4. Discussion
This study investigated the effects of L-β-galactoglucan supplementation on growth performance, nutrient digestibility, palatability, hematology, serum biochemistry, fecal quality, and intestinal flora composition in beagle dogs. In recent decades, extensive research has explored the role of prebiotics in enhancing canine growth, immune function, and intestinal balance [
17,
18]. However, the specific impact of L-β-galactoglucan in dogs has not been previously documented, leaving a gap in the literature, which this study aimed to address. Our investigation focused on how L-β-galactoglucan influences palatability and overall canine health. The findings of this study demonstrate the positive effects of L-β-galactoglucan on both palatability and intestinal microbiota in dogs.
When introducing new components to pet food, it is crucial to ensure they do not negatively impact palatability. In this study, dogs in the Low_Gal (0.25%) group showed a significantly higher feed intake ratio as compared with the control (Con) group (
p < 0.05), indicating a positive impact on dietary acceptability in dogs. The Mid_Gal (0.5%) group showed an increasing trend in feed intake as compared with the High_Gal (1%) group, indicating a dose-dependent effect on palatability within the tested range. These findings align with previous research, which has shown that the addition of modest amounts of prebiotic fibers can enhance the palatability of pet food [
19,
20]. It should be emphasized that several factors, including the intrinsic properties of ingredients, food processing techniques, physical structure, and the interaction between aroma, texture, and taste, contribute to the overall palatability of pet food [
21]. Nevertheless, the two-day palatability assessment used in this study is limited, as it may not fully capture long-term dietary preferences or adaptation effects. Prolonged assessments, such as week-long trials, may prove to provide deeper insights into sustained dietary acceptance and better reflect real-world feeding scenarios. This limitation is recognized, and it is recommended that future studies incorporate longer evaluation periods to address this issue.
Fecal quality is often viewed by pet owners as a key indicator of gastrointestinal function and overall digestive health in dogs [
17]. In this experiment, L-β-galactoglucan did not appear to affect fecal characteristics or metabolite concentrations. Canine health was assessed through parameters such as fecal mass, body weight, and nutrient digestibility, which are critical for nutrition and metabolism. Our results indicate that the inclusion of L-β-galactoglucan in the diet did not significantly impact the digestibility of nutrients, which is consistent with previous studies showing that prebiotics had no effect on nutrient digestibility in healthy dogs [
18,
21,
22,
23,
24]. L-β-galactoglucan, as a prebiotic, is not digested in the small intestine; instead, it is mainly utilized by gut microbes. It makes sense that L-β-galactoglucan does not directly alter the apparent digestibility of other nutrients. However, a study reported a decrease in the apparent digestibility of dry matter, organic matter, and minerals in dogs supplemented with beta-glucan [
25]. Conversely, the addition of dehydrated yeast cultures increases the apparent nutrient digestibility of crude fiber and decreases the digestibility of crude protein and nitrogen-free extract in dogs (
p < 0.05) [
26]. The discrepancies between our data and data from previous studies are currently unknown. The age, sex, life stage, and diet composition, as well as the capability of animals to utilize L-β-galactoglucan, might be involved in and contribute to discrepancies, as shown in previous studies [
19,
25]. In this experiment, the dogs in all the groups supplemented with L-β-galactoglucan showed a statistically significant trend toward a reduction in body weight as compared with the control (Con) group. Further studies are required to uncover the potential mechanisms responsible for this phenomenon.
Serum biochemical profiles and immunoglobulin levels provide essential insights into an animal’s overall health and immunological status. LDL-C is an apolipoprotein that primarily transports cholesterol from the liver to the rest of the body. In this experiment, LDL-C levels in the Mid_Gal (0.5%) group showed a trend toward lower levels as compared with the control group, indicating a potential effect of L-β-galactoglucan on lipid metabolism. Previous research has shown that dogs administered 1% oat β-glucan exhibited lower serum total cholesterol concentrations as compared with the control group, along with decreased low-density lipoprotein and very-low-density lipoprotein concentrations (
p < 0.05) [
25]. Zhou et al. [
20] showed that Modified Highland Barley supplementation significantly reduced TC, TG, and LDL-C levels, while HDL-C levels showed an increasing trend in mice. A meta-analysis found that a daily intake of at least 3 g of oat beta-glucan reduced serum total cholesterol and LDL-C levels [
27]. Additionally, mannan oligosaccharides combined with 1,3 β-glucan significantly reduced plasma cholesterol (
p < 0.001) and LDL levels (
p < 0.05) in hens [
28]. These findings indicate a potential dose-dependent effect of L-β-galactoglucan on LDL-C levels. Alternatively, gut microorganisms may metabolize galactoglucan, enhancing gut microbial composition, which may affect hepatic lipid metabolism and decrease LDL-C synthesis. The trend toward lower triglyceride levels in the supplemented groups (
p = 0.06) further reinforces the role of L-β-galactoglucan in lipid regulation, likely through its prebiotic effects and subsequent impact on hepatic metabolism.
Regarding immune function, the dogs in our study showed no significant changes in serum immunoglobulin levels (IgA, IgM, and IgG) regardless of the supplementation level. A previous study showed that β-glucan supplementation enhanced immunoglobulin levels in mice [
29], dogs [
30], and calves [
31]. These findings indicate that L-β-galactoglucan is typically well-tolerated and does not adversely affect the immune system in dogs, although its effects on immunomodulation may vary by species or dosage.
The gut microbiota, which exists in symbiosis with the host, plays an important role in maintaining health [
32]. Gut microbiota diversity is typically assessed using alpha and beta diversity metrics. Alpha diversity quantifies species richness and evenness within individual samples. In this experiment, the Shannon index was significantly higher in the Mid_Gal (0.5%) group as compared with the control group (
p < 0.05), indicating an increase in species richness and a more complex and possibly healthier microbial ecosystem. Similarly, the Simpson index, which quantifies species evenness, was considerably lower in the Mid_Gal (0.5%) group. The decrease in the Simpson index suggests a more balanced microbial population, reducing the dominance of a few species and contributing to a stable and functional gut environment. Greater diversity and richness in the intestinal flora are typically associated with improved health outcomes [
33], although the specific mechanisms and conditions under which this occurs require further investigation. For instance, the addition of Artemisia ordosica crude polysaccharide reduced the Simpson estimator in rumen fluid as compared with the control group (
p < 0.05) [
34]. Likewise, Schutte et al. [
35] reported enhanced microbial diversity and positive metabolic effects after consuming high-fiber or whole-grain diets.
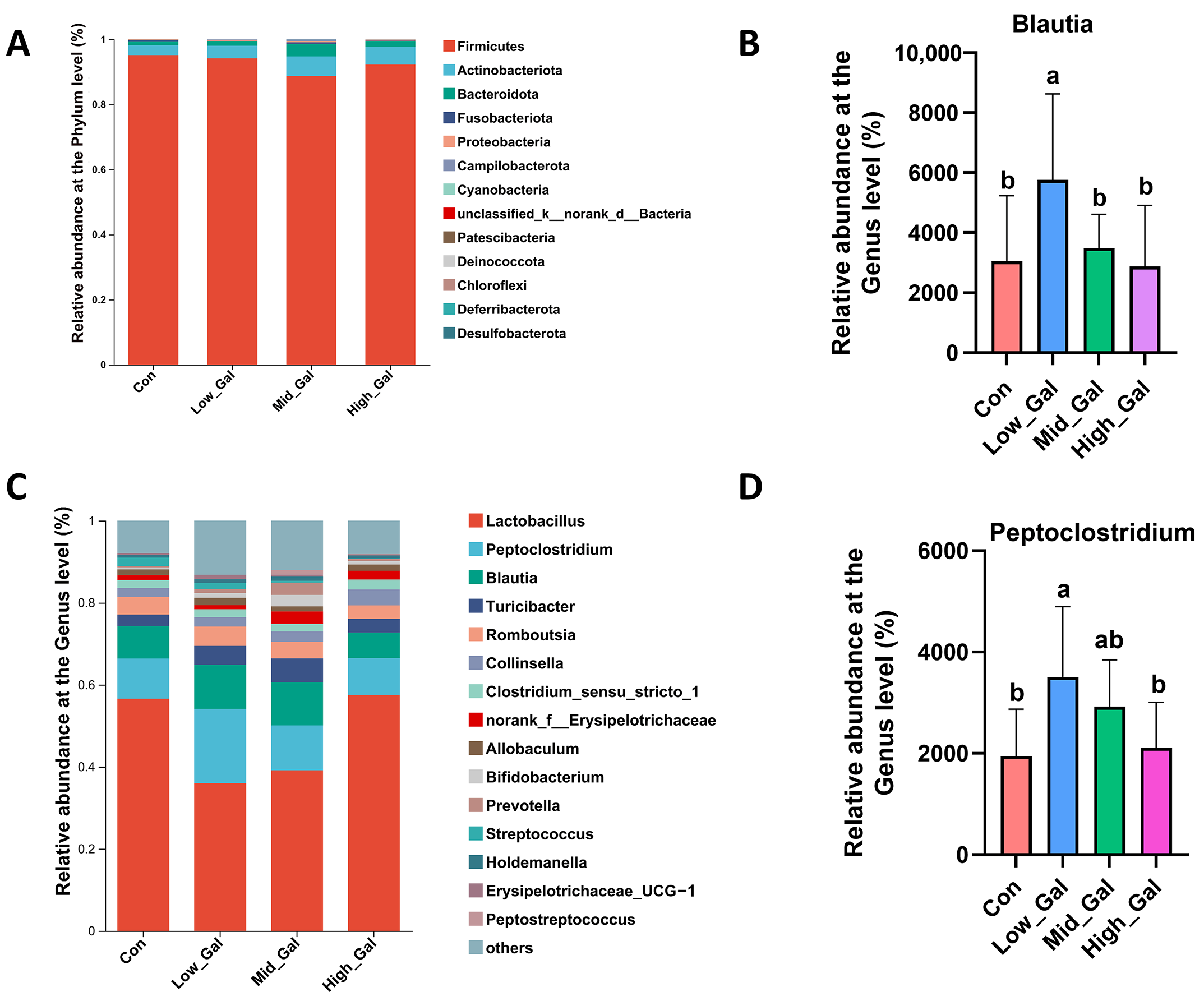
In healthy dogs, Firmicutes, Bacteroidetes, Proteobacteria, Fusobacteria, and Actinobacteria are recognized as the dominant phyla [
36]. Several
Blautia species have recently been shown to have beneficial effects on host function, such as suppressing
Vibrio cholera colonization [
37], improving inflammatory responses in a dextran sulfate sodium-induced colitis mouse model [
38], ameliorating obesity and type-2 diabetes [
39], and protecting against enteric viral infection [
40].
Peptoclostridium species, particularly those within the Clostridium genus, have been identified as beneficial bacteria with significant roles in gut health and homeostasis. Research indicates that these bacteria can help modulate immune responses, reduce inflammation, and promote the production of short-chain fatty acids like butyrate, which are crucial for intestinal health. Furthermore, some studies indicate their potential as probiotics due to their ability to restore gut microbiota balance, especially following antibiotic treatment or gastrointestinal disturbances [
41]. The results of this study also showed that L-β-galactoglucan did not alter the core composition of the normal flora of fecal microflora. However, at the genus level, the relative abundance of the beneficial bacterium
Blautia was significantly higher in the Low_Gal group (0.25%) as compared with the control group (
p < 0.05). Similarly, the relative abundance of the beneficial bacterium
Peptoclostridium was significantly higher in the Low_Gal group (0.25%) as compared with the control group (
p < 0.05). Previous studies have shown that β-glucan modulates microbiota composition, and supplementation with β-glucan derived from cereal or microbial sources increases the intestinal population of beneficial bacteria in both pigs [
42] and chickens [
43]. Santos et al. [
26] reported an increase in the proportion of
Actinobacteria and
Sterolactobacilli and a decrease in
Fusobacteria in response to the addition of dehydrated yeast cultures. However, the gut microbiota composition can vary significantly between individuals, which may lead to different responses to dietary interventions [
44]. These findings indicate that L-β-galactoglucan may act as a fermentable substrate for specific gut microbes, leading to the production of SCFAs such as butyrate, which supports gut barrier integrity and modulates host immune responses.
To further validate the prebiotic effects of L-β-galactoglucan, we conducted a Linear Discriminant Analysis (LDA) Effect Size (LEfSe) analysis. This analysis revealed increased LDA scores for certain genera in response to L-β-galactoglucan supplementation, indicating that L-β-galactoglucan can modulate gut microbiota by promoting beneficial genera while possibly suppressing harmful ones.
The long-term effects of L-β-galactoglucan supplementation in canines merit further investigation. The data presented in the current and previous studies with polysaccharides indicated that the application of L-β-galactoglucan is unlikely to exert adverse effects; instead, it may provide benefits, including improved gut health, weight management, and immune function. However, the short duration of this study emphasizes the necessity for long-term research to validate its safety and efficacy in chronic dietary use.