Purine Metabolism Pathway Influence on Running Capacity in Rats
Abstract
1. Introduction
2. Methods
2.1. Materials and Equipment
2.2. Experimental Design
2.3. Collection and Processing of Rat Serum Samples
Liquid Chromatography-Mass Spectrometry Analysis Conditions (LC-MS/MS)
2.4. Metabolomics Data Extraction
2.5. Metabolomics Data Analysis Methods
2.6. ITP Intervention Experimental Method
2.7. Data Analysis
3. Results
3.1. Rat Treadmill Test Analysis
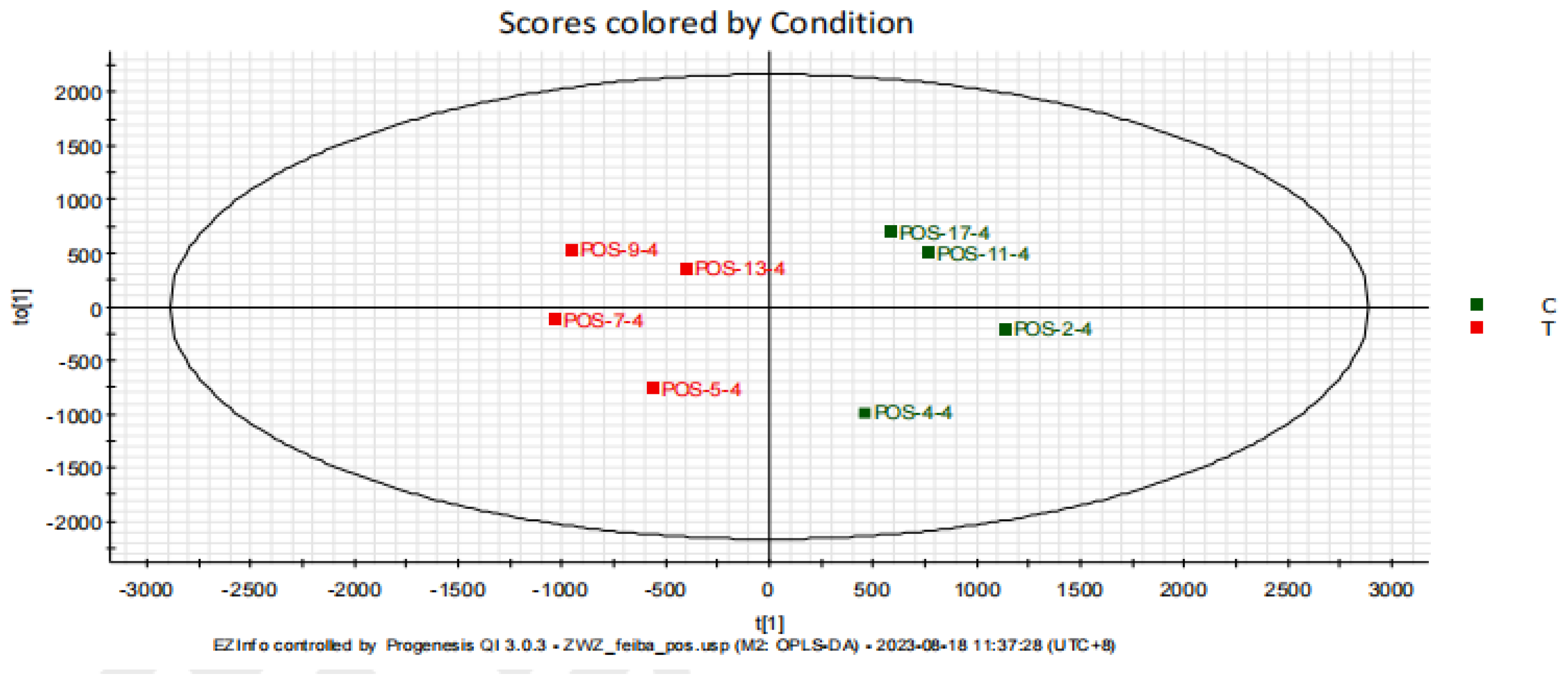
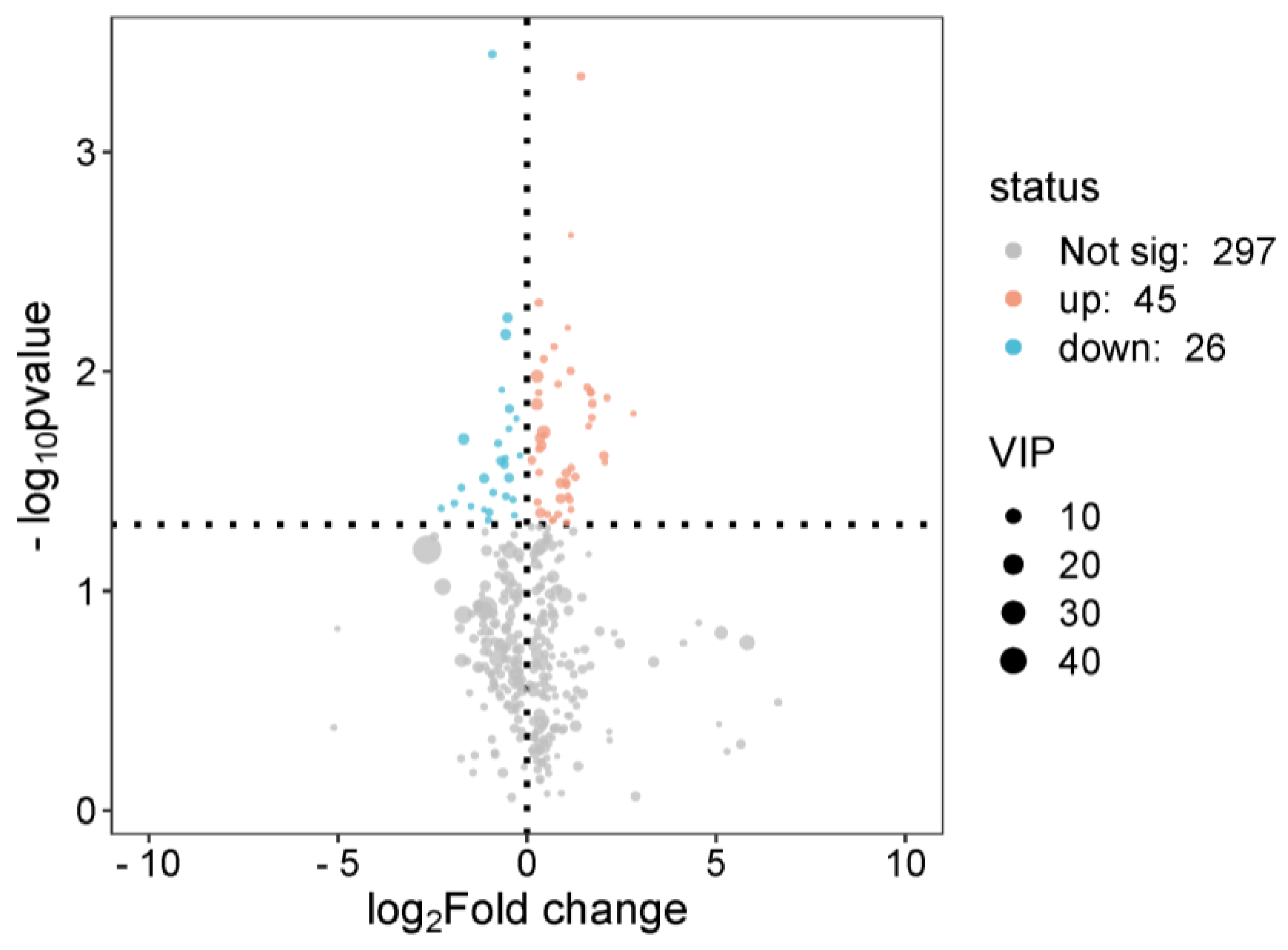
3.2. Rat Serum Metabolomics Analysis
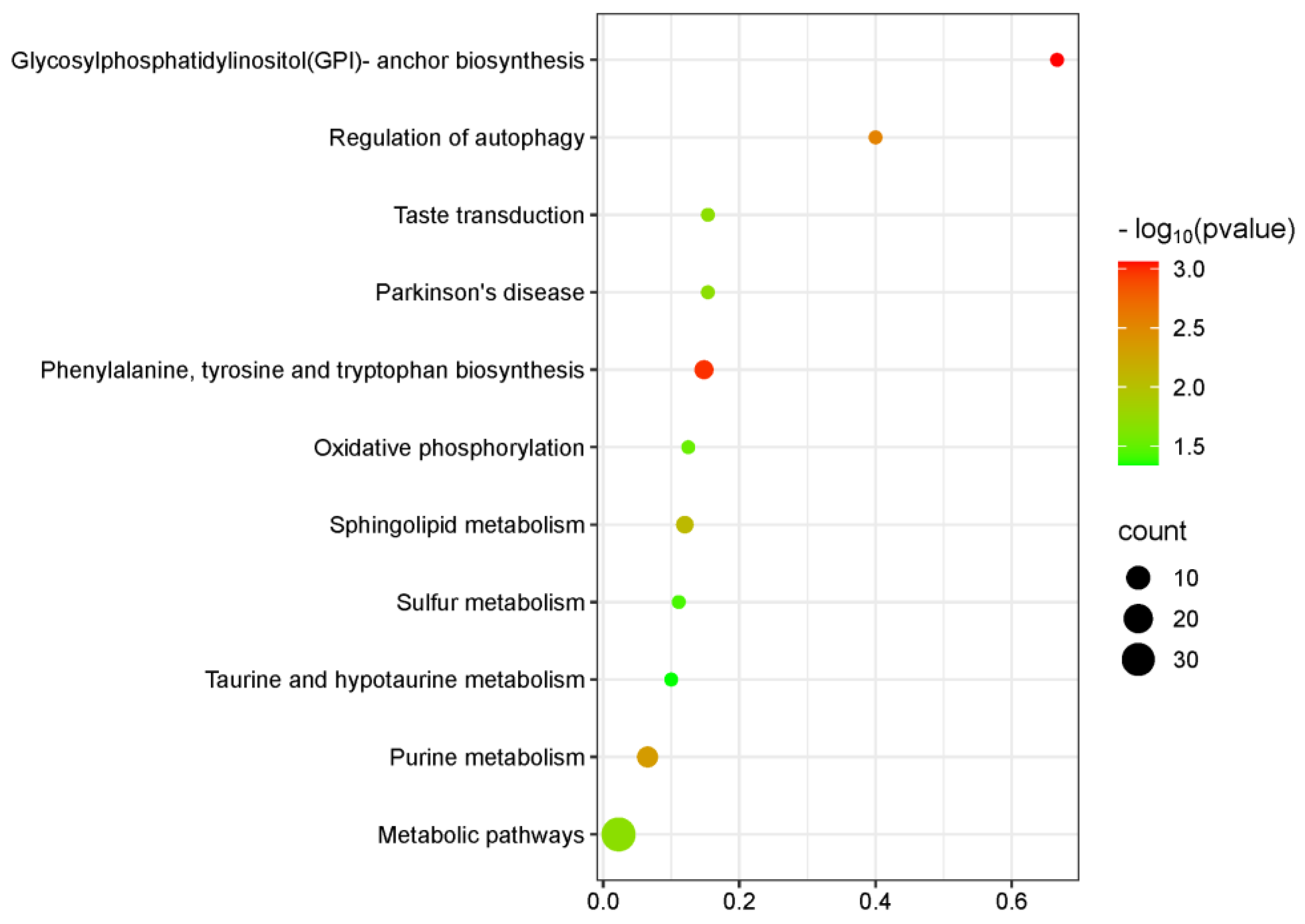
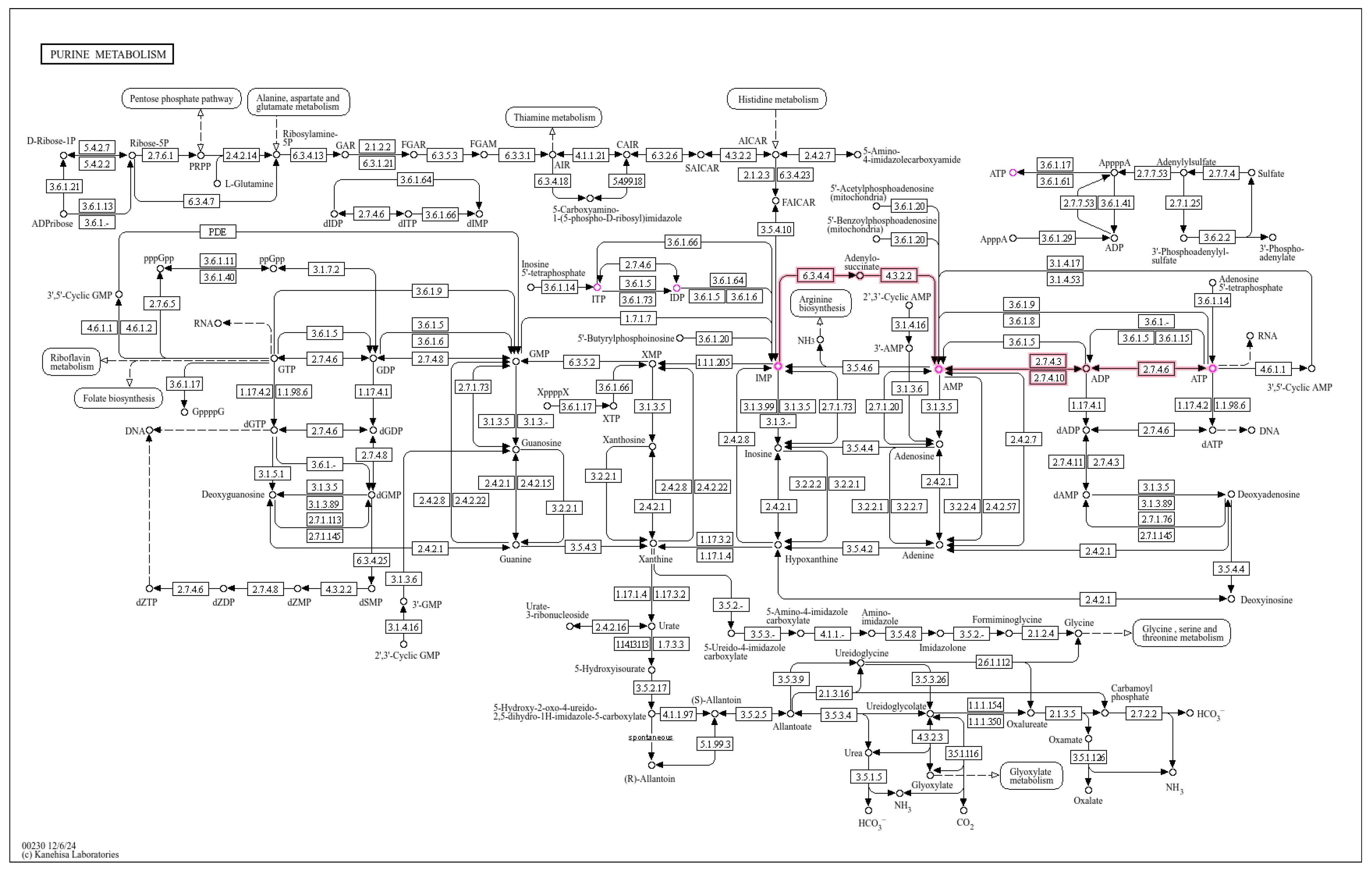
3.3. KEGG Pathway Analysis
3.4. ITP Intervention Experiment
4. Discussion
4.1. Key Differential Metabolites and Their Functional Roles
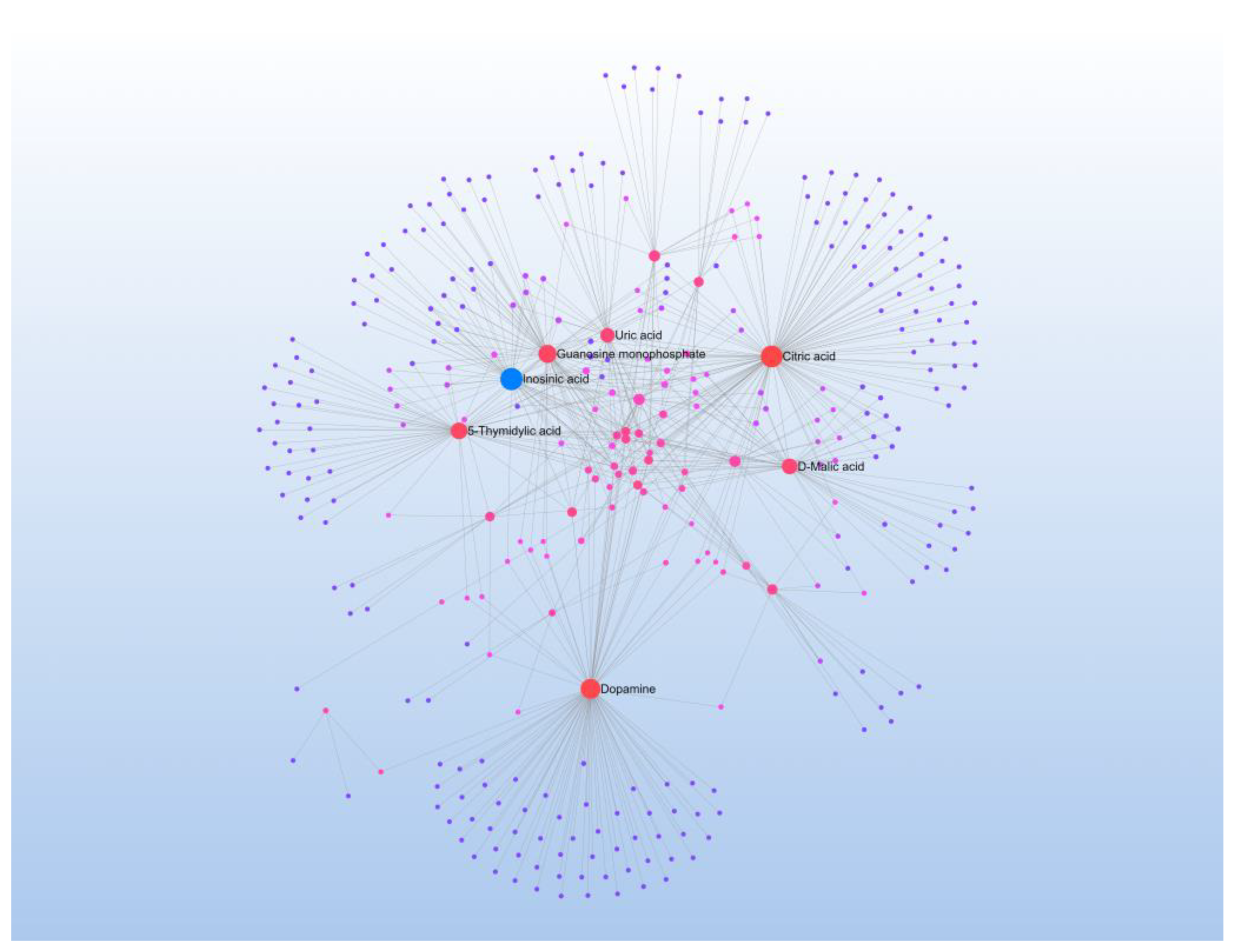
4.2. Metabolic Pathway Crosstalk and Network Dynamics
4.3. Validation Through ITP Intervention
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise sustains the hallmarks of health. J. Sport Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Miko, H.C.; Zillmann, N.; Ring-Dimitriou, S.; Dorner, T.E.; Titze, S.; Bauer, R. Effects of Physical Activity on Health. Gesundheitswesen 2020, 82, S184–S195. [Google Scholar] [PubMed]
- Raczkowska, E.; Bienkiewicz, M.; Gajda, R. Modulation of the glycaemic index value of shortbread cookies by the use of erythritol and fruit pomace. Sci. Rep. 2024, 14, 14215. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Egorova, E.S.; Gabdrakhmanova, L.J.; Fedotovskaya, O.N. Genes and Athletic Performance: An Update. Med. Sports Sci. 2016, 61, 41–54. [Google Scholar]
- Jang, S.; Drescher, M.J.; Armstrong, T.A.; Neil, E.R.; Eberman, L.E. Exploring Secondary School Athletic Trainers’ Perspectives in Managing Mental Health Situations. Int. J. Environ. Res. Public Health 2024, 21, 577. [Google Scholar] [CrossRef]
- Yang, X.; He, M.; Tang, Q.; Cao, J.; Wei, Z.; Li, T.; Sun, M. Metabolomics as a promising technology for investigating external therapy of traditional Chinese medicine: A review. Medicine 2024, 103, e40719. [Google Scholar] [CrossRef]
- Kell, P.; Sidhu, R.; Qian, M.; Mishra, S.; Nicoli, E.R.; D’Souza, P.; Tifft, C.J.; Gross, A.L.; Gray-Edwards, H.L.; Martin, D.R.; et al. A pentasaccharide for monitoring pharmacodynamic response to gene therapy in GM1 gangliosidosis. EBioMedicine 2023, 92, 104627. [Google Scholar]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Morville, T.; Sahl, R.E.; Moritz, T.; Helge, J.W.; Clemmensen, C. Plasma Metabolome Profiling of Resistance Exercise and Endurance Exercise in Humans. Cell Rep. 2020, 33, 108554. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.G.; Sokolowska, I.; Ngounou Wetie, A.G.; Channaveerappa, D.; Dupree, E.J.; Jayathirtha, M.; Aslebagh, R.; Wormwood, K.L.; Darie, C.C. Mass Spectrometry for Proteomics-Based Investigation. Adv. Exp. Med. Biol. 2019, 1140, 1–26. [Google Scholar] [PubMed]
- Cendali, F.; D’Alessandro, A.; Nemkov, T. Dried blood spot characterization of sex-based metabolic responses to acute running exercise. Anal. Sci. Adv. 2023, 4, 37–48. [Google Scholar] [PubMed]
- Klinker, J.F.; Seifert, R. Functionally nonequivalent interactions of guanosine 5′-triphosphate, inosine 5′-triphosphate, and xanthosine 5′-triphosphate with the retinal G-protein, transducin, and with Gi-proteins in HL-60 leukemia cell membranes. Biochem. Pharmacol. 1997, 54, 551–562. [Google Scholar]
- Devries, M.C. Sex-based differences in endurance exercise muscle metabolism: Impact on exercise and nutritional strategies to optimize health and performance in women. Exp. Physiol. 2016, 101, 243–249. [Google Scholar] [PubMed]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So you think you can PLS-DA? BMC Bioinform. 2020, 21, 2. [Google Scholar]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar]
- Nishijo, N.; Hayama, T.; Tomita, R.; Fujioka, T. Accurate LC-MS/MS Analysis of Diacylglycerols in Human Plasma with Eliminating Matrix Effect by Phospholipids Using Fluorous Biphasic Extraction. Anal. Chem. 2023, 95, 14898–14904. [Google Scholar]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Sakumi, K.; Abolhassani, N.; Behmanesh, M.; Iyama, T.; Tsuchimoto, D.; Nakabeppu, Y. ITPA protein, an enzyme that eliminates deaminated purine nucleoside triphosphates in cells. Mutat. Res. 2010, 703, 43–50. [Google Scholar] [PubMed]
- Blomstrand, E. A role for branched-chain amino acids in reducing central fatigue. J. Nutr. 2006, 136, 544s–547s. [Google Scholar]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar]
- Wu, D.; Yang, S.; Yuan, C.; Zhang, K.; Tan, J.; Guan, K.; Zeng, H.; Huang, C. Targeting purine metabolism-related enzymes for therapeutic intervention: A review from molecular mechanism to therapeutic breakthrough. Int. J. Biol. Macromol. 2024, 282, 136828. [Google Scholar]
- van der Vusse, G.J.; Glatz, J.F.; Stam, H.C.; Reneman, R.S. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol. Rev. 1992, 72, 881–940. [Google Scholar]
- Galloway, S.D.; Talanian, J.L.; Shoveller, A.K.; Heigenhauser, G.J.; Spriet, L.L. Seven days of oral taurine supplementation does not increase muscle taurine content or alter substrate metabolism during prolonged exercise in humans. J. Appl. Physiol. (1985) 2008, 105, 643–651. [Google Scholar]



| Group | Weight (g) | First Time | Second Time | Third Time | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Distance (m) | p | Time (min) | Distance (m) | p | Time (min) | Distance (m) | p | ||
| Low | 441 ± 21 | 6.5 (3.9–10.8) | 116.1 (67.4–197.2) | 0.014 * | 5.3 (2.2–8.2) | 94.1 (33.8–146.5) | 0.014 * | 5.2 (4.2–6.8) | 89.4 (71.6–121.9) | 0.028 * |
| High | 446 ± 23 | 30.3 (29.1–32.1) | 564.6 (560.9–582.5) | 28.9 (27.2–29.9) | 537.9 (502.1–560.1) | 30.8 (29.7–30.2) | 557.6 (549.8–563.6) | |||
| No. | Compound | Molecular Formula | Precise Molecular Weight (Da) | RT (min) | Score | p Value | Fold Change |
|---|---|---|---|---|---|---|---|
| 1 | 1,2,10-Trihydroxydihydro-trans-linalyl oxide 7-O-beta-D-glucopyranoside | C16H30O10 | 382.18 | 12.09 | 34.1 | <0.05 | 2.84 |
| 2 | Tryptophan | C11H12N2O2 | 204.23 | 7.3 | 48.2 | <0.05 | 2.12 |
| 3 | Deacetylnomilinic acid | C26H34O9 | 490.22 | 6.91 | 37 | <0.05 | 1.75 |
| 4 | Inosine triphosphate | C10H15N4O14P3 | 574.06 | 9.90 | 36.8 | <0.05 | 1.74 |
| 5 | Edetic acid | C10H16N2O8 | 292.08 | 0.89 | 39.7 | <0.05 | 1.65 |
| 6 | Lactosylceramide (d18:1/26:1(17Z)) | C56H105NO13 | 999.75 | 0.83 | 31.4 | <0.05 | 1.62 |
| 7 | Tetrahydrodeoxycortisol | C21H34O4 | 350.24 | 12.09 | 42.1 | <0.05 | 1.59 |
| 8 | Tocladesine | C10H11ClN5O6P | 363.01 | 0.89 | 34.7 | <0.05 | 1.56 |
| 9 | Gamma-glutamyltyrosine | C14H18N2O6 | 310.11 | 4.34 | 37.6 | <0.05 | 1.56 |
| 10 | Chalepin acetate | C21H24O5 | 356.16 | 11.48 | 36.7 | <0.05 | 1.51 |
| No. | Compound | Molecular Formula | Precise Molecular Weight (Da) | RT (min) | Score | p Value | Fold Change |
|---|---|---|---|---|---|---|---|
| 1 | PE(20:1(11Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C47H80NO8P | 817.56 | 11.35 | 37.6 | <0.05 | 0.65 |
| 2 | PC(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C46H80NO8P | 805.56 | 13.53 | 35.1 | <0.05 | 0.60 |
| 3 | PC(o-16:0/20:4(8Z,11Z,14Z,17Z)) | C44H82NO7P | 767.57 | 11.37 | 34.1 | <0.05 | 0.59 |
| 4 | PC(18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C48H84NO8P | 833.59 | 13.54 | 36.3 | <0.05 | 0.55 |
| 5 | Farnesyl triphosphate | C15H29O10P3 | 562.09 | 4.12 | 35.6 | <0.05 | 0.51 |
| 6 | S-methyl-5-thio-D-ribulose 1-phosphate(2-) | HMDB0062647 | 785.57 | 13.52 | 33.2 | <0.05 | 0.45 |
| 7 | PC(22:4(7Z,10Z,13Z,16Z)/14:0) | C44H80NO8P | 781.56 | 13.52 | 40.2 | <0.05 | 0.41 |
| 8 | PC(16:1(9Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C46H78NO8P | 803.54 | 11.51 | 53.1 | <0.05 | 0.39 |
| 9 | PC(18:0/18:3(6Z,9Z,12Z)) | C44H82NO8P | 783.57 | 12.46 | 38 | <0.05 | 0.37 |
| 10 | Aclarubicin | CSID397638 | 857.56 | 12.37 | 39.6 | <0.05 | 0.36 |
| Gender | Group | Before Experiment | After Experiment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight (g) | Time (min) | Distance (m) | p | Weight (g) | Time (min) | Distance (m) | p | ||
| Female | Control group | 328 ± 19 | 3.8 (2.6–7.5) | 70.1 (43.7–142.2) | 0.201 | 333 ± 22 | 3.6 (2.6–6.6) | 64.5 (44.1–121.1) | 0.039 * |
| ITP group | 329 ± 19 | 7.8 (3.1–9.3) | 160.7 (53.1–176.1) | 332 ± 16 | 10.9 (5.3–14.7) | 206.9 (96.7–277.6) | |||
| Male | Control group | 359 ± 39 | 6.87 (2.2–9.1) | 155.7 (38.4–190.3) | 0.305 | 368 ± 34 | 5.7 (2.7–6.9) | 103.9 (52.9–27.7) | 0.009 ** |
| ITP group | 353 ± 31 | 8.8 (3.1–11.9) | 181.8 (58.8–268.7) | 380 ± 38 | 15.9 (6.4–27.7) | 299.9 (114.9–523.3) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Biney, C.N.; Wang, Q.; Cai, M.; Cheng, S.; Chen, W.; Zhang, J.; Zhao, J.; Zhang, Y.; Zhang, W. Purine Metabolism Pathway Influence on Running Capacity in Rats. Metabolites 2025, 15, 241. https://doi.org/10.3390/metabo15040241
Chen D, Biney CN, Wang Q, Cai M, Cheng S, Chen W, Zhang J, Zhao J, Zhang Y, Zhang W. Purine Metabolism Pathway Influence on Running Capacity in Rats. Metabolites. 2025; 15(4):241. https://doi.org/10.3390/metabo15040241
Chicago/Turabian StyleChen, Dengbo, Christian Noble Biney, Qian Wang, Mingzheng Cai, Shi Cheng, Wentao Chen, Jinrui Zhang, Junran Zhao, Yuhan Zhang, and Wenzhong Zhang. 2025. "Purine Metabolism Pathway Influence on Running Capacity in Rats" Metabolites 15, no. 4: 241. https://doi.org/10.3390/metabo15040241
APA StyleChen, D., Biney, C. N., Wang, Q., Cai, M., Cheng, S., Chen, W., Zhang, J., Zhao, J., Zhang, Y., & Zhang, W. (2025). Purine Metabolism Pathway Influence on Running Capacity in Rats. Metabolites, 15(4), 241. https://doi.org/10.3390/metabo15040241






