Metal Ion Reduction, Chelation, and Cytotoxicity of Selected Bicyclic Monoterpenes and Their Binary Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Ferric Reducing Antioxidant Power—FRAP Method
2.2.2. FRAP—Synergism Studies
2.2.3. Fe(II) Chelation Assay—Ferrozine Based Study
2.2.4. Fe(II) Chelation Assay—Synergism Studies
2.2.5. Cu(II) Reduction—CUPRAC Assay
2.2.6. CUPRAC—Synergism Assay
2.2.7. Cytotoxicity Evaluation
2.2.8. Statistical Analysis
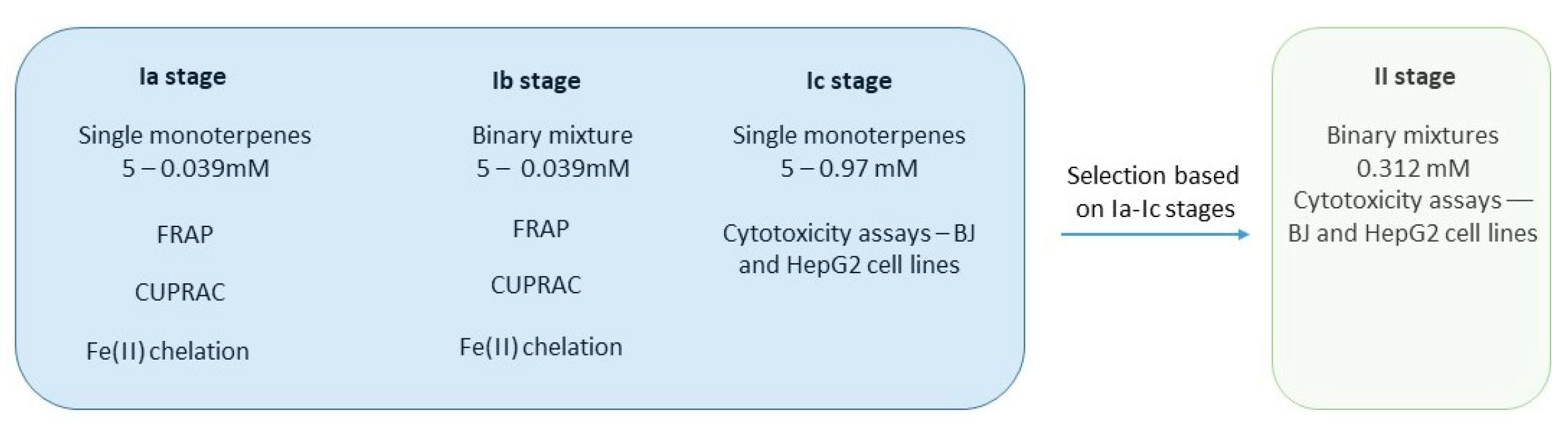
3. Results
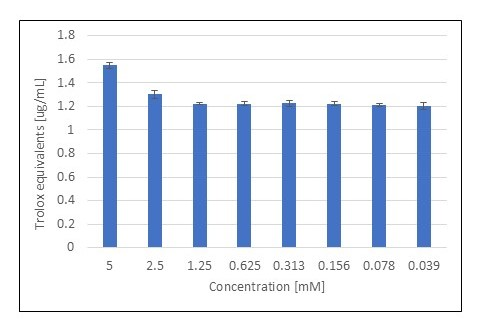
3.1. FRAP Assay
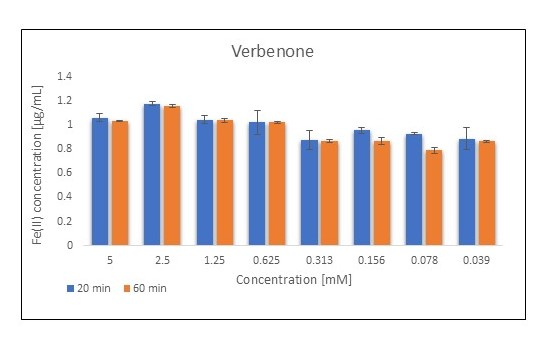
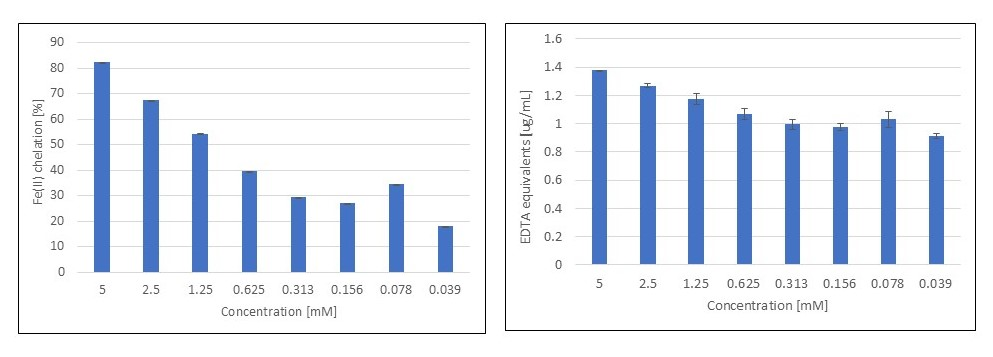
3.2. Fe(II) Chelation Assay
3.3. CUPRAC Assay
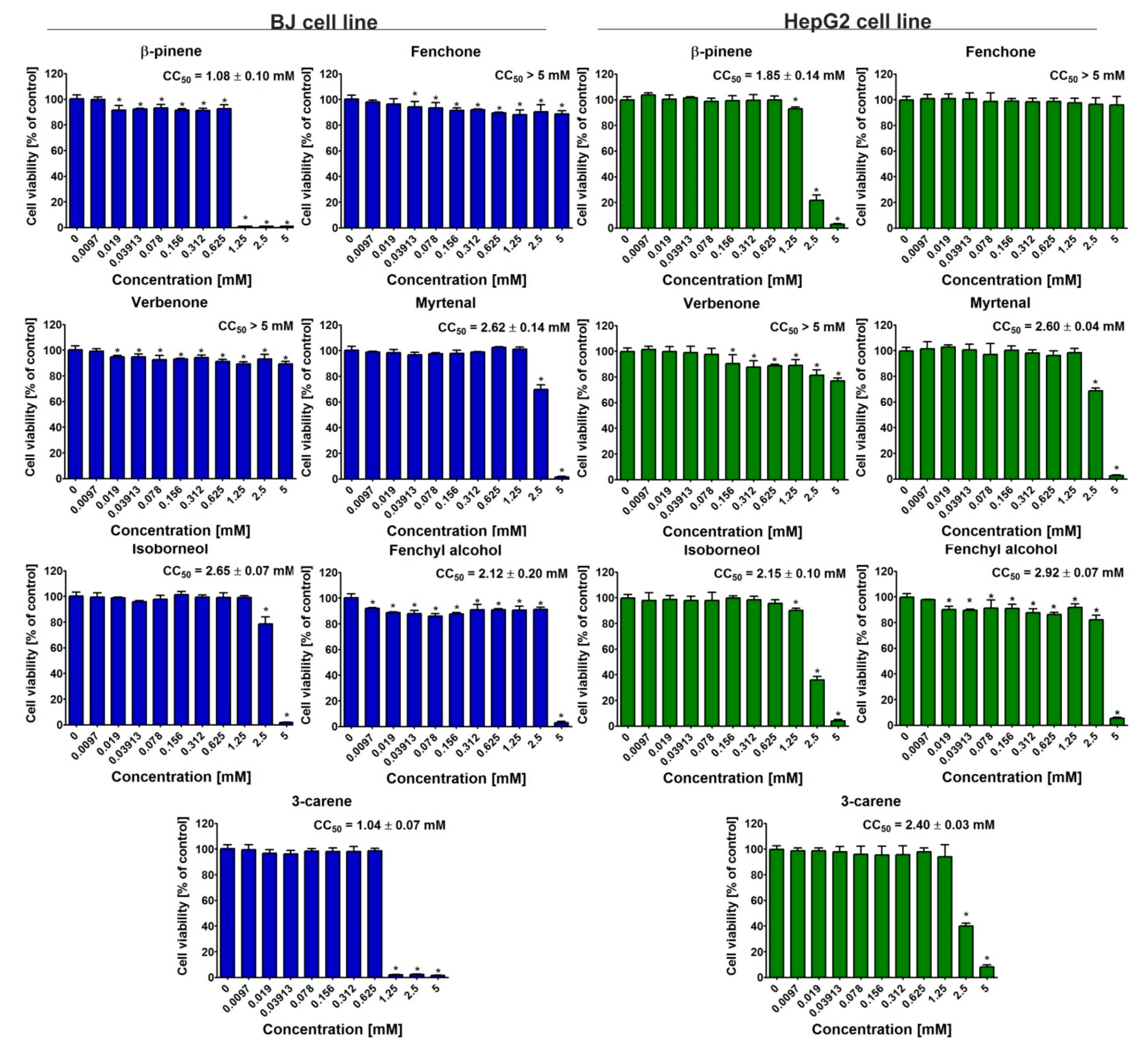
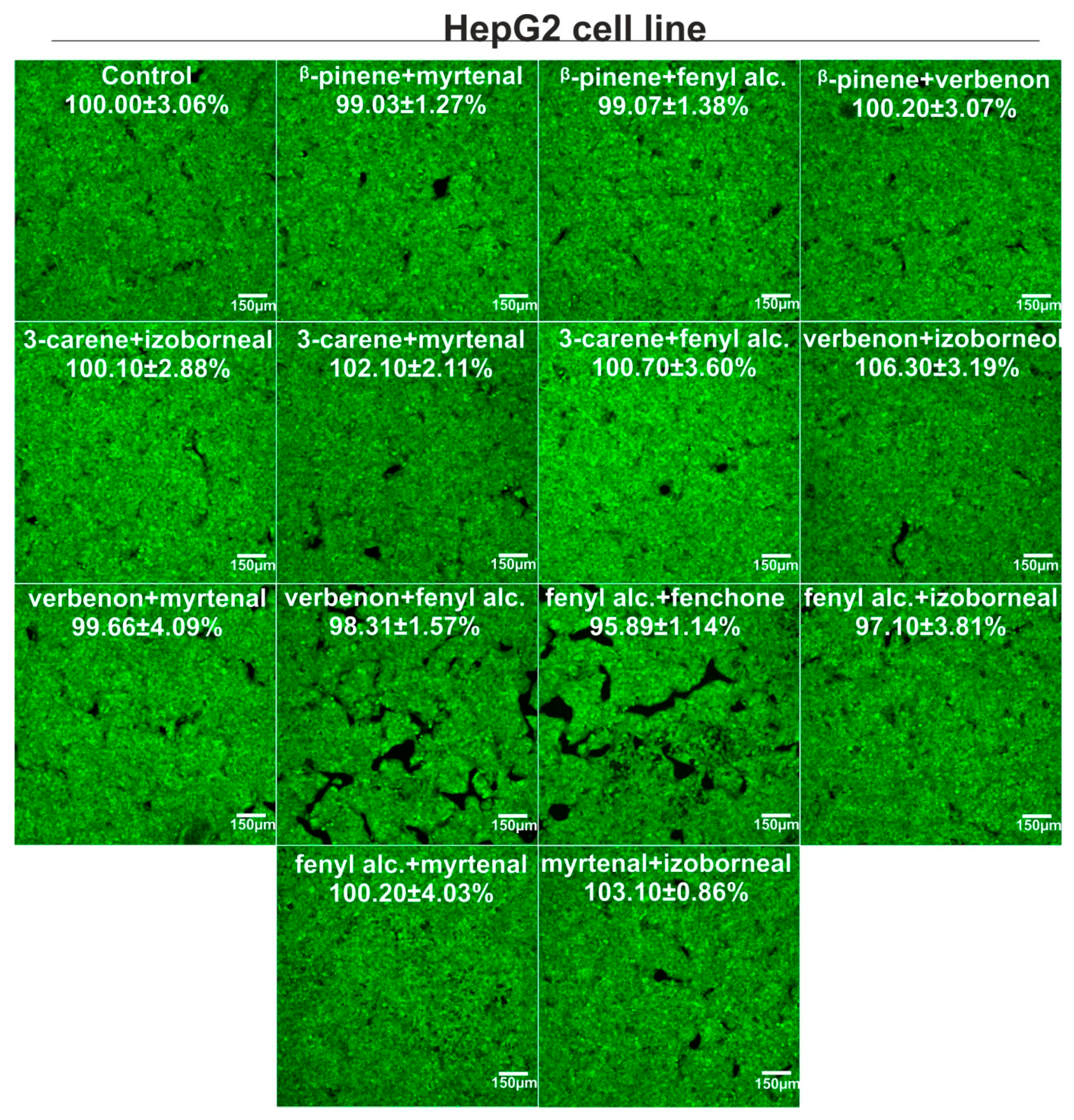
3.4. Cytotoxicity Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.Y.; Trizna, E.Y.; Sulaiman, R.K.; Pavelyev, R.S.; Gilfanov, I.R.; Lisovskaya, S.A.; Ostolopovskaya, O.V.; Frolova, L.L.; Kutchin, A.V.; Guseva, G.B.; et al. Increasing the Efficacy of Treatment of Staphylococcus aureus–Candida albicans Mixed Infections with Myrtenol. Antibiotics 2022, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.I.M.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer activity of monoterpenes: A systematic review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Boopathy, L.K.; Roy, A.; Gopal, T.; Kandy, R.R.K.; Arumugam, M.K. Potential molecular mechanisms of myrtenal against colon cancer: A systematic review. J. Biochem. Mol. Toxicol. 2024, 38, e23525. [Google Scholar] [CrossRef]
- Somade, O.T. Camphor Toxicity: A Review of Recent Findings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 775–790. [Google Scholar] [CrossRef]
- Ciesla, L.M.; Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Waksmundzka-Hajnos, M. Antioxidant synergism and antagonism between selected monoterpenes using the 2,2-diphenyl-1-picrylhydrazyl method. Flavour Fragr. J. 2016, 31, 412–419. [Google Scholar] [CrossRef]
- Charlebois, E.; Pantopoulos, K. Nutritional Aspects of Iron in Health and Disease. Nutrients 2023, 15, 2441. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, X.; Li, J.; Xie, Z.; Wu, Q.; Chen, J.; Wang, Y.; Chen, Z.; Cao, X.; Li, T.; et al. Insights Into the Role of Copper in Neurodegenerative Diseases and the Therapeutic Potential of Natural Compounds. Curr. Neuropharmacol. 2024, 22, 1650–1671. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Approach to Optimization of FRAP Methodology for Studies Based on Selected Monoterpenes. Molecules 2020, 25, 5267. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Wiśniewska, R. Interactions of Selected Monoterpenes with Iron and Copper Ions Based on Ferrozine and CUPRAC Methods—The Preliminary Studies. Chem. Biodivers. 2022, 19, e202200461. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Strubińska, J.; Czernel, G.; Ginalska, G.; Gagoś, M. In vitro evaluation of antifungal and cytotoxic activities as also the therapeutic safety of the oxidized form of amphotericin B. Chem. Biol. Interact. 2016, 256, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Cetin, S.; Knez, D.; Gobec, S.; Kos, J.; Pišlar, A. Cell models for Alzheimer’s and Parkinson’s disease: At the interface of biology and drug discovery. Biomed. Pharmacother. 2022, 149, 112924. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, M.; Kumar, A.; Yadav, M. Ameliorative effect of β-pinene targeting mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, e076020. [Google Scholar] [CrossRef]
- Woo, J.; Yang, H.; Yoon, M.; Gadhe, C.G.; Pae, A.N.; Cho, S.; Lee, C.J. 3-Carene, a Phytoncide from Pine Tree Has a Sleep-enhancing Effect by Targeting the GABAA-benzodiazepine Receptors. Exp. Neurobiol. 2019, 28, 593–601. [Google Scholar] [CrossRef]
- Anchimowicz, J.; Zielonka, P.; Jakiela, S. Plant Secondary Metabolites as Modulators of Mitochondrial Health: An Overview of Their Anti-Oxidant, Anti-Apoptotic, and Mitophagic Mechanisms. Int. J. Mol. Sci. 2025, 26, 380. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Mlicka, A.; Zielińska, W.; Mikołajczyk, K.; Hałas-Wiśniewska, M.; Izdebska, M.; Grzanka, A. Synergistic effect of oxymatrine and 5-fluorouracil on the migratory potential in A549 non-small cell lung cancer cells. Med. Res. J. 2022, 7, 293–300. [Google Scholar] [CrossRef]
- Gao, J.; Yin, Z.; Wu, Z.; Sheng, Z.; Ma, C.; Chen, R.; Zhang, X.; Tang, K.; Fei, J.; Cao, Z. Probing Synergistic Targets by Natural Compounds for Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2021, 9, 715762. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Bouchmaa, N.; Ouedrhiri, W.; Ennoury, A.; ZouaouI, Z.; Kabach, I.; Nhiri, M.; El Fatimy, R. Synergistic antioxidant effects of natural compounds on H2O2-induced cytotoxicity of human monocytes. Front. Pharmacol. 2022, 13, 830323. [Google Scholar] [CrossRef]



| Monoterpene | Concentration (mM) | Trolox Equivalents [µg/mL] | Fe(II) Concentration [µg/mL] | ||
|---|---|---|---|---|---|
| 20 min | 60 min | 20 min | 60 min | ||
| β-Pinene | 5 | 2.487 ± 0.016 | 1.507 ± 0.464 | 1.868 ± 0.012 | 1.137 ± 0.350 |
| 0.312 | 1.585 ± 0.293 | 1.578 ± 0.324 | 1.196 ± 0.221 | 1.190 ± 0.244 | |
| Fenchone | 5 | 1.266 ± 0.073 | 1.243 ± 0.060 | 0.957 ± 0.055 | 0.940 ± 0.048 |
| 0.312 | 1.039 ± 0.021 | 1.048 ± 0.020 | 0.788 ± 0.016 | 0.795 ± 0.016 | |
| Verbenone | 5 | 1.511 ± 0.051 | 1.472 ± 0.004 | 1.140 ± 0.038 | 1.112 ± 0.003 |
| 0.312 | 1.249 ± 0.101 | 1.233 ± 0.015 | 0.945 ± 0.077 | 0.933 ± 0.011 | |
| Myrtenal | 5 | 1.360 ± 0.009 | 1.336 ± 0.010 | 1.028 ± 0.007 | 1.009 ± 0.008 |
| 0.312 | 1.223 ± 0.044 | 1.210 ± 0.058 | 0.925 ± 0.033 | 0.916 ± 0.044 | |
| Isoborneol | 5 | 1.236 ± 0.066 | 1.227 ± 0.084 | 0.935 ± 0.050 | 0.929 ± 0.063 |
| 0.312 | 1.193 ± 0.109 | 1.165 ± 0.085 | 0.903 ± 0.082 | 0.882 ± 0.064 | |
| Fenchyl alcohol | 5 | 1.274 ± 0.034 | 1.257 ± 0.050 | 0.964 ± 0.026 | 0.951 ± 0.038 |
| 0.312 | 1.357 ± 0.013 | 1.319 ± 0.012 | 1.025 ± 0.010 | 0.997 ± 0.009 | |
| 3-Carene | 5 | 1.926 ± 0.036 | 1.525 ± 0.131 | 1.450 ± 0.027 | 1.151 ± 0.099 |
| 0.312 | 0.954 ± 0.039 | 0.894 ± 0.037 | 0.725 ± 0.030 | 0.680 ± 0.028 | |
| β-pinene | fenchone | 3-carene | verbenone | myrtenal | isoborneol | fenchyl alcohol | |
|---|---|---|---|---|---|---|---|
| β-pinene | x | 1.075 ± 0.038 | 1.072 ± 0.057 | 1.048 ± 0.048 | 1.070 ± 0.064 | 1.085 ± 0.082 | 1.050 ± 0.104 |
| 1.124 ± 0.032 | 1.059 ± 0.051 | 1.032 ± 0.036 | 1.059 ± 0.073 | 1.039 ± 0.045 | 1.055 ± 0.106 | ||
| fenchone | 1.457 ± 0.051 | x | 0.954 ± 0.048 | 1.075 ± 0.122 | 0.917 ± 0.019 | 0.966 ± 0.039 | 0.971 ± 0.032 |
| 1.522 ± 0.043 | 0.933 ± 0.049 | 1.059 ± 0.122 | 0.906 ± 0.022 | 0.941 ± 0.042 | 0.951 ± 0.019 | ||
| 3-carene | 1.453 ± 0.078 | 1.294 ± 0.065 | x | 0.728 ± 0.012 | 0.896 ± 0.084 | 0.902 ± 0.033 | 0.995 ± 0.106 |
| 1.435 ± 0.069 | 1.266 ± 0.066 | 0.723 ± 0.010 | 0.879 ± 0.090 | 0.878 ± 0.022 | 0.968 ± 0.094 | ||
| verbenone | 1.420 ± 0.065 | 1.457 ± 0.166 | 0.991 ± 0.017 | x | 0.832 ± 0.039 | 0.792 ± 0.069 | 1.031 ± 0.059 |
| 1.398 ± 0.049 | 1.435 ± 0.166 | 0.985 ± 0.013 | 0.802 ± 0.036 | 0.800 ± 0.064 | 1.013 ± 0.072 | ||
| myrtenal | 1.450 ± 0.087 | 1.244 ± 0.026 | 1.216 ± 0.114 | 1.130 ± 0.053 | x | 0.887 ± 0.037 | 0.917 ± 0.023 |
| 1.435 ± 0.099 | 1.230 ± 0.030 | 1.195 ± 0.122 | 1.091 ± 0.049 | 0.865 ± 0.038 | 0.872 ± 0.014 | ||
| isoborneol | 1.470 ± 0.112 | 1.310 ± 0.053 | 1.224 ± 0.045 | 1.076 ± 0.094 | 1.205 ± 0.051 | x | 0.971 ± 0.058 |
| 1.408 ± 0.062 | 1.277 ± 0.056 | 1.192 ± 0.030 | 1.088 ± 0.087 | 1.175 ± 0.052 | 0.937 ± 0.052 | ||
| fenchyl alcohol | 1.423 ± 0.141 | 1.317 ± 0.043 | 1.348 ± 0.143 | 1.397 ± 0.081 | 1.244 ± 0.031 | 1.317 ± 0.079 | x |
| 1.430 ± 0.143 | 1.290 ± 0.026 | 1.313 ± 0.128 | 1.373 ± 0.097 | 1.185 ± 0.019 | 1.272 ± 0.070 |
| Monoterpene | Concentration [mM] | EDTA Equivalents [µg/mL] |
|---|---|---|
| β-Pinene | 5 | 1.376 ± 0.002 |
| 0.312 | 0.995 ± 0.031 | |
| Fenchone | 5 | 1.076 ± 0.022 |
| 0.312 | 0.912 ± 0.126 | |
| Verbenone | 5 | 1.247 ± 0.037 |
| 0.312 | 0.970 ± 0.011 | |
| Myrtenal | 5 | 1.292 ± 0.023 |
| 0.312 | 0.987 ± 0.044 | |
| Isoborneol | 5 | 0.911 ± 0.004 |
| 0.312 | 0.832 ± 0.020 | |
| Fenchyl alcohol | 5 | 1.011 ± 0.005 |
| 0.312 | 0.994 ± 0.018 | |
| 3-Carene | 5 | 1.361 ± 0.014 |
| 0.312 | 1.341 ± 0.032 |
| Monoterpene | Concentration [mM] | EDTA Equivalents [µg/mL] |
|---|---|---|
| β-pinene | 5 | 1.877 ± 0.075 |
| 0.312 | 0.611 ± 0.015 | |
| Fenchone | 5 | 0.809 ± 0.017 |
| 0.312 | 0.796 ± 0.006 | |
| Verbenone | 5 | 1.265 ± 0.026 |
| 0.312 | 0.524 ± 0.006 | |
| Myrtenal | 5 | 0.741 ± 0.019 |
| 0.312 | 0.698 ± 0.020 | |
| Isoborneol | 5 | 0.340 ± 0.006 |
| 0.312 | 0.630 ± 0.022 | |
| Fenchyl alcohol | 5 | 0.722 ± 0.014 |
| 0.312 | 0.864 ± 0.014 | |
| 3-Carene | 5 | 3.062 ± 0.085 |
| 0.312 | 0.654 ± 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtunik-Kulesza, K.; Dubiel, M.; Klimek, K. Metal Ion Reduction, Chelation, and Cytotoxicity of Selected Bicyclic Monoterpenes and Their Binary Mixtures. Metabolites 2025, 15, 199. https://doi.org/10.3390/metabo15030199
Wojtunik-Kulesza K, Dubiel M, Klimek K. Metal Ion Reduction, Chelation, and Cytotoxicity of Selected Bicyclic Monoterpenes and Their Binary Mixtures. Metabolites. 2025; 15(3):199. https://doi.org/10.3390/metabo15030199
Chicago/Turabian StyleWojtunik-Kulesza, Karolina, Marcela Dubiel, and Katarzyna Klimek. 2025. "Metal Ion Reduction, Chelation, and Cytotoxicity of Selected Bicyclic Monoterpenes and Their Binary Mixtures" Metabolites 15, no. 3: 199. https://doi.org/10.3390/metabo15030199
APA StyleWojtunik-Kulesza, K., Dubiel, M., & Klimek, K. (2025). Metal Ion Reduction, Chelation, and Cytotoxicity of Selected Bicyclic Monoterpenes and Their Binary Mixtures. Metabolites, 15(3), 199. https://doi.org/10.3390/metabo15030199








