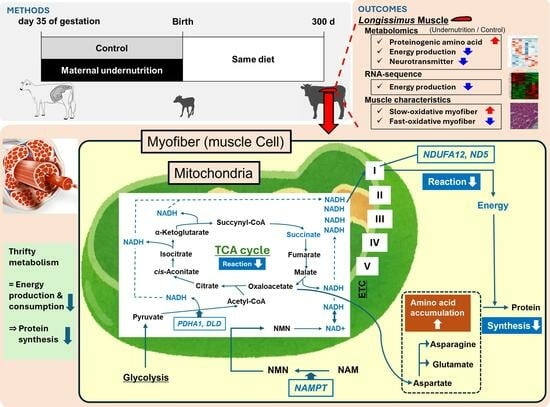
A Pilot Study: Maternal Undernutrition Programs Energy Metabolism and Alters Metabolic Profile and Morphological Characteristics of Skeletal Muscle in Postnatal Beef Cattle
Abstract
1. Introduction
2. Materials and Methods
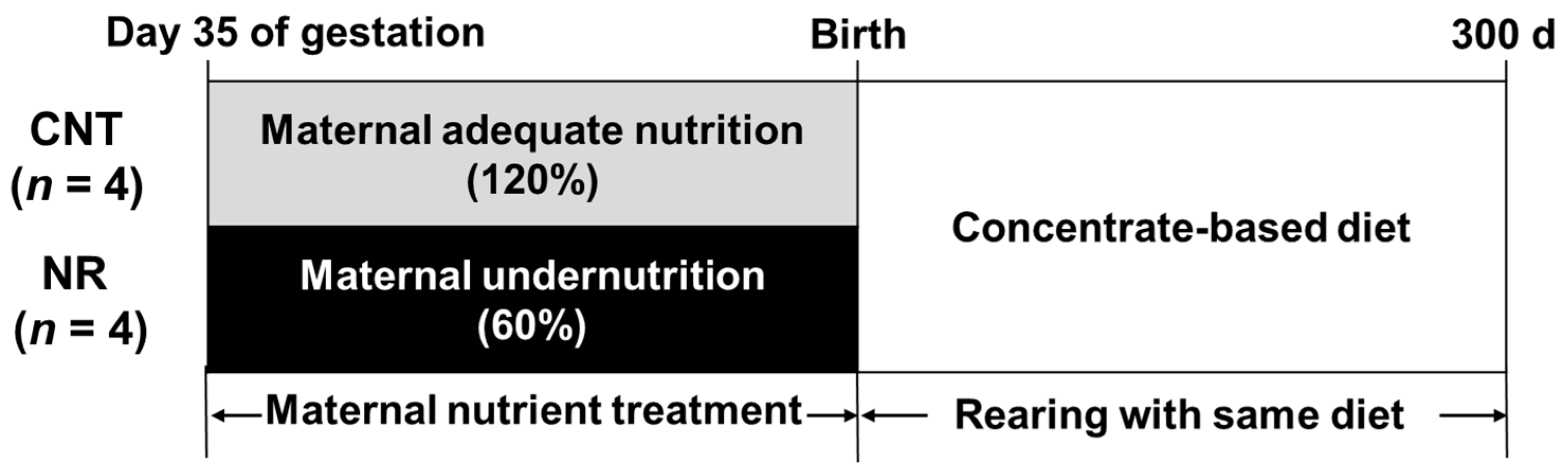
2.1. Animals and Experimental Design
2.2. Longissimus Thoracis Muscle Sampling
2.3. Metabolomics and Pathway Analysis
2.4. RNA Sequencing and Pathway Analysis
2.5. Muscle and Adipocyte Histochemical Properties and Myofiber Type Composition
2.6. Statistical Analyses
3. Results
3.1. Growth Performance
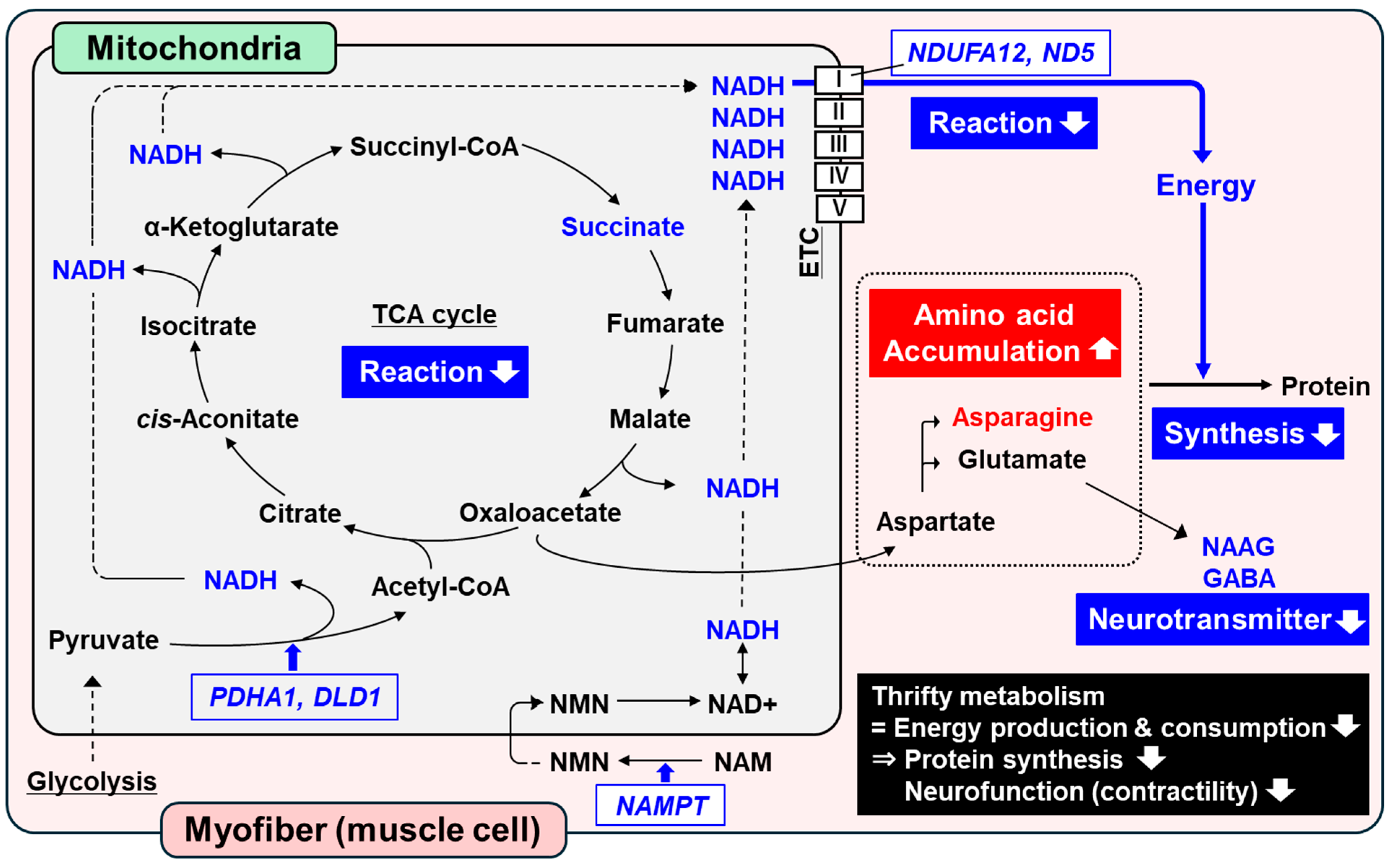
3.2. Metabolomics and Pathway Analysis
3.3. Transcriptomics and Pathway Analysis
3.4. Muscle and Adipocyte Histochemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, P.L.; Bell, A.W. Developmental Programming and Growth of Livestock Tissues for Meat Production. Vet. Clin. N. A. -Food Anim. Pract. 2019, 35, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Huang, Y.; Das, A.K.; Yang, Q.; Duarte, M.S.; Dodson, M.V.; Zhu, M.J. Meat Science and Muscle Biology Symposium: Manipulating Mesenchymal Progenitor Cell Differentiation to Optimize Performance and Carcass Value of Beef Cattle. J. Anim. Sci. 2013, 91, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L.; Café, L.M.; Hearnshaw, H.; Hennessy, D.W. Consequences of Nutrition and Growth Retardation Early in Life for Growth and Composition of Cattle and Eating Quality of Beef. Recent Adv. Anim. Nutr. Aust. 2005, 15, 183–195. [Google Scholar]
- Webb, M.J.; Block, J.J.; Funston, R.N.; Underwood, K.R.; Legako, J.F.; Harty, A.A.; Salverson, R.R.; Olson, K.C.; Blair, A.D. Influence of Maternal Protein Restriction in Primiparous Heifers during Mid- and/or Late-Gestation on Meat Quality and Fatty Acid Profile of Progeny. Meat Sci. 2019, 152, 31–37. [Google Scholar] [CrossRef]
- Zumbaugh, M.D.; Johnson, S.E.; Shi, T.H.; Gerrard, D.E. Molecular and Biochemical Regulation of Skeletal Muscle Metabolism. J. Anim. Sci. 2022, 100, skac035. [Google Scholar] [CrossRef]
- Bonnet, M.; Cassar-Malek, I.; Chilliard, Y.; Picard, B. Ontogenesis of Muscle and Adipose Tissues and Their Interactions in Ruminants and Other Species. Animal 2010, 4, 1093–1109. [Google Scholar] [CrossRef]
- Zhang, Y.; Otomaru, K.; Oshima, K.; Goto, Y.; Oshima, I.; Muroya, S.; Sano, M.; Saneshima, R.; Nagao, Y.; Kinoshita, A.; et al. Effects of Low and High Levels of Maternal Nutrition Consumed for the Entirety of Gestation on the Development of Muscle, Adipose Tissue, Bone, and the Organs of Wagyu Cattle Fetuses. Anim. Sci. J. 2021, 92, e13600. [Google Scholar] [CrossRef]
- Muroya, S.; Zhang, Y.; Kinoshita, A.; Otomaru, K.; Oshima, K.; Gotoh, Y.; Oshima, I.; Sano, M.; Roh, S.; Oe, M.; et al. Maternal Undernutrition during Pregnancy Alters Amino Acid Metabolism and Gene Expression Associated with Energy Metabolism and Angiogenesis in Fetal Calf Muscle. Metabolites 2021, 11, 582. [Google Scholar] [CrossRef]
- Chang, E.I.; Wesolowski, S.R.; Gilje, E.A.; Baker, P.R.; Reisz, J.A.; D’Alessandro, A.; Hay, W.W.; Rozance, P.J.; Brown, L.D. Skeletal Muscle Amino Acid Uptake Is Lower and Alanine Production Is Greater in Late Gestation Intrauterine Growth-Restricted Fetal Sheep Hindlimb. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R615–R629. [Google Scholar] [CrossRef]
- Smith, B.I.; Vásquez-Hidalgo, M.A.; Li, X.; Vonnahme, K.A.; Grazul-Bilska, A.T.; Swanson, K.C.; Moore, T.E.; Reed, S.A.; Govoni, K.E. The Effects of Maternal Nutrient Restriction during Mid to Late Gestation with Realimentation on Fetal Metabolic Profiles in the Liver, Skeletal Muscle, and Blood in Sheep. Metabolites 2024, 14, 465. [Google Scholar] [CrossRef]
- Martin, D.E.; Jones, A.K.; Pillai, S.M.; Hoffman, M.L.; McFadden, K.K.; Zinn, S.A.; Govoni, K.E.; Reed, S.A. Maternal Restricted- And Overfeeding during Gestation Result in Distinct Lipid and Amino Acid Metabolite Profiles in the Longissimus Muscle of the Offspring. Front. Physiol. 2019, 10, 448206. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, A.; Wishart, D.S.; Fitzsimmons, C. Exploring Biological Impacts of Prenatal Nutrition and Selection for Residual Feed Intake on Beef Cattle Using Omics Technologies: A Review. Front. Genet. 2021, 12, 720268. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, B.; Ghosh, S.; Dysart, M.W.; Kanaan, G.N.; Chu, A.; Blais, A.; Rajamanickam, K.; Tsai, E.C.; Patti, M.E.; Harper, M.E. Low Birth Weight Is Associated with Adiposity, Impaired Skeletal Muscle Energetics and Weight Loss Resistance in Mice. Int. J. Obes. 2014, 39, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.A.; Storey, B.T.; Peterside, I.; Simmons, R.A. Impaired Oxidative Phosphorylation in Skeletal Muscle of Intrauterine Growth-Retarded Rats. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E130–E137. [Google Scholar] [CrossRef]
- Beauchamp, B.; Harper, M.E. In Utero Undernutrition Programs Skeletal and Cardiac Muscle Metabolism. Front. Physiol. 2016, 6, 171634. [Google Scholar] [CrossRef]
- National Agriculture and Food Research Organization. Japanese Feeding Standard for Beef Cattle, 2008th ed.; Japan Livestock Industry Association: Tokyo, Japan, 2009. [Google Scholar]
- Wagyu Registry Association. The Handbook for Wagyu Registration; Wagyu Registry Association: Kyoto, Japan, 2017. (In Japanese) [Google Scholar]
- Phomvisith, O.; Muroya, S.; Otomaru, K.; Oshima, K.; Oshima, I.; Nishino, D.; Haginouchi, T.; Gotoh, T. Maternal Undernutrition Affects Fetal Thymus DNA Methylation, Gene Expression, and, Thereby, Metabolism and Immunopoiesis in Wagyu (Japanese Black) Cattle. Int. J. Mol. Sci. 2024, 25, 9242. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Brooke, M.H.; Kaiser, K.K. Muscle Fiber Types: How Many and What Kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef]
- Brooke, M.H.; Kaiser, K.K. Three “Myosin Adenosine Triphosphatase” Systems: The Nature of Their PH Lability and Sulfhydryl Dependence. J. Histochem. Cytochem. 1970, 18, 670–672. [Google Scholar] [CrossRef]
- Funston, R.N.; Larson, D.M.; Vonnahme, K.A. Effects of Maternal Nutrition on Conceptus Growth and Offspring Performance: Implications for Beef Cattle Production. J. Anim. Sci. 2010, 88, E205–E215. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal Programming of Skeletal Muscle Development in Ruminant Animals. J. Anim. Sci. 2010, 88. [Google Scholar] [CrossRef]
- Freetly, H.C.; Ferrell, C.L.; Jenkins, T.G. Timing of Realimentation of Mature Cows That Were Feed-Restricted during Pregnancy Influences Calf Birth Weights and Growth Rates. J. Anim. Sci. 2000, 78, 2790–2796. [Google Scholar] [CrossRef]
- Marquez, D.C.; Paulino, M.F.; Rennó, L.N.; Villadiego, F.C.; Ortega, R.M.; Moreno, D.S.; Martins, L.S.; De Almeida, D.M.; Gionbelli, M.P.; Manso, M.R.; et al. Supplementation of Grazing Beef Cows during Gestation as a Strategy to Improve Skeletal Muscle Development of the Offspring. Animal 2017, 11, 2184–2192. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Gomez, N.A.; Gao, Y.; Son, J.S.; Chae, S.A.; Zhu, M.J.; Du, M. Stage-Specific Nutritional Management and Developmental Programming to Optimize Meat Production. J. Anim. Sci. Biotechnol. 2023, 14, 2. [Google Scholar] [CrossRef]
- Holeček, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef]
- Balasubramanian, M.N.; Butterworth, E.A.; Kilberg, M.S. Asparagine Synthetase: Regulation by Cell Stress and Involvement in Tumor Biology. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E789–E799. [Google Scholar] [CrossRef]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine Promotes Cancer Cell Proliferation through Use as an Amino Acid Exchange Factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef] [PubMed]
- Wirth, C.; Brandt, U.; Hunte, C.; Zickermann, V. Structure and Function of Mitochondrial Complex I. Biochim. Biophys. Acta (BBA) -Bioenerg. 2016, 1857, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Basse, A.L.; Agerholm, M.; Farup, J.; Dalbram, E.; Nielsen, J.; Ørtenblad, N.; Altıntaş, A.; Ehrlich, A.M.; Krag, T.; Bruzzone, S.; et al. Nampt Controls Skeletal Muscle Development by Maintaining Ca2+ Homeostasis and Mitochondrial Integrity. Mol. Metab. 2021, 53, 101271. [Google Scholar] [CrossRef] [PubMed]
- Pavlu-Pereira, H.; Silva, M.J.; Florindo, C.; Sequeira, S.; Ferreira, A.C.; Duarte, S.; Rodrigues, A.L.; Janeiro, P.; Oliveira, A.; Gomes, D.; et al. Pyruvate Dehydrogenase Complex Deficiency: Updating the Clinical, Metabolic and Mutational Landscapes in a Cohort of Portuguese Patients. Orphanet J. Rare Dis. 2020, 15, 298. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Effect of Maternal Nutrient Restriction in Sheep on the Development of Fetal Skeletal Muscle. Biol. Reprod. 2004, 71, 1968–1973. [Google Scholar] [CrossRef]
- Pendleton, A.L.; Antolic, A.T.; Kelly, A.C.; Davis, M.A.; Camacho, L.E.; Doubleday, K.; Anderson, M.J.; Langlais, P.R.; Lynch, R.M.; Limesand, S.W. Lower Oxygen Consumption and Complex I Activity in Mitochondria Isolated from Skeletal Muscle of Fetal Sheep with Intrauterine Growth Restriction. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E67–E80. [Google Scholar] [CrossRef]
- Zhao, W.; Kelly, A.C.; Luna-Ramirez, R.I.; Bidwell, C.A.; Anderson, M.J.; Limesand, S.W. Decreased Pyruvate but Not Fatty Acid Driven Mitochondrial Respiration in Skeletal Muscle of Growth Restricted Fetal Sheep. Int. J. Mol. Sci. 2023, 24, 15760. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Polizel, G.H.G.; Cracco, R.C.; Cançado, F.A.C.Q.; Baldin, G.C.; Poleti, M.D.; Ferraz, J.B.S.; de Almeida Santana, M.H. Metabolomics Changes in Meat and Subcutaneous Fat of Male Cattle Submitted to Fetal Programming. Metabolites 2024, 14, 9. [Google Scholar] [CrossRef]
- Neale, J.H.; Bzdega, T.; Wroblewska, B. N-Acetylaspartylglutamate. J. Neurochem. 2000, 75, 443–452. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Transitions of Muscle Fiber Phenotypic Profiles. Histochem. Cell Biol. 2001, 115, 359–372. [Google Scholar] [CrossRef]
- van Vliet, E.; Eixarch, E.; Illa, M.; Arbat-Plana, A.; González-Tendero, A.; Hogberg, H.T.; Zhao, L.; Hartung, T.; Gratacos, E. Metabolomics Reveals Metabolic Alterations by Intrauterine Growth Restriction in the Fetal Rabbit Brain. PLoS ONE 2013, 8, e64545. [Google Scholar] [CrossRef] [PubMed]
- Peerboom, C.; Wierenga, C.J. The Postnatal GABA Shift: A Developmental Perspective. Neurosci. Biobehav. Rev. 2021, 124, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New Insights in Muscle Biology That Alter Meat Quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Fahey, A.J.; Brameld, J.M.; Parr, T.; Buttery, P.J. The Effect of Maternal Undernutrition before Muscle Differentiation on the Muscle Fiber Development of the Newborn Lamb. J. Anim. Sci. 2005, 83, 2564–2571. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Means, W.J.; Hess, B.W.; Nathanielsz, P.W.; Du, M. Maternal Nutrient Restriction Affects Properties of Skeletal Muscle in Offspring. J. Physiol. 2006, 575, 241–250. [Google Scholar] [CrossRef]
- Alvarenga, T.I.R.C.; Copping, K.J.; Han, X.; Clayton, E.H.; Meyer, R.J.; Rodgers, R.J.; McMillen, I.C.; Perry, V.E.A.; Geesink, G. The Influence of Peri-Conception and First Trimester Dietary Restriction of Protein in Cattle on Meat Quality Traits of Entire Male Progeny. Meat Sci. 2016, 121, 141–147. [Google Scholar] [CrossRef]
- Costa, T.C.; Du, M.; Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Schultz, E.B.; Gionbelli, M.P.; Duarte, M.d.S. Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation. Animals 2021, 11, 860. [Google Scholar] [CrossRef]
- Greenwood, P.L.; Cafe, L.M. Prenatal and Pre-Weaning Growth and Nutrition of Cattle: Long-Term Consequences for Beef Production. Animal 2007, 1, 1283–1296. [Google Scholar] [CrossRef]
- Ahmadzadeh-Gavahan, L.; Hosseinkhani, A.; Hamidian, G.; Jarolmasjed, S.; Yousefi-Tabrizi, R. Restricted Maternal Nutrition and Supplementation of Propylene Glycol, Monensin Sodium and Rumen-Protected Choline Chloride during Late Pregnancy Does Not Affect Muscle Fibre Characteristics of Offspring. Vet. Med. Sci. 2023, 9, 2260–2268. [Google Scholar] [CrossRef]
- Tygesen, M.P.; Harrison, A.P.; Therkildsen, M. The Effect of Maternal Nutrient Restriction during Late Gestation on Muscle, Bone and Meat Parameters in Five Month Old Lambs. Livest. Sci. 2007, 110, 230–241. [Google Scholar] [CrossRef]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and Adipogenesis Originate from a Common Mesenchymal Progenitor in Skeletal Muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, B.; Fu, X.; Yang, Q.; Zhu, M.J. Fetal Programming in Meat Production. Meat Sci. 2015, 109, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; Heasman, L.; Juniper, D.T.; Symonds, M.E. Maternal Nutrition in Early-Mid Gestation and Placental Size in Sheep. Br. J. Nutr. 1998, 79, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Vonnahme, K.A.; Hess, B.W.; Hansen, T.R.; McCormick, R.J.; Rule, D.C.; Moss, G.E.; Murdoch, W.J.; Nijland, M.J.; Skinner, D.C.; Nathanielsz, P.W.; et al. Maternal Undernutrition from Early- to Mid-Gestation Leads to Growth Retardation, Cardiac Ventricular Hypertrophy, and Increased Liver Weight in the Fetal Sheep. Biol. Reprod. 2003, 69, 133–140. [Google Scholar] [CrossRef]
| Treatment 1 | p-Value | |||||
|---|---|---|---|---|---|---|
| Items | CNT | NR | SEM | Diet | Time | Diet × Time |
| Nutrient intake, kg | ||||||
| Milk replacer | ||||||
| CP 2, kg/d | 0.14 | 0.14 | 0.01 | 0.73 | – | – |
| TDN 3, kg/d | 0.53 | 0.52 | 0.02 | 0.73 | – | – |
| Total mixed ration | ||||||
| CP 2, kg/d | ||||||
| 0–120 d | 0.27 | 0.28 | 0.02 | 0.45 | <0.01 | 0.71 |
| 121–300 d | 1.19 | 1.19 | 0.04 | |||
| TDN 3, kg/d | ||||||
| 0–120 d | 1.15 | 1.21 | 0.07 | 0.43 | <0.01 | 0.77 |
| 121–300 d | 4.77 | 4.81 | 0.16 | |||
| Birth weight, kg | 36.1 a | 32.4 b | 1.2 | 0.03 | – | – |
| Body weight, kg | ||||||
| 30 d | 63.2 | 55.9 | 1.2 | 0.94 | <0.01 | 0.01 |
| 60 d | 82.3 | 78.8 | 2.6 | |||
| 120 d | 140.0 | 139.2 | 5.0 | |||
| 180 d | 198.4 | 205.9 | 7.0 | |||
| 240 d | 249.3 b | 278.3 a | 11.1 | |||
| 300 d | 311.1 | 328.8 | 12.5 | |||
| ADG 4, kg/d | ||||||
| 0–30 d | 0.90 | 0.78 | 0.03 | 0.34 | 0.03 | 0.03 |
| 31–60 d | 0.64 | 0.76 | 0.09 | |||
| 61–120 d | 0.96 | 1.01 | 0.05 | |||
| 121–180 d | 0.97 | 1.11 | 0.05 | |||
| 181–240 d | 0.85 b | 1.21 a | 0.16 | |||
| 241–300 d | 1.03 | 0.84 | 0.16 | |||
| CPCR 5, kg CP intake /kg BW gain | ||||||
| 0–120 d | 0.47 | 0.48 | 0.02 | 0.86 | <0.01 | 0.03 |
| 121–300 d | 1.26 x | 1.11 y | 0.07 | |||
| TDNCR 6, kg TDN intake /kg BW gain | ||||||
| 0–120 d | 1.94 | 1.99 | 0.08 | 0.85 | <0.01 | 0.03 |
| 121–300 d | 5.07 x | 4.47 y | 0.31 | |||
| Treatment 3 | ||||
|---|---|---|---|---|
| Items 2 | CNT | NR | SEM | p-Value |
| Increased compound in NR offspring LM | ||||
| Pyroglutamine | 100.0 | 145.1 | 8.0 | 0.01 |
| N6,N6-Dimethyllysine | 100.0 | 141.3 | 11.0 | 0.03 |
| 11-Aminoundecanoic acid | 100.0 | 140.7 | 11.2 | 0.03 |
| 3′,5′-ADP | 100.0 | 203.4 | 27.6 | 0.03 |
| Asparagine | 100.0 | 141.4 | 13.4 | 0.05 |
| Taurine | 100.0 | 140.3 | 14.3 | 0.06 |
| Methylguanidine | 100.0 | 139.0 | 16.1 | 0.09 |
| 2-Amino-2-methyl-1-propanol | 100.0 | 120.7 | 8.9 | 0.10 |
| Glycerophosphorylethanolamine | 100.0 | 171.5 | 30.4 | 0.10 |
| Decreased compound in NR offspring LM | ||||
| NADH | 100.0 | 22.2 | 5.7 | <0.01 |
| 2-Deoxyribonic acid | 100.0 | 72.6 | 4.6 | 0.01 |
| N-Acetylglucosamine 1-phosphate | 100.0 | 33.2 | 12.6 | 0.01 |
| myo-Inositol 2-phosphate | 100.0 | 68.9 | 8.9 | 0.04 |
| 3-Methylcytidine | 100.0 | 55.2 | 12.0 | 0.04 |
| Allantoic acid | 100.0 | 57.8 | 13.2 | 0.05 |
| Tyrosine methyl ester | 100.0 | 69.4 | 9.5 | 0.06 |
| N-Acetylaspartylglutamate | 100.0 | 55.3 | 16.4 | 0.07 |
| Fructose 6-phosphate | 100.0 | 62.6 | 12.0 | 0.07 |
| Succinate | 100.0 | 60.5 | 13.7 | 0.07 |
| GABA | 100.0 | 72.4 | 11.2 | 0.08 |
| Glucosaminic acid | 100.0 | 63.2 | 15.2 | 0.10 |
| Metabolites 3 | ||||
|---|---|---|---|---|
| Pathway 2 | p-Value | FDR | Increased in NR LM | Decreased in NR LM |
| Alanine, aspartate and glutamate metabolism | <0.01 | <0.01 | Asparagine | Succinate, N-Acetylaspartylglutamate, GABA |
| Butanoate metabolism | <0.01 | 0.15 | Succinate, GABA | |
| Amino sugar and nucleotide sugar metabolism | 0.03 | 0.76 | Fructose 6-phosphate, N-Acetyl-glucosamine 1-phosphate | |
| Taurine and hypotaurine metabolism | 0.05 | 1.00 | Taurine | |
| Starch and sucrose metabolism | 0.11 | 1.00 | Fructose 6-phosphate | |
| TCA cycle | 0.12 | 1.00 | Succinate | |
| Fructose and mannose metabolism | 0.12 | 1.00 | Fructose 6-phosphate | |
| Ether lipid metabolism | 0.12 | 1.00 | Glycerylphosphorylethanolamine | |
| Pantothenate and CoA biosynthesis | 0.12 | 1.00 | 3′,5′-ADP | |
| Propanoate metabolism | 0.13 | 1.00 | Succinate | |
| Pentose phosphate pathway | 0.14 | 1.00 | Fructose 6-phosphate | |
| Glycolysis/Gluconeogenesis | 0.16 | 1.00 | Fructose 6-phosphate | |
| Arginine and proline metabolism | 0.21 | 1.00 | GABA | |
| Glycerophospholipid metabolism | 0.21 | 1.00 | Glycerylphosphorylethanolamine | |
| Primary bile acid biosynthesis | 0.26 | 1.00 | Taurine | |
| Purine metabolism | 0.37 | 1.00 | Allantoic acid | |
| Biological Process | Gene Symbol | Gene Name | FC 2 | p-Value |
|---|---|---|---|---|
| Energy metabolism | NDUFA12 | NADH:ubiquinone oxidoreductase subunit A12 | 0.49 | <0.01 |
| ND5 | NADH dehydrogenase subunit 5 | 0.78 | 0.01 | |
| NAMPT | nicotinamide phosphoribosyltransferase | 0.77 | 0.01 | |
| PDHA1 | pyruvate dehydrogenase E1 subunit alpha 1 | 0.79 | 0.01 | |
| DLD | dihydrolipoamide dehydrogenase | 0.80 | 0.03 | |
| Cytoskeletal organization | COL1A1 | collagen type I alpha 1 chain | 1.43 | 0.02 |
| COL6A1 | collagen type VI alpha 1 chain | 1.33 | 0.02 | |
| COL6A2 | collagen type VI alpha 2 chain | 1.35 | 0.04 | |
| ITGA11 | integrin subunit alpha 11 | 1.59 | 0.01 |
| Treatment | ||||
|---|---|---|---|---|
| Items 2 | CNT | NR | SEM | p-Value |
| Myofiber diameter at 300 d, µm | ||||
| Type I | 37.5 | 41.1 | 3.0 | 0.32 |
| Type ⅡA | 49.9 | 54.3 | 5.5 | 0.48 |
| Type ⅡX | 64.7 | 64.5 | 4.3 | 0.95 |
| Adipocyte size at 300 d, μm2 | ||||
| Cross sectional area | 2365.0 | 1947.4 | 306.3 | 0.35 |
| 75 d | 180 d | 300 d | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | CNT | NR | SEM | CNT | NR | SEM | CNT | NR | SEM | Diet | Time | Diet × Time |
| Fiber type, % | ||||||||||||
| Type I | 24.7 | 23.8 | 2.2 | 23.3 | 21.2 | 1.6 | 21.6 y | 29.0 x | 2.7 | 0.72 | 0.38 | 0.01 |
| Type IIA | 26.0 | 26.7 | 1.2 | 24.8 | 27.9 | 2.1 | 30.2 a | 24.1 b | 1.3 | 0.77 | 0.05 | <0.01 |
| Type IIX | 49.2 | 49.5 | 2.9 | 51.9 | 50.9 | 1.9 | 48.2 | 46.9 | 1.8 | 0.92 | 0.27 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishino, D.; Haginouchi, T.; Shimogiri, T.; Muroya, S.; Kawabata, K.; Urasoko, S.; Oshima, I.; Yasuo, S.; Gotoh, T. A Pilot Study: Maternal Undernutrition Programs Energy Metabolism and Alters Metabolic Profile and Morphological Characteristics of Skeletal Muscle in Postnatal Beef Cattle. Metabolites 2025, 15, 209. https://doi.org/10.3390/metabo15030209
Nishino D, Haginouchi T, Shimogiri T, Muroya S, Kawabata K, Urasoko S, Oshima I, Yasuo S, Gotoh T. A Pilot Study: Maternal Undernutrition Programs Energy Metabolism and Alters Metabolic Profile and Morphological Characteristics of Skeletal Muscle in Postnatal Beef Cattle. Metabolites. 2025; 15(3):209. https://doi.org/10.3390/metabo15030209
Chicago/Turabian StyleNishino, Daichi, Taketo Haginouchi, Takeshi Shimogiri, Susumu Muroya, Kenji Kawabata, Saki Urasoko, Ichiro Oshima, Shinobu Yasuo, and Takafumi Gotoh. 2025. "A Pilot Study: Maternal Undernutrition Programs Energy Metabolism and Alters Metabolic Profile and Morphological Characteristics of Skeletal Muscle in Postnatal Beef Cattle" Metabolites 15, no. 3: 209. https://doi.org/10.3390/metabo15030209
APA StyleNishino, D., Haginouchi, T., Shimogiri, T., Muroya, S., Kawabata, K., Urasoko, S., Oshima, I., Yasuo, S., & Gotoh, T. (2025). A Pilot Study: Maternal Undernutrition Programs Energy Metabolism and Alters Metabolic Profile and Morphological Characteristics of Skeletal Muscle in Postnatal Beef Cattle. Metabolites, 15(3), 209. https://doi.org/10.3390/metabo15030209