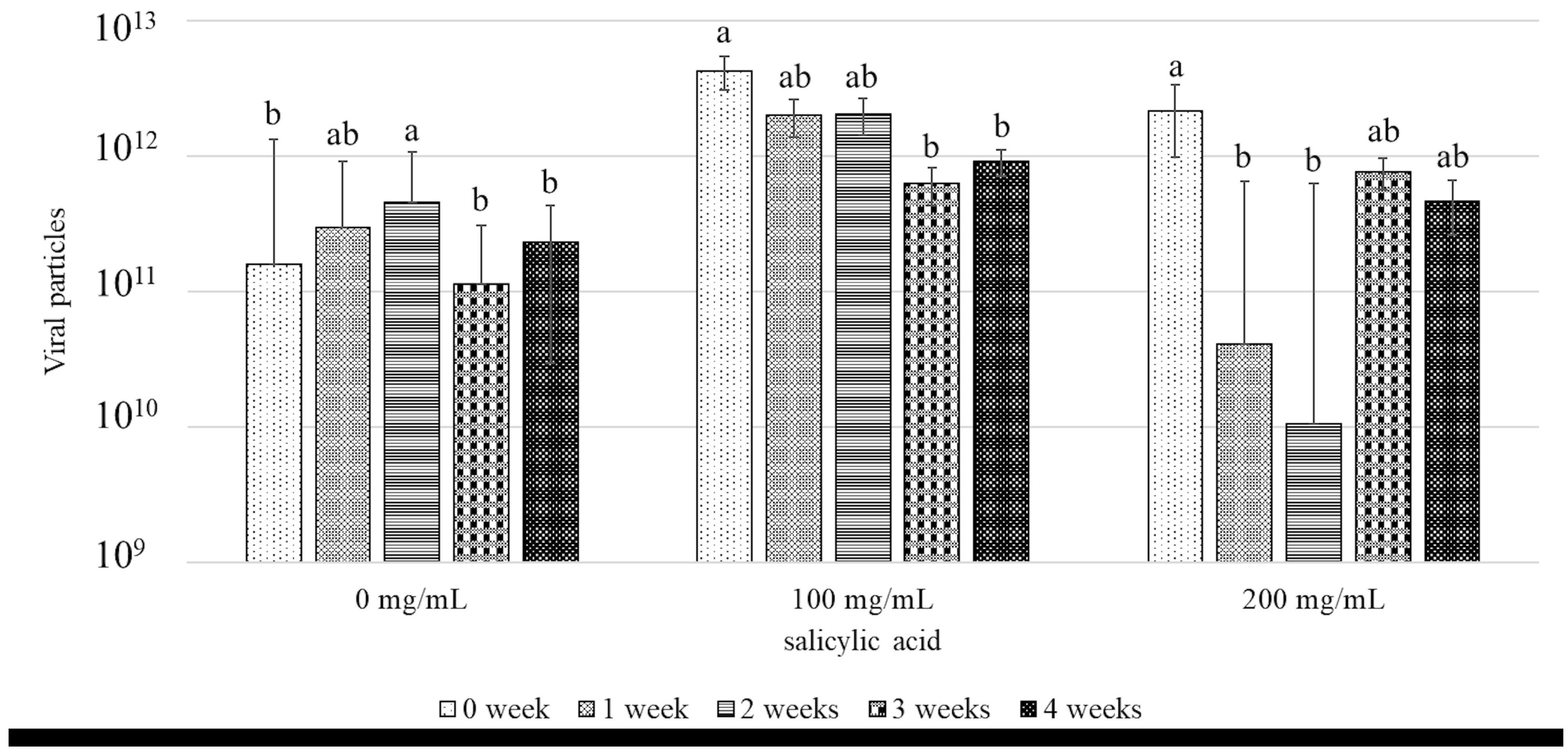
3.1. Effects of SA Against SLCMV
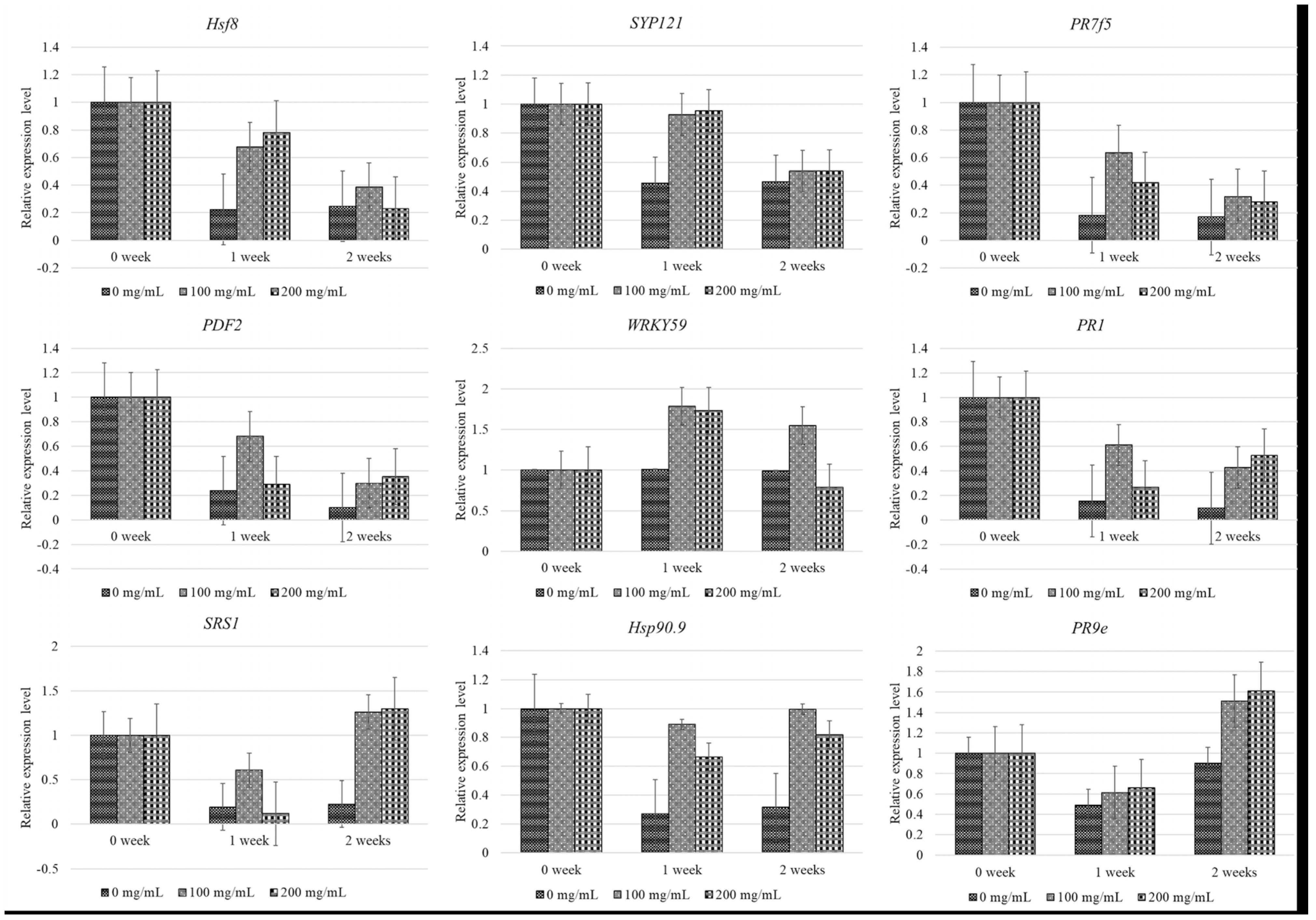
3.2. Relative Gene Expression Levels of Resistance Genes Following SA Treatment
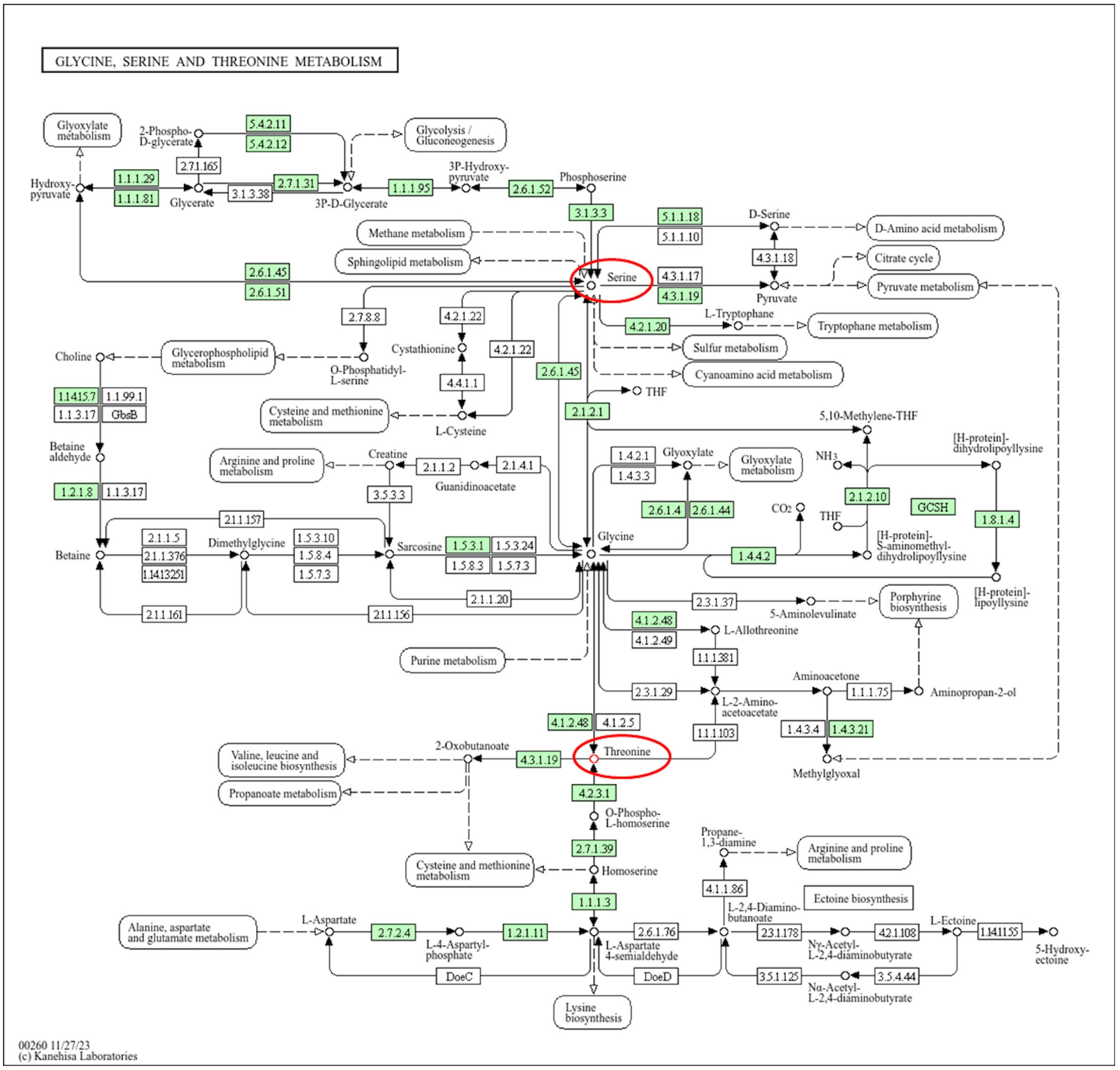
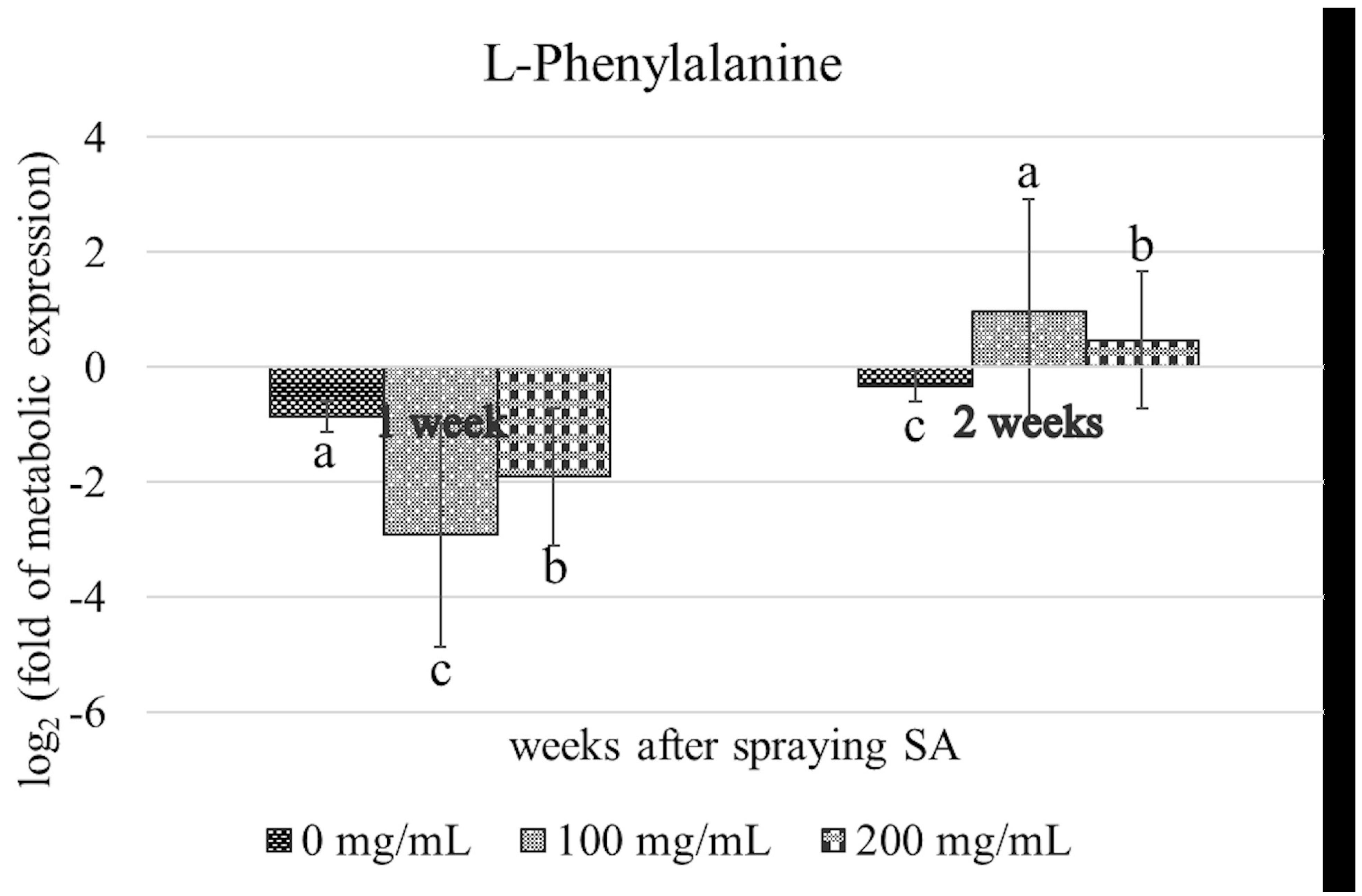
3.3. Identification of Metabolites Associated with SAR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis and one-way analysis of variance |
| CMD | Cassava mosaic disease |
| CTAB | Cetyl trimethylammonium bromide |
| HESI | Heated electrospray ionization |
| HR | Hypersensitive response |
| IR | Induced resistance |
| JA | Jasmonic acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LSD | Least significant difference |
| PR | Pathogenesis-related |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SAR | Systemic acquired resistance |
| SLCMV | Sri Lankan cassava mosaic virus |
| TF | Transcription factors |
| TMV | Tobacco mosaic virus |
| TYLCV | Tomato yellow leaf curl virus |
References
- Wanapat, M.; Kang, S. Cassava chip (Manihot esculenta Crantz) as an energy source for ruminant feeding. Anim. Nutr. 2015, 1, 266–270. [Google Scholar] [CrossRef]
- Legg, J.; Alvarez, E. Diseases Affecting Cassava. In Achieving Sustainable Cultivation of Cassava; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 2, pp. 213–244. [Google Scholar] [CrossRef]
- Chikoti, P.C.; Mulenga, R.M.; Tembo, M.; Sseruwagi, P. Cassava mosaic disease: A review of a threat to cassava production in Zambia. J. Plant Pathol. 2019, 101, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Zerbini, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.C.F.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A.; et al. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef]
- Siriwan, W.; Jimenez, J.; Hemniam, N.; Saokham, K.; Lopez-Alvarez, D.; Leiva, A.M.; Martinez, A.; Mwanzia, L.; Becerra Lopez-Lavalle, L.A.; Cuellar, W.J. Surveillance and diagnostics of the emergent Sri Lankan cassava mosaic virus (Fam. Geminiviridae) in Southeast Asia. Virus Res. 2020, 285, 197959. [Google Scholar] [CrossRef]
- Saokham, K.; Hemniam, N.; Roekwan, S.; Hunsawattanakul, S.; Thawinampan, J.; Siriwan, W. Survey and Molecular Detection of Sri Lankan Cassava Mosaic Virus in Thailand. bioRxiv 2021, 16, e0252846. [Google Scholar] [CrossRef]
- Wang, H.-L.; Cui, X.-Y.; Wang, X.-W.; Liu, S.-S.; Zhang, Z.-H.; Zhou, X. First Report of Sri Lankan cassava mosaic virus Infecting Cassava in Cambodia. Plant Dis. 2016, 100, 1029. [Google Scholar] [CrossRef]
- Mabasso, S. Tobacco Whitefly on Cassava; PlantwisePlus Knowl. Bank: Wallingford, UK, 2019. [Google Scholar] [CrossRef]
- Chant, S.R. Studies on the transmission of cassava mosaic virus by bemisia spp. (aleyrodidae). Ann. Appl. Biol. 1958, 46, 210–215. [Google Scholar] [CrossRef]
- Pradhan, B.; Vu, T.; Dey, N.; Mukherjee, S. Molecular Biology of Geminivirus DNA Replication. In Viral Replication; Avid Science: Hyderabad, India, 2017; pp. 2–31. [Google Scholar]
- Houngue, J.A.; Houédjissin, S.S.; Ahanhanzo, C.; Pita, J.S.; Houndénoukon, M.S.E.; Zandjanakou-Tachin, M. Cassava mosaic disease (CMD) in Benin: Incidence, severity and its whitefly abundance from field surveys in 2020. Crop. Prot. 2022, 158, 106007. [Google Scholar] [CrossRef]
- Bennett, A.; Agbandje-McKenna, M. Geminivirus structure and assembly. Adv. Virus Res. 2020, 108, 1–32. [Google Scholar] [CrossRef]
- Legg, J.P.; Owor, B.; Sseruwagi, P.; Ndunguru, J. Cassava Mosaic Virus Disease in East and Central Africa: Epidemiology and Management of A Regional Pandemic. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2006; Volume 67, pp. 355–418. [Google Scholar]
- Dos Santos, C.; Franco, O.L. Pathogenesis-Related Proteins (PRs) with Enzyme Activity Activating Plant Defense Responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Mahawer, S.; Arya, S.; Kabdal, T.; Kumar, R.; Prakash, O.; Chitara, M.; Koli, P. Plant Defense Systems: Mechanism of Self-Protection by Plants Against Pathogens. In Plant Protection: From Chemicals to Biologicals Edited; De Gruyter: Berlin, Germany; Boston, MA, USA, 2022; pp. 115–140. [Google Scholar]
- Aboulila, A.A. Efficiency of plant growth regulators as inducers for improve systemic acquired resistance against stripe rust disease caused by Puccinia striiformis f. sp. tritici in wheat through up-regulation of PR-1 and PR-4 genes expression. Physiol. Mol. Plant Pathol. 2022, 121, 101882. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, I.; Cohen, J.D.; Culler, A.H.; Quint, M.; Slovin, J.P.; Nakajima, M.; Yamaguchi, S.; Sakakibara, H.; Kuroha, T.; Hirai, N.; et al. 4.02—Plant Hormones. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 9–125. [Google Scholar]
- Raskin, I. Role of Salicylic Acid in Plants. Annu. Rev. Plant Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Ullah, C.; Chen, Y.-H.; Ortega, M.A.; Tsai, C.-J. The diversity of salicylic acid biosynthesis and defense signaling in plants: Knowledge gaps and future opportunities. Curr. Opin. Plant Biol. 2023, 72, 102349. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthes©is of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Ge, Y.; Bi, Y.; Guest, D.I. Defence responses in leaves of resistant and susceptible melon (Cucumis melo L.) cultivars infected with Colletotrichum lagenarium. Physiol. Mol. Plant Pathol. 2013, 81, 13–21. [Google Scholar] [CrossRef]
- Sangpueak, R.; Phansak, P.; Thumanu, K.; Siriwong, S.; Wongkaew, S.; Buensanteai, N. Effect of Salicylic AcidFormulations on Induced Plant Defense against Cassava Anthracnose Disease. Plant Pathol. J. 2021, 37, 356–364. [Google Scholar] [CrossRef]
- Wang, F.; Feng, G.; Chen, K. Burdock fructooligosaccharide induces resistance to tobacco mosaic virus in tobacco seedlings. Physiol. Mol. Plant Pathol. 2009, 74, 34–40. [Google Scholar] [CrossRef]
- Király, L.; Künstler, A.; Höller, K.; Fattinger, M.; Juhász, C.; Müller, M.; Gullner, G.; Zechmann, B. Sulfate supply influences compartment specific glutathione metabolism and confers enhanced resistance to Tobacco mosaic virus during a hypersensitive response. Plant Physiol. Biochem. 2012, 59, 44–54. [Google Scholar] [CrossRef]
- Wang, P.; Sun, S.; Peng, R.; Li, N.; Liu, K.; Wang, L.; Geng, X.; Hu, B.; Wang, H.; Afzal, A.J. Physiological and transcriptomic analyses revealed gene networks involved in heightened resistance against tomato yellow leaf curl virus infection in salicylic acid and jasmonic acid treated tomato plants. Front. Microbiol. 2022, 13, 970139. [Google Scholar] [CrossRef]
- Obata, T.; Klemens, P.A.W.; Rosado-Souza, L.; Schlereth, A.; Gisel, A.; Stavolone, L.; Zierer, W.; Morales, N.; Mueller, L.A.; Zeeman, S.C.; et al. Metabolic profiles of six African cultivars of cassava (Manihot esculenta Crantz) highlight bottlenecks of root yield. Plant J. 2020, 102, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Chaowongdee, S.; Malichan, S.; Pongpamorn, P.; Paemanee, A.; Siriwan, W. Metabolic profiles of Sri Lankan cassava mosaic virus-infected and healthy cassava (Manihot esculenta Crantz) cultivars with tolerance and susceptibility phenotypes. BMC Plant Biol. 2023, 23, 178. [Google Scholar] [CrossRef] [PubMed]
- Sseruwagi, P.; Sserubombwe, W.S.; Legg, J.P.; Ndunguru, J.; Thresh, J.M. Methods of surveying the incidence and severity of cassava mosaic disease and whitefly vector populations on cassava in Africa: A review. Virus Res. 2004, 100, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Olasanmi, B.; Kyallo, M.; Yao, N. Marker-assisted selection complements phenotypic screening at seedling stage to identify cassava mosaic disease-resistant genotypes in African cassava populations. Sci. Rep. 2021, 11, 2850. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Behnam, B.; Bohorquez-Chaux, A.; Castaneda-Mendez, O.F.; Tsuji, H.; Ishitani, M.; Becerra Lopez-Lavalle, L.A. An optimized isolation protocol yields high-quality RNA from cassava tissues (Manihot esculenta Crantz). FEBS Open Bio 2019, 9, 814–825. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular Characterization of the Alfalfa mosaic virus Infecting Solanum melongena in Egypt and the Control of Its Deleterious Effects with Melatonin and Salicylic Acid. Plants 2021, 10, 459. [Google Scholar] [CrossRef]
- Sambandham, V.T.; Shankar, P.; Mukhopadhaya, S. Early Onset Yellow Rust Detection Guided By Remote Sensing Indices. Agriculture 2022, 12, 1206. [Google Scholar] [CrossRef]
- Basit, A.; Farhan, M.; Mo, W.-D.; Ding, H.-X.; Ikram, M.; Farooq, T.; Ahmed, S.; Yang, Z.-F.; Wang, Y.; Hashem, M.; et al. Enhancement of resistance by poultry manure and plant hormones (salicylic acid & citric acid) against tobacco mosaic virus. Saudi J. Biol. Sci. 2021, 28, 3526–3533. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, Y.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Salicylic acid-induced differential resistance to the Tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef]
- Radojičić, A.; Li, X.; Zhang, Y. Salicylic Acid: A Double-Edged Sword for Programed Cell Death in Plants. Front. Plant Sci. 2018, 9, 1133. [Google Scholar] [CrossRef]
- Singh, D.P.; Moore, C.A.; Gilliland, A.; Carr, J.P. Activation of multiple antiviral defence mechanisms by salicylic acid. Mol. Plant Pathol. 2004, 5, 57–63. [Google Scholar] [CrossRef]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Gao, Q.-M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef]
- Singh, A.; Lim, G.H.; Kachroo, P. Transport of chemical signals in systemic acquired resistance. J. Integr. Plant Biol. 2017, 59, 336–344. [Google Scholar] [CrossRef]
- Wendehenne, D.; Gao, Q.-m.; Kachroo, A.; Kachroo, P. Free radical-mediated systemic immunity in plants. Curr. Opin. Plant Biol. 2014, 20, 127–134. [Google Scholar] [CrossRef]
- Kim, T.-J.; Lim, G.-H. Salicylic Acid and Mobile Regulators of Systemic Immunity in Plants: Transport and Metabolism. Plants 2023, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Roychowdhury, R.; Ray, S.; Hada, A.; Kumar, A.; Sarker, U.; Aftab, T.; Das, R. Salicylic acid (SA)-mediated plant immunity against biotic stresses: An insight on molecular components and signaling mechanism. Plant Stress 2024, 11, 100427. [Google Scholar] [CrossRef]
- Malichan, S.; Vannatim, N.; Chaowongdee, S.; Pongpamorn, P.; Paemanee, A.; Siriwan, W. Comparative analysis of salicylic acid levels and gene expression in resistant, tolerant, and susceptible cassava varieties following whitefly-mediated SLCMV infection. Sci. Rep. 2023, 13, 13610. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, B.; Liu, W.; Cheng, X.; Lin, D.; He, C.; Shi, H. Heat shock protein 90 co-chaperone modules fine-tune the antagonistic interaction between salicylic acid and auxin biosynthesis in cassava. Cell Rep. 2021, 34, 108717. [Google Scholar] [CrossRef]
- Loon, L.C.v.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Irigoyen, M.L.; Garceau, D.C.; Bohorquez-Chaux, A.; Lopez-Lavalle, L.A.B.; Perez-Fons, L.; Fraser, P.D.; Walling, L.L. Genome-wide analyses of cassava Pathogenesis-related (PR) gene families reveal core transcriptome responses to whitefly infestation, salicylic acid and jasmonic acid. BMC Genom. 2020, 21, 93. [Google Scholar] [CrossRef]
- Pandey, S.P.; Somssich, I.E. The Role of WRKY Transcription Factors in Plant Immunity. Plant Physiol. 2009, 150, 1648–1655. [Google Scholar] [CrossRef]
- Deslandes, L.; Olivier, J.; Theulieres, F.; Hirsch, J.; Feng, D.X.; Bittner-Eddy, P.; Beynon, J.; Marco, Y. Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1-R gene, a member of a novel family of resistance genes. Proc. Natl. Acad. Sci. USA 2002, 99, 2404–2409. [Google Scholar] [CrossRef]
- Oh, S.K.; Baek, K.H.; Park, J.M.; Yi, S.Y.; Yu, S.H.; Kamoun, S.; Choi, D. Capsicum annuum WRKY protein CaWRKY1 is a negative regulator of pathogen defense. New Phytol. 2008, 177, 977–989. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, L.; Ma, X.; Zhao, X.; Jiao, B.; Li, C.; Luo, K. The WRKY transcription factors PtrWRKY18 and PtrWRKY35 promote Melampsora resistance in Populus. Tree Physiol. 2017, 37, 665–675. [Google Scholar] [CrossRef]
- Wang, H.; Hao, J.; Chen, X.; Hao, Z.; Wang, X.; Lou, Y.; Peng, Y.; Guo, Z. Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol. Biol. 2007, 65, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; De Luca, V.; Després, C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid. Nutrients 2022, 14, 1987. [Google Scholar] [CrossRef]
- Ros, R.; Muñoz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar] [CrossRef]
- Stuttmann, J.; Hubberten, H.M.; Rietz, S.; Kaur, J.; Muskett, P.; Guerois, R.; Bednarek, P.; Hoefgen, R.; Parker, J.E. Perturbation of Arabidopsis amino acid metabolism causes incompatibility with the adapted biotrophic pathogen Hyaloperonospora arabidopsidis. Plant Cell 2011, 23, 2788–2803. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Krishnan, S.R.; Pandian, S.; Mareeswaran, N.; Aruni, W.; Pandian, S.K.; Ramesh, M. Global analysis of threonine metabolism genes unravel key players in rice to improve the abiotic stress tolerance. Sci. Rep. 2018, 8, 9270. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Du, K.; Chen, H.; Cao, Y.; Xie, J.; Li, M.; Carr, J.P.; Wu, B.; Fan, Z. Maize phenylalanine ammonia-lyases contribute to resistance to Sugarcane mosaic virus infection, most likely through positive regulation of salicylic acid accumulation. Mol. Plant Pathol. 2019, 20, 1365–1378. [Google Scholar] [CrossRef]
- Creager, A.N.; Scholthof, K.-B.G.; Citovsky, V.; Scholthof, H.B. Tobacco mosaic virus: Pioneering research for a century. Plant Cell 1999, 11, 301–308. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.; Hollander, J.G.; Lefeber, A.W.; Erkelens, C.; Nuzillard, J.-M.; Verpoorte, R. NMR metabolomics to revisit the tobacco mosaic virus infection in nicotiana t abacum leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanavongsawat, C.; Malichan, S.; Vannatim, N.; Chaowongdee, S.; Hemniam, N.; Paemanee, A.; Siriwan, W. Enhancing Plant Resistance to Sri Lankan Cassava Mosaic Virus Using Salicylic Acid. Metabolites 2025, 15, 261. https://doi.org/10.3390/metabo15040261
Pattanavongsawat C, Malichan S, Vannatim N, Chaowongdee S, Hemniam N, Paemanee A, Siriwan W. Enhancing Plant Resistance to Sri Lankan Cassava Mosaic Virus Using Salicylic Acid. Metabolites. 2025; 15(4):261. https://doi.org/10.3390/metabo15040261
Chicago/Turabian StylePattanavongsawat, Chonnipa, Srihunsa Malichan, Nattachai Vannatim, Somruthai Chaowongdee, Nuannapa Hemniam, Atchara Paemanee, and Wanwisa Siriwan. 2025. "Enhancing Plant Resistance to Sri Lankan Cassava Mosaic Virus Using Salicylic Acid" Metabolites 15, no. 4: 261. https://doi.org/10.3390/metabo15040261
APA StylePattanavongsawat, C., Malichan, S., Vannatim, N., Chaowongdee, S., Hemniam, N., Paemanee, A., & Siriwan, W. (2025). Enhancing Plant Resistance to Sri Lankan Cassava Mosaic Virus Using Salicylic Acid. Metabolites, 15(4), 261. https://doi.org/10.3390/metabo15040261






