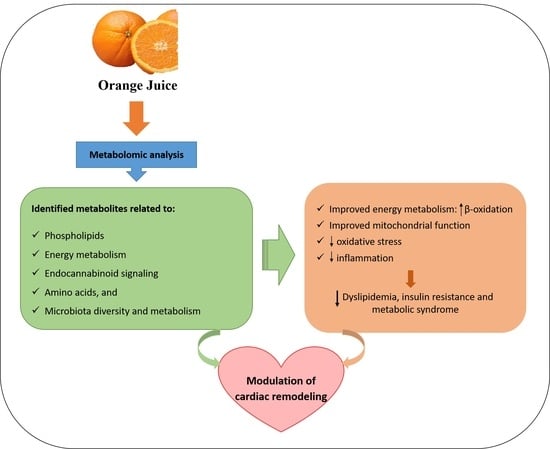
The Therapeutic Potential of Orange Juice in Cardiac Remodeling: A Metabolomics Approach
Abstract
1. Introduction
2. Orange Juice Consumption and the Metabolomics Approach
3. Metabolic Pathways Modulated by Orange Juice Intake and Potential Attenuation of Cardiac Remodeling
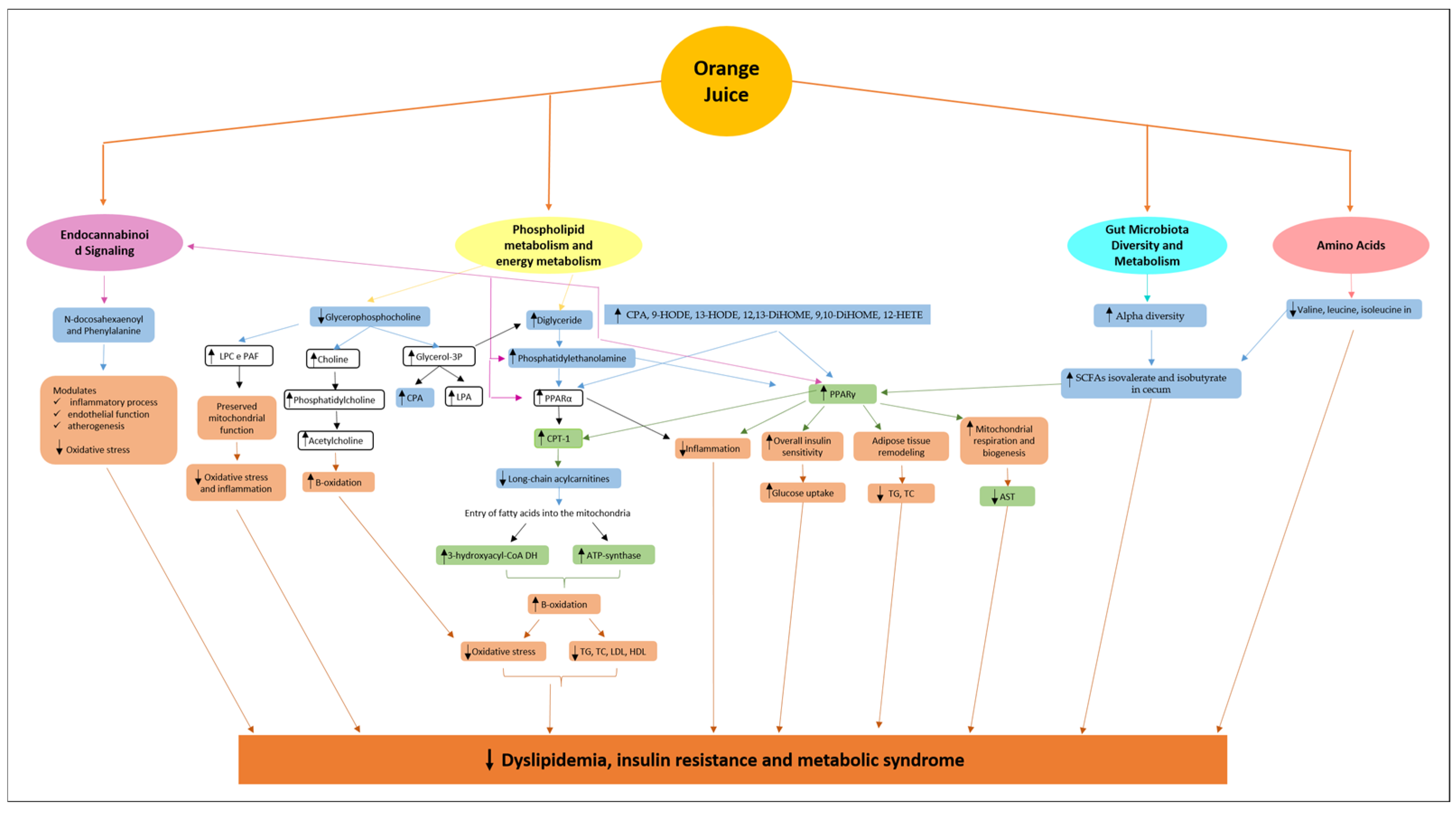
3.1. Phospholipids and Alterations in Energy Metabolism
3.2. Endocannabinoid Signaling
3.3. Modulation of Gut Microbiota Diversity and Metabolism
3.4. Amino Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration; 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling–concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Maliken, B.D.; Jones, S.M.; Ivey, M.J.; Wu, Z.; Wang, Y.; Kanisicak, O. Cardiac Remodeling and Repair: Recent Approaches; Advancements; and Future Perspective. Int. J. Mol. Sci. 2021, 22, 13104. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012, 95, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.C.; Santos, P.P.; Figueiredo, A.M.; Rafacho, B.P.M.; Ishikawa, L.; Zanati, S.G.; Fernandes, A.A.H.; Azevedo, P.S.; Polegato, B.F.; Zornoff, L.A.M.; et al. Influence of Consumption of Orange Juice (Citrus sinensis) on Cardiac Remodeling of Rats Submitted to Myocardial Infarction. Arq. Bras. Cardiol. 2021, 116, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Pla-Pagà, L.; Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Martín-Luján, F.; Moragas, A.; Canela, N.; Puiggròs, F.; et al. Effects of Hesperidin Consumption on the Cardiovascular System in Pre- and Stage 1 Hypertensive Subjects: Targeted and Non-Targeted Metabolomic Approaches (CITRUS Study). Mol. Nutr. Food Res. 2021, 65, e2001175. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.D.; Pereira, A.G.; Todo, M.C.; Fujimori, A.S.S.; Dos Santos, P.P.; Dantas, D.; Fernandes, A.A.; Zanati, S.G.; Hassimotto, N.M.A.; Zornoff, L.A.M.; et al. Pera Orange (Citrus sinensis) and Moro Orange (Citrus sinensis (L.) Osbeck) Juices Attenuate Left Ventricular Dysfunction and Oxidative Stress and Improve Myocardial Energy Metabolism in Acute Doxorubicin-Induced Cardiotoxicity in Rats. Nutrition 2021, 91–92, 111350. [Google Scholar] [CrossRef]
- Cabral, R.P.; Ribeiro, A.P.D.; Monte, M.G.; Fujimori, A.S.S.; Tonon, C.R.; Ferreira, N.F.; Zanatti, S.G.; Minicucci, M.F.; Zornoff, L.A.M.; Paiva, S.A.R.; et al. Pera Orange Juice (Citrus sinensis L. Osbeck) Alters Lipid Metabolism and Attenuates Oxidative Stress in the Heart and Liver of Rats Treated with Doxorubicin. Heliyon 2024, 10, e36834. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.Q.; Dourado, G.K.; Cesar, T.B. Red-Fleshed Sweet Orange Juice Improves the Risk Factors for Metabolic Syndrome. Int. J. Food Sci. Nutr. 2015, 66, 830–836. [Google Scholar] [CrossRef]
- Brasili, E.; Hassimotto, N.M.A.; Del Chierico, F.; Marini, F.; Quagliariello, A.; Sciubba, F.; Miccheli, A.; Putignani, L.; Lajolo, F. Daily Consumption of Orange Juice from Citrus sinensis L. Osbeck cv. Cara Cara and cv. Bahia Differently Affects Gut Microbiota Profiling as Unveiled by an Integrated Meta-Omics Approach. J. Agric. Food Chem. 2019, 67, 1381–1391. [Google Scholar] [CrossRef]
- Santos, K.G.D.; Yoshinaga, M.Y.; Glezer, I.; Chaves-Filho, A.B.; Santana, A.A.; Kovacs, C.; Magnoni, C.D.; Lajolo, F.M.; Miyamoto, S.; Aymoto Hassimotto, N.M. Orange Juice Intake by Obese and Insulin-Resistant Subjects Lowers Specific Plasma Triglycerides: A Randomized Clinical Trial. Clin. Nutr. ESPEN 2022, 51, 336–344. [Google Scholar] [CrossRef]
- Tadros, F.J.; Andrade, J.M. Impact of Hesperidin in 100% Orange Juice on Chronic Disease Biomarkers: A Narrative Systematic Review and Gap Analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 8335–8354. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, N.; Ji, K.; He, Y.; Li, H.; Liu, X. Does Chronic Consumption of Orange Juice Improve Cardiovascular Risk Factors in Overweight and Obese Adults? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2022, 13, 11945–11953. [Google Scholar] [CrossRef]
- Pineda-Lozano, J.E.; Fonseca-Bustos, V.; Martinez-Moreno, A.G.; Virgen-Carrillo, C.A. The Biological Effect of Orange (Citrus sinensis L.) By-Products on Metabolic Biomarkers: A Systematic Review. Front. Sustain. Food Syst. 2022, 6, 1003144. [Google Scholar] [CrossRef]
- Capetini, V.C.; Quintanilha, B.J.; de Oliveira, D.C.; Nishioka, A.H.; de Matos, L.A.; Ferreira, L.R.P.; Ferreira, F.M.; Sampaio, G.R.; Hassimotto, N.M.A.; Lajolo, F.M.; et al. Blood orange juice intake modulates plasma and PBMC microRNA expression in overweight and insulin-resistant women: Impact on MAPK and NFκB signaling pathways. J. Nutr. Biochem. 2023, 112, 109240. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, Y.; Xu, L.; Liao, X.; Xu, Z. Health outcomes of 100% orange juice and orange flavored beverage: A comparative analysis of gut microbiota and metabolomics in rats. Curr. Res. Food Sci. 2023, 6, 100454. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Perez-de-la-Cruz, A.; Vallejo, F.; Tomas-Barberan, F.; Gil, A.; Mesa, M.D. A serum metabolomics-driven approach predicts orange juice consumption and its impact on oxidative stress and inflammation in subjects from the BIONAOS study. Mol. Nutr. Food Res. 2017, 61, 1600120. [Google Scholar] [CrossRef]
- Moreira, V.; Brasili, E.; Fiamoncini, J.; Marini, F.; Miccheli, A.; Daniel, H.; Lee, J.J.H.; Hassimotto, N.M.A.; Lajolo, F.M. Orange juice affects acylcarnitine metabolism in healthy volunteers as revealed by a mass-spectrometry based metabolomics approach. Food Res. Int. 2018, 107, 346–352. [Google Scholar] [CrossRef]
- Fujimori, A.S.S.; Ribeiro, A.P.D.; Pereira, A.G.; Dias-Audibert, F.L.; Tonon, C.R.; Dos Santos, P.P.; Dantas, D.; Zanati, S.G.; Catharino, R.R.; Zornoff, L.A.M.; et al. Effects of Pera Orange Juice and Moro Orange Juice in Healthy Rats: A Metabolomic Approach. Metabolites 2023, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, H.B.; Walker, J.M. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005, 144, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M.; Hoskins, A.A. Lehninger Principles of Biochemistry, 8th ed.; WH Freeman: New York, NY, USA, 2021; p. 1248. ISBN 978-1-319-38149-3. [Google Scholar]
- Ito, C.; Fujiwara, K.; Kajita, M.; Ju-Ichi, M.; Takemura, Y.; Suzuki, Y.; Furukawa, H. New coumarins from Citrus plants. Chem. Pharm. Bull. 1991, 39, 2509–2513. [Google Scholar] [CrossRef]
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2015–2016). Expert Opin. Ther. Pat. 2017, 27, 1201–1226. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.X.; Shi, C.Y.; Liu, X.; Ning, D.Y.; Jing, L.F.; Yang, H.; Liu, Y.Z. Citrate Accumulation-Related Gene Expression and/or Enzyme Activity Analysis Combined with Metabolomics Provide a Novel Insight for an Orange Mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Zuzarte, M.; Marques, C.; Salgueiro, L.; Girao, H. Protective Effects of Terpenes on the Cardiovascular System: Current Advances and Future Perspectives. Curr. Med. Chem. 2016, 23, 4559–4600. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Wang, G.; Yang, L.; Qiu, H.; Wu, H.; Du, M.; Chen, J.; Song, J.; Jia, X.; Feng, L. Crude terpene glycoside component from Radix paeoniae rubra protects against isoproterenol-induced myocardial ischemic injury via activation of the PI3K/AKT/mTOR signaling pathway. J. Ethnopharmacol. 2017, 206, 160–169. [Google Scholar] [CrossRef]
- Yahara, S.; Yamashita, T.; Nozawa, N.; Nohara, T. Steroidal glycosides from Solanum torvum. Phytochemistry 1996, 43, 1069–1074. [Google Scholar] [CrossRef]
- Agrawal, A.D.; Bajpei, P.S.; Patil, A.A.; Bavaskar, S.R. Solanum torvum Sw.—A phytopharmacological review. Der Pharm. Lett. 2010, 2, 403–407. [Google Scholar]
- Ellinger, P.; Kader, M.M. Nicotinamide metabolism in mammals. Biochem. J. 1949, 44, 77–87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roussos, P.A.; Efstathios, N.; Intidhar, B.; Denaxa, N.K.; Tsafouros, A. Plum (Prunus domestica L. and P. salicina Lindl.). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 639–666. [Google Scholar] [CrossRef]
- Chellappa, K.; McReynolds, M.R.; Lu, W.; Zeng, X.; Makarov, M.; Hayat, F.; Mukherjee, S.; Bhat, Y.R.; Lingala, S.R.; Shima, R.T.; et al. NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 2022, 34, 1947–1959.e5. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, S.; Li, K.; Yu, H. Microbial Degradation of Nicotinamide by a Strain Alcaligenes sp. P156. Sci. Rep. 2019, 9, 3647. [Google Scholar] [CrossRef]
- Nikiforov, A.; Kulikova, V.; Ziegler, M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.B. Niacin status, NAD distribution and ADP-ribose metabolism. Curr. Pharm. Des. 2009, 15, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Onuh, J.O.; Girgih, A.T.; Nwachukwu, I.; Ievari-Shariati, S.; Raj, P.; Netticadan, T.; Aluko, R.E.; Aliani, M. A metabolomics approach for investigating urinary and plasma changes in spontaneously hypertensive rats (SHR) fed with chicken skin protein hydrolysates diets. J. Funct. Foods 2016, 22, 20–33. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.; Tan, L.; Zhou, Q.; Wu, X. Abnormalities in lysine degradation are involved in early cardiomyocyte hypertrophy development in pressure-overloaded rats. BMC Cardiovasc. Disord. 2021, 21, 403. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bo, Y.; Wu, X.; Wang, Q.; Qin, F.; Zhao, L.; Xiong, Z. An intergated serum and urinary metabonomic research based on UPLC-MS and therapeutic effects of Gushudan on prednisolone-induced osteoporosis rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1027, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Yu, Q.; Tang, N.; Mei, C.; Zhang, H.; Wang, G.; Lu, J.; Chen, W. Characteristics of the Gut Microbiome and Serum Metabolome in Patients with Functional Constipation. Nutrients 2023, 15, 1779. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Muthyala, S.D.V.; Klemashevich, C.; Ufondu, A.U.; Menon, R.; Chen, Z.; Devaraj, S.; Jayaraman, A.; Sun, Y. Age-dependent remodeling of gut microbiome and host serum metabolome in mice. Aging 2021, 13, 6330–6345. [Google Scholar] [CrossRef]
- Fujiwara, Y. Cyclic phosphatidic acid—A unique bioactive phospholipid. Biochim. Biophys. Acta 2008, 1781, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Haniu, H.; Matsuda, Y. Cyclic phosphatidic acid inhibits alkyl-glycerophosphate-induced downregulation of histone deacetylase 2 expression and suppresses the inflammatory response in human coronary artery endothelial cells. Int. J. Med. Sci. 2014, 11, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, Y.; Xue, C.; Wang, J.; Wang, Y.; Xu, J.; Li, Z. Exogenous natural EPA-enriched phosphatidylcholine and phosphatidylethanolamine ameliorate lipid accumulation and insulin resistance via activation of PPARα/γ in mice. Food Funct. 2020, 11, 8248–8258. [Google Scholar] [CrossRef]
- Tsukahara, T. PPARγ networks in cell signaling: Update and impact of cyclic phosphatidic acid. J. Lipids 2013, 2013, 246597. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid-regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARγ in metabolism, immunity, and cancer: Unified and diverse mechanisms of action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines—Old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, B.; Alterås, E.K.; Lindquist, C.; Svardal, A.; Skorve, J.; Berge, R.K. Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutr. Metab. 2018, 15, 10. [Google Scholar] [CrossRef]
- Han, X.Q.; Zhang, L.Y.; Ding, L.; Shi, H.H.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Synergistic effect of sea cucumber saponins and EPA-enriched phospholipids on insulin resistance in high-fat diet-induced obese mice. Food Funct. 2019, 10, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, R.; Wang, S.; Yuan, C.; Yuan, Q.; Zhang, Y.; Ren, J.; He, Y.; Wu, X.; Dai, W.; et al. Selenium-enriched polysaccharides from Pyracantha fortuneana (SePFP) ameliorated hepatic lipid accumulation through enhancing mitochondrial fatty acid β-oxidation in aging mice. J. Oleo Sci. 2023, 72, 929–938. [Google Scholar] [CrossRef]
- Dávalos, A.; Goedeke, L.; Smibert, P.; Ramírez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, Y.; Hu, X.; Peng, X.; Wei, H.; Peng, J.; Jiang, S. Activation of PPARγ2 by PPARγ1 through a Functional PPRE in Transdifferentiation of Myoblasts to Adipocytes Induced by EPA. Cell Cycle 2015, 14, 1830–1841. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Ołdakowska, M.; Dobosz, T. Exploring PPAR Gamma and PPAR Alpha’s Regulation Role in Metabolism via Epigenetics Mechanism. Biomolecules 2024, 14, 1445. [Google Scholar] [CrossRef]
- Liss, K.H.; Finck, B.N. PPARs and Nonalcoholic Fatty Liver Disease. Biochimie 2017, 136, 65–74. [Google Scholar] [CrossRef] [PubMed]
- AlNafea, H.M.; Korish, A.A. Activation of the Peroxisome Proliferator-Activated Receptors (PPAR-α/γ) and the Fatty Acid Metabolizing Enzyme Protein CPT1A by Camel Milk Treatment Counteracts the High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease. PPAR Res. 2021, 2021, 5558731. [Google Scholar] [CrossRef] [PubMed]
- de Lima, L.P.; de Paula Barbosa, A. A Review of the Lipolytic Effects and the Reduction of Abdominal Fat from Bioactive Compounds and Moro Orange Extracts. Heliyon 2021, 7, e07695. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Chalasani, N. 5–Laboratory Tests in Liver Disease. In Practical Hepatic Pathology: A Diagnostic Approach; Saunders: Philadelphia, PA, USA, 2011; pp. 55–62. [Google Scholar] [CrossRef]
- Sun, X.; Alford, J.; Qiu, H. Structural and Functional Remodeling of Mitochondria in Cardiac Diseases. Int. J. Mol. Sci. 2021, 22, 4167. [Google Scholar] [CrossRef] [PubMed]
- Maulik, N.; Kagan, V.E.; Tyurin, V.A.; Das, D.K. Redistribution of phosphatidylethanolamine and phosphatidylserine precedes reperfusion-induced apoptosis. Am. J. Physiol. 1998, 274, H242–H248. [Google Scholar] [CrossRef]
- Post, J.A.; Bijvelt, J.J.; Verkleij, A.J. Phosphatidylethanolamine and sarcolemmal damage during ischemia or metabolic inhibition of heart myocytes. Am. J. Physiol. 1995, 268, H773–H780. [Google Scholar] [CrossRef] [PubMed]
- Tasseva, G.; Bai, H.D.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J.E. Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 2013, 288, 4158–4173. [Google Scholar] [CrossRef] [PubMed]
- Vianello, E.; Ambrogi, F.; Kalousová, M.; Badalyan, J.; Dozio, E.; Tacchini, L.; Schmitz, G.; Zima, T.; Tsongalis, G.J.; Corsi-Romanelli, M.M. Circulating perturbation of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) is associated to cardiac remodeling and NLRP3 inflammasome in cardiovascular patients with insulin resistance risk. Exp. Mol. Pathol. 2024, 137, 104895. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Tsukahara, R.; Haniu, H.; Matsuda, Y.; Murakami-Murofushi, K. Cyclic phosphatidic acid inhibits the secretion of vascular endothelial growth factor from diabetic human coronary artery endothelial cells through peroxisome proliferator-activated receptor gamma. Mol. Cell. Endocrinol. 2015, 412, 320–329. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.W.; Han, J.; Liang, H.; Wang, N.; Cheng, Y. 12/15-Lipoxygenase Metabolites of Arachidonic Acid Activate PPARγ: A Possible Neuroprotective Effect in Ischemic Brain. J. Lipid Res. 2015, 56, 502–514. [Google Scholar] [CrossRef]
- Shen, W.; Yang, L.; Yang, Y.; Wang, P.; Tao, X.; Shen, Y.; Wang, S.; Shen, Y. PRDX6 Promotes Fatty Acid Oxidation via PLA2-Dependent PPARα Activation in Rats Fed High-Fat Diet. Antioxid. Redox Signal 2023, 38, 1184–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, Y.; Guo, M.; Tang, L.; Xing, Y.; Zhou, J.; Guo, Y.; Gu, Y.; Wen, Q.; Gao, N.; et al. Q11, a CYP2E1 Inhibitor, Exerts Anti-Hepatocellular Carcinoma Effect by Inhibiting M2 Macrophage Polarization. Cancer Immunol. Immunother. CII 2024, 74, 35. [Google Scholar] [CrossRef]
- Thompson, D.A.; Hammock, B.D. Dihydroxyoctadecamonoenoate Esters Inhibit the Neutrophil Respiratory Burst. J. Biosci. 2007, 32, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic Acids: Novel Regulators of Macrophage Differentiation and Atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Porro, B.; Songia, P.; Squellerio, I.; Tremoli, E.; Cavalca, V. Analysis, Physiological and Clinical Significance of 12-HETE: A Neglected Platelet-Derived 12-Lipoxygenase Product. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 964, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Marchan, R.; Lesjak, M.S.; Stewart, J.D.; Winter, R.; Seeliger, J.; Hengstler, J.G. Choline-Releasing Glycerophosphodiesterase EDI3 Links the Tumor Metabolome to Signaling Network Activities. Cell Cycle 2012, 11, 4499–4506. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Okubo, T.; Sato, K.; Fujita, S.; Goto, K.; Hamaoka, T.; Iemitsu, M. Glycerophosphocholine Enhances Growth Hormone Secretion and Fat Oxidation in Young Adults. Nutrition 2012, 28, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Marathe, G.K.; Pandit, C.; Lakshmikanth, C.L.; Chaithra, V.H.; Jacob, S.P.; D’Souza, C.J. To Hydrolyze or Not to Hydrolyze: The Dilemma of Platelet-Activating Factor Acetylhydrolase. J. Lipid Res. 2014, 55, 1847–1854. [Google Scholar] [CrossRef]
- Syme, C.; Czajkowski, S.; Shin, J.; Abrahamowicz, M.; Leonard, G.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Strug, L.; et al. Glycerophosphocholine Metabolites and Cardiovascular Disease Risk Factors in Adolescents: A Cohort Study. Circulation 2016, 134, 1629–1636. [Google Scholar] [CrossRef]
- Scribner, D.M.; Witowski, N.E.; Mulier, K.E.; Lusczek, E.R.; Wasiluk, K.R.; Beilman, G.J. Liver Metabolomic Changes Identify Biochemical Pathways in Hemorrhagic Shock. J. Surg. Res. 2010, 164, e131–e139. [Google Scholar] [CrossRef] [PubMed]
- Strifler, G.; Tuboly, E.; Görbe, A.; Boros, M.; Pécz, D.; Hartmann, P. Targeting Mitochondrial Dysfunction with L-Alpha Glycerylphosphorylcholine. PLoS ONE 2016, 11, e0166682. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Luquin, N.; Navarro-Otano, J. The endocannabinoid system in cardiovascular function: Novel insights and clinical implications. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2018, 28, 35–52. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Umesh, S.V. Endocannabinoids and atherosclerosis: The future of therapeutic strategies—A review. Cardiol. Plus 2024, 9, 283–290. [Google Scholar] [CrossRef]
- COMPOUND: C00350. Retrograde Endocannabinoid Signaling—Reference Pathway: KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/pathway/map04723+C00350 (accessed on 9 September 2023).
- Leishman, E.; Bradshaw, H.B. N-acyl amides: Ubiquitous endogenous cannabimimetic lipids that are in the right place at the right time. In The Endocannabinoidome; Elsevier: Oxford, UK, 2015; pp. 33–48. [Google Scholar] [CrossRef]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Reviews. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; van den Munckhof, I.C.L.; Chen, L.; Bonder, M.J.; Schraa, K.; Rutten, J.H.W.; Riksen, N.P.; de Graaf, J.; Oosting, M.; Sanna, S.; et al. Gut Microbial Associations to Plasma Metabolites Linked to Cardiovascular Phenotypes and Risk. Circ. Res. 2019, 124, 1808–1820. [Google Scholar] [CrossRef]
- Gong, Y.; Lv, J.; Pang, X.; Zhang, S.; Zhang, G.; Liu, L.; Wang, Y.; Li, C. Advances in the Metabolic Mechanism and Functional Characteristics of Equol. Foods 2023, 12, 2334. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Gojda, J.; Cahova, M. Gut Microbiota as the Link between Elevated BCAA Serum Levels and Insulin Resistance. Biomolecules 2021, 11, 1414. [Google Scholar] [CrossRef]
- Yu, Y.; Yoon, S.O.; Poulogiannis, G.; Yang, Q.; Ma, X.M.; Villén, J.; Kubica, N.; Hoffman, G.R.; Cantley, L.C.; Gygi, S.P.; et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 2011, 332, 1322–1326. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Chen, Y.; Lian, G.; Wang, J.; Zhang, J.; Shan, K.; Shang, L.; Tian, F.; Jing, C. Role of Gut Microbiome and Microbial Metabolites in Alleviating Insulin Resistance After Bariatric Surgery. Obes. Surg. 2021, 31, 327–336. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Cho, K. Lipid metabolism, inflammation, and foam cell formation in health and metabolic disorders: Targeting mTORC1. J. Mol. Med. 2021, 99, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Ni, M.; Cao, H.; Signer, R.A.J.; Li, D.; Li, M.; Gu, Z.; Hu, Z.; Dickerson, K.E.; et al. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat. Cell Biol. 2017, 19, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Dong, J.; Zhao, H.; Li, H.; Guo, H.; Wang, S.; Zhang, C.; Wang, S.; Wang, M.; Yu, S.; et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS ONE 2014, 9, e99598. [Google Scholar] [CrossRef]
- Mirmiran, P.; Teymoori, F.; Asghari, G.; Azizi, F. Dietary Intakes of Branched Chain Amino Acids and the Incidence of Hypertension: A Population-Based Prospective Cohort Study. Arch. Iran. Med. 2019, 22, 182–188. [Google Scholar] [PubMed]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
| Metabolites | Biochemical Class → Function | Reference |
|---|---|---|
| Acyl-carnitines | Acyl group (derived from fatty acids or related compounds) + carnitine → fatty acid β-oxidation | Moreira et al. [19] |
| Hydroxyoctadecadienoic acid (9-HODE + 13-HODE) | Oxygenated metabolites of polyunsaturated fatty acids (linoleic acid) → PPAR ligands | Rangel-Huerta et al. [18] |
| Dihydroxyoctadecanoic acid (12,13-DiHOME and 9,10-DiHOME) | Oxygenated metabolites of polyunsaturated fatty acids (linoleic acid) → PPAR ligands | |
| 12-hydroxyeicosatetraenoic acid (12-HETE) | Oxygenated metabolites of polyunsaturated fatty acids (arachidonic acid) → PPAR ligands | |
| N-docosahexaenoyl-phenylalanine | N-acylamides (fatty acid—acyl group- attached to a simple amine) → participate in endocannabinoid signaling | Fujimori et al. [20] |
| Diglyceride (DG, 20:4/24:1) | Glycerolipids → synthesis pathways of the main phospholipids and triacylglycerols in eukaryotes | |
| Phosphatidylethanolamine (PE, O-20:0/16:0) | Glycerophospholipid → PPAR ligands and participate in endocannabinoid signaling | |
| Casegravol isovalerate | Coumarins → possibly derived from oranges | |
| Abscisic alcohol 11-glucoside | Terpene glycosides → possibly derived from oranges | |
| Torvoside C | Steroidal saponins → possibly derived from oranges | |
| N-formylmaleamic acid | metabolites of oral and intestinal microflora → precursor in NAD synthesis | |
| N2-acetyl-L-ornithine | Metabolites of oral and intestinal microflora → de novo ornithine biosynthesis pathway | |
| Cyclic phosphatidic acid (CPA, 18:2) | Glycerophospholipid → PPAR ligands | |
| Proline betaine | Direct marker of citrus fruit intake | Pla-Pagà et al. [6] |
| Glycerophosphocholine | Small phosphodiester → compound derived from phosphatidylcholine (phospholipid) metabolism | |
| Acetate, valine, isoleucine, leucine | Branched-chain amino acids | |
| N-acetyl glycoproteins | Novel biomarker of systemic inflammation and cardiovascular disease risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.P.d.; Fujimori, A.S.S.; Polegato, B.F.; Okoshi, M.P. The Therapeutic Potential of Orange Juice in Cardiac Remodeling: A Metabolomics Approach. Metabolites 2025, 15, 198. https://doi.org/10.3390/metabo15030198
Santos PPd, Fujimori ASS, Polegato BF, Okoshi MP. The Therapeutic Potential of Orange Juice in Cardiac Remodeling: A Metabolomics Approach. Metabolites. 2025; 15(3):198. https://doi.org/10.3390/metabo15030198
Chicago/Turabian StyleSantos, Priscila Portugal dos, Anderson Seiji Soares Fujimori, Bertha Furlan Polegato, and Marina Politi Okoshi. 2025. "The Therapeutic Potential of Orange Juice in Cardiac Remodeling: A Metabolomics Approach" Metabolites 15, no. 3: 198. https://doi.org/10.3390/metabo15030198
APA StyleSantos, P. P. d., Fujimori, A. S. S., Polegato, B. F., & Okoshi, M. P. (2025). The Therapeutic Potential of Orange Juice in Cardiac Remodeling: A Metabolomics Approach. Metabolites, 15(3), 198. https://doi.org/10.3390/metabo15030198