Role of Dietary Ceramide 2-Aminoethylphosphonate on Aberrant Crypt Foci Formation and Colon Inflammation in 1,2-Dimethylhydrazine-Treated Mice: A Comparison with the Role of Sphingomyelin
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CAEP from Octopus
2.2. Analysis of CAEP Purified from Octopus
2.3. Animals
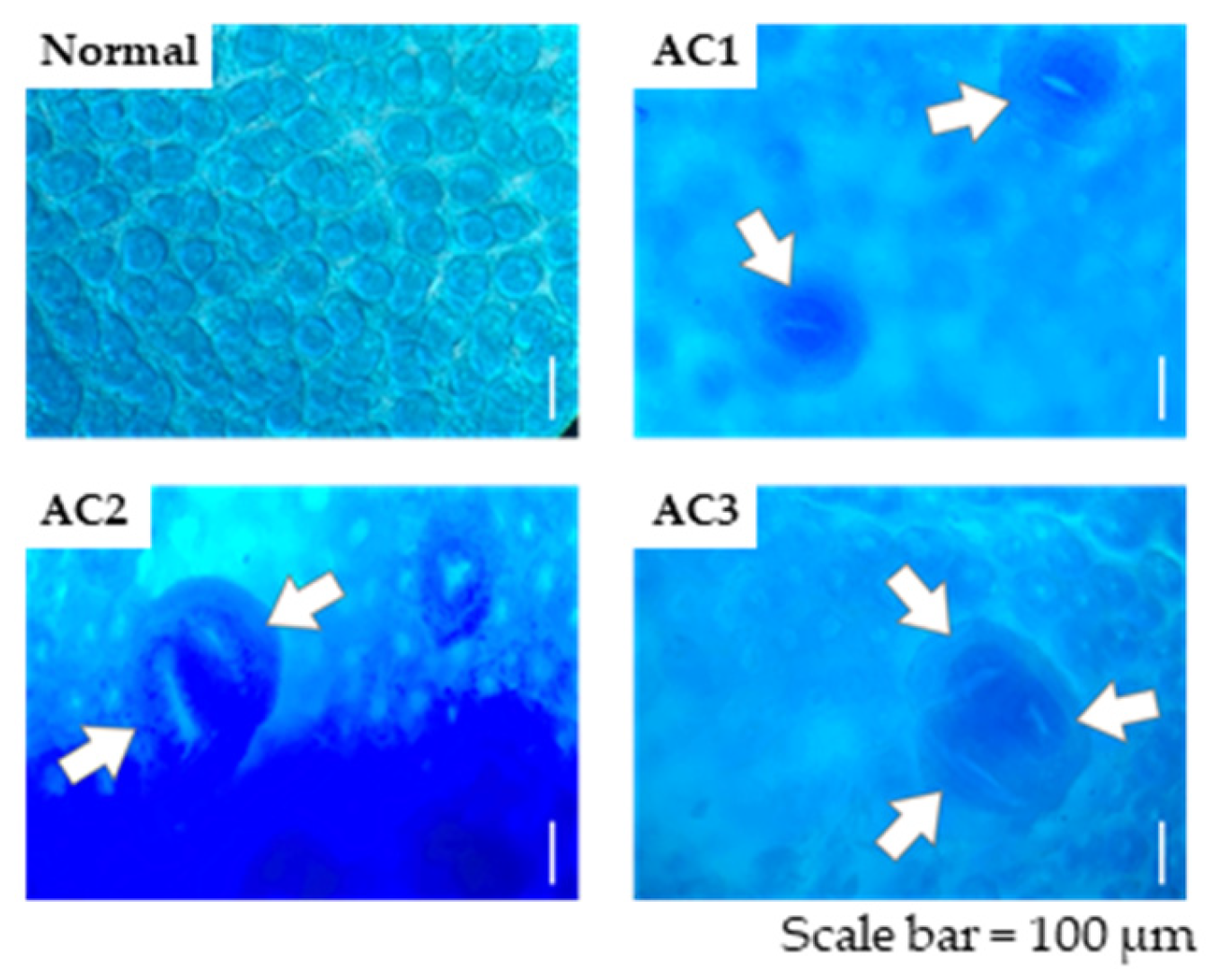
2.4. ACF Identification
2.5. Inflammation Assay
2.6. Statistical Analysis
3. Results
3.1. Fatty Chain Composition of Sphingolipids—CAEP and LCB
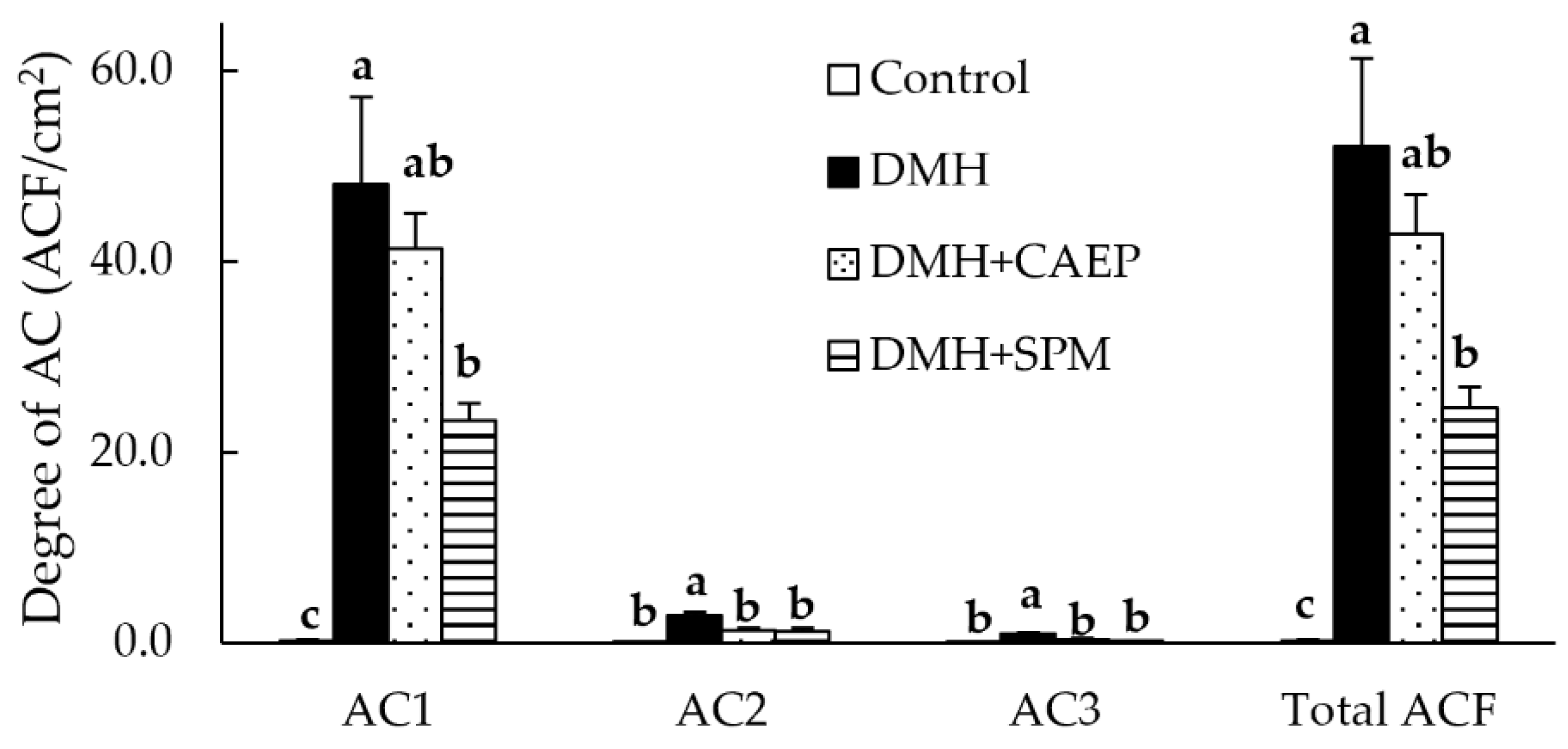
3.2. ACF Formation
3.3. Expressions of Inflammation-Related Cytokines in the Colon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, Z.; Wan, X. Global, regional, and national burden of inflammatory bowel disease and its associated anemia, 1990 to 2019 and predictions to 2050: An analysis of the global burden of disease study 2019. Autoimmun. Rev. 2024, 23, 103498. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e713. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef]
- Jiang, Y.; Jarr, K.; Layton, C.; Gardner, C.D.; Ashouri, J.F.; Abreu, M.T.; Sinha, S.R. Therapeutic Implications of Diet in Inflammatory Bowel Disease and Related Immune-Mediated Inflammatory Diseases. Nutrients 2021, 13, 890. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Kinoshita, M.; Miyazawa, T. Dietary Sphingolipids Contribute to Health via Intestinal Maintenance. Int. J. Mol. Sci. 2021, 22, 7052. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Arakawa, I.; Sugita, M. Distribution of Ceramide 2-Aminoethylphosphonate and Ceramide Aminoethylphosphate (Sphingoethanolamine) in Some Aquatic Animals. J. Biochem. 1967, 62, 67–70. [Google Scholar] [CrossRef]
- Wang, R.; Chen, Q.; Song, Y.; Ding, Y.; Cong, P.; Xu, J.; Xue, C. Identification of ceramide 2-aminoethylphosphonate molecular species from different aquatic products by NPLC/Q-Exactive-MS. Food Chem. 2020, 304, 125425. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, Å. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1968, 164, 575–584. [Google Scholar] [CrossRef]
- Nilsson, Å. Metabolism of cerebroside in the intestinal tract of the rat. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1969, 187, 113–121. [Google Scholar] [CrossRef]
- Sugawara, T. Sphingolipids as Functional Food Components: Benefits in Skin Improvement and Disease Prevention. J. Agric. Food Chem. 2022, 70, 9597–9609. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Renaguli, M.; Kinoshita, M.; Matsuyama, H.; Mawatari, S.; Fujino, T.; Kodama, Y.; Sugiyama, M.; Ohnishi, M. Dietary Sphingolipids Ameliorate Disorders of Lipid Metabolism in Zucker Fatty Rats. J. Agric. Food Chem. 2010, 58, 7030–7035. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, N.; Manabe, Y.; Sugawara, T. Digestion of Ceramide 2-Aminoethylphosphonate, a Sphingolipid from the Jumbo Flying Squid Dosidicus gigas, in Mice. Lipids 2017, 52, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, N.; Tsuduki, T.; Manabe, Y.; Sugawara, T. Sphingoid bases of dietary ceramide 2-aminoethylphosphonate, a marine sphingolipid, absorb into lymph in rats. J. Lipid Res. 2019, 60, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, N.; Manabe, Y.; Aida, K.; Sugawara, T. Dietary ceramide 2-aminoethylphosphonate, a marine sphingophosphonolipid, improves skin barrier function in hairless mice. Sci. Rep. 2020, 10, 13891. [Google Scholar] [CrossRef]
- Sugimoto, K.; Kosaka, A.; Hosomi, R.; Itonori, S.; Yoshida, M.; Fukunaga, K. Scallop ceramide 2-aminoethylphosphonate is more effective than egg yolk sphingomyelin in improving cholesterol metabolism in obese/type II diabetic KK-A mice. J. Funct. Foods 2024, 123, 106569. [Google Scholar] [CrossRef]
- Dillehay, D.L.; Webb, S.K.; Schmelz, E.-M.; Merrill, A.H. Dietary Sphingomyelin Inhibits 1,2-Dimethylhydrazine–Induced Colon Cancer in CF1 Mice. J. Nutr. 1994, 124, 615–620. [Google Scholar] [CrossRef]
- Schmelz, E.M.; Sullards, M.C.; Dillehay, D.L.; Merrill, A.H. Colonic Cell Proliferation and Aberrant Crypt Foci Formation Are Inhibited by Dairy Glycosphingolipids in 1,2-Dimethylhydrazine-Treated CF1 Mice. J. Nutr. 2000, 130, 522–527. [Google Scholar] [CrossRef]
- Aida, K.; Kinoshita, M.; Tanji, M.; Sugawara, T.; Tamura, M.; Ono, J.; Ueno, N.; Ohnishi, M. Prevention of Aberrant Crypt Foci Formation by Dietary Maize and Yeast Cerebrosides in 1,2-Dimethyihydrazine-treated Mice. J. Oleo Sci. 2005, 54, 45–49. [Google Scholar] [CrossRef]
- Yamashita, S.; Sakurai, R.; Hishiki, K.; Aida, K.; Kinoshita, M. Effects of Dietary Plant-origin Glucosylceramide on Colon Cytokine Contents in DMH-treated Mice. J. Oleo Sci. 2017, 66, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Miyazawa, T. Separation and determination of glycolipids from edible plant sources by high-performance liquid chromatography and evaporative light-scattering detection. Lipids 1999, 34, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Eto, T.; Ichikawa, Y.; Nishimura, K.; Ando, S.; Yamakawa, T. Chemistry of lipid of the posthemyolytic residue or stroma of erythrocytes. XVI. Occurrence of ceramide pentasaccharide in the membrane of erythrocytes and reticulocytes of rabbit. J. Biochem. 1968, 64, 205–213. [Google Scholar] [CrossRef]
- Ohnishi, M.; Ito, S.; Fujino, Y. Characterization of sphingolipids in spinach leaves. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1983, 752, 416–422. [Google Scholar] [CrossRef]
- Imai, H.; Ohnishi, M.; Hotsubo, K.; Kojima, M.; Ito, S. Sphingoid Base Composition of Cerebrosides from Plant Leaves. Biosci. Biotechnol. Biochem. 1997, 61, 351–353. [Google Scholar] [CrossRef][Green Version]
- Ohnishi, M.; Nakano, M.; Fujino, Y. Detection of molecular ions of cerebroside by gas chromatography -mass spectrometry. Agric. Biol. Chem. 1975, 39, 743–744. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Inamine, M.; Suzui, M.; Morioka, T.; Kinjo, T.; Kaneshiro, T.; Sugishita, T.; Okada, T.; Yoshimi, N. Inhibitory effect of dietary monoglucosylceramide 1-O-β-glucosyl-N-2′-hydroxyarachidoyl-4,8-sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in F344 rats. Cancer Sci. 2005, 96, 876–881. [Google Scholar] [CrossRef]
- Yamashita, S.; Tsuruma, T.; Honda, T.; Hashimoto, S.; Kaneda, S.; Ezure, T.; Kubota, T.; Yumoto, E.; Miyashita, K.; Koga, J.; et al. Dietary Fungal Glucosylceramide and Ceramide Reduce the Formation of Aberrant Crypt Foci in 1,2-Dimethylhydrazine-Treated Mice: Differences in the Role of Glucosylceramide and Ceramide. ACS Food Sci. Technol. 2023, 3, 85–91. [Google Scholar] [CrossRef]
- McLellan, E.; Bird, R.P. Effect of disulfiram on 1,2-dimethylhydrazine- and azoxymethane-induced aberrant crypt foci. Carcinogenesis 1991, 12, 969–972. [Google Scholar] [CrossRef]
- McLellan, E.A.; Medline, A.; Bird, R.P. Sequential analyses of the growth and morphological characteristics of aberrant crypt foci: Putative preneoplastic lesions. Cancer Res. 1991, 51, 5270–5274. [Google Scholar] [PubMed]
- Aida, K.; Kinoshita, M.; Sugawara, T.; Ono, J.; Miyazawa, T.; Ohnishi, M. Apoptosis Inducement by Plant and Fungus Sphingoid Bases in Human Colon Cancer Cells. J. Oleo Sci. 2004, 53, 503–510. [Google Scholar] [CrossRef]
- Symolon, H.; Bushnev, A.; Peng, Q.; Ramaraju, H.; Mays, S.G.; Allegood, J.C.; Pruett, S.T.; Sullards, M.C.; Dillehay, D.L.; Liotta, D.C.; et al. Enigmol: A Novel Sphingolipid Analogue with Anticancer Activity against Cancer Cell Lines and In vivo Models for Intestinal and Prostate Cancer. Mol. Cancer Ther. 2011, 10, 648–657. [Google Scholar] [CrossRef]
- Sugawara, T.; Zaima, N.; Yamamoto, A.; Sakai, S.; Noguchi, R.; Hirata, T. Isolation of Sphingoid Bases of Sea Cucumber Cerebrosides and Their Cytotoxicity against Human Colon Cancer Cells. Biosci. Biotechnol. Biochem. 2006, 70, 2906–2912. [Google Scholar] [CrossRef]
- Yamashita, S.; Soga, M.; Nguma, E.; Kinoshita, M.; Miyazawa, T. Protective Mechanism of Rice-Derived Lipids and Glucosylceramide in an In Vitro Intestinal Tract Model. J. Agric. Food Chem. 2021, 69, 10206–10214. [Google Scholar] [CrossRef]
- Lutgens, M.W.M.D.; van Oijen, M.G.H.; van der Heijden, G.J.M.G.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining Risk of Colorectal Cancer in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef]
- Fischbeck, A.; Leucht, K.; Frey-Wagner, I.; Bentz, S.; Pesch, T.; Kellermeier, S.; Krebs, M.; Fried, M.; Rogler, G.; Hausmann, M.; et al. Sphingomyelin induces cathepsin D-mediated apoptosis in intestinal epithelial cells and increases inflammation in DSS colitis. Gut 2010, 60, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, B.; Leppimäki, P.; Axberg, M.; Slotte, J.P. Analysis of natural and synthetic sphingomyelins using high-performance thin-layer chromatography. Eur. J. Biochem. 2001, 266, 997–1002. [Google Scholar] [CrossRef]
- Mazzei, J.C.; Zhou, H.; Brayfield, B.P.; Hontecillas, R.; Bassaganya-Riera, J.; Schmelz, E.M. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: Importance of peroxisome proliferator-activated receptor γ expression. J. Nutr. Biochem. 2011, 22, 1160–1171. [Google Scholar] [CrossRef]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yokoi, K.; Inoue, T.; Asano, S.; Hatano, R.; Shinohara, R.; Itonori, S.; Sugita, M. Sphingomyelins in Four Ascidians, Ciona intestinalis, Halocynthia roretzi, Halocynthia aurantium, and Styela clava. J. Oleo Sci. 2009, 58, 473–480. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, S.M.; Feeney, R.H.; Prasoodanan, P.K.V.; Puértolas-Balint, F.; Singh, D.K.; Wongkuna, S.; Zandbergen, L.; Hauner, H.; Brandl, B.; Nieminen, A.I.; et al. The gut commensal Blautia maintains colonic mucus function under low-fiber consumption through secretion of short-chain fatty acids. Nat. Commun. 2024, 15, 3502. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, E.; Keto, J.; Nikkilä, J.; Mättö, J.; Lähteenmäki, K. Cytokine response of human mononuclear cells induced by intestinal Clostridium species. Anaerobe 2013, 19, 70–76. [Google Scholar] [CrossRef]
- Hamajima, H.; Matsunaga, H.; Fujikawa, A.; Sato, T.; Mitsutake, S.; Yanagita, T.; Nagao, K.; Nakayama, J.; Kitagaki, H. Japanese traditional dietary fungus koji Aspergillus oryzae functions as a prebiotic for Blautia coccoides through glycosylceramide: Japanese dietary fungus koji is a new prebiotic. Springerplus 2016, 5, 1321. [Google Scholar] [CrossRef]
- Dai, H.; Otsuka, A.; Tanabe, K.; Yanagita, T.; Nakayama, J.; Kitagaki, H. Glucosylceramide Changes Bacterial Metabolism and Increases Gram-Positive Bacteria through Tolerance to Secondary Bile Acids In Vitro. Int. J. Mol. Sci. 2022, 23, 5300. [Google Scholar] [CrossRef]
- Gaetani, S.; Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.J.; Magness, S.; Jobin, C.; Lund, P.K. High-Fat Diet: Bacteria Interactions Promote Intestinal Inflammation Which Precedes and Correlates with Obesity and Insulin Resistance in Mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.H.; Milard, M.; Michalski, M.-C.; Blesso, C.N. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J. Nutr. Biochem. 2019, 73, 108224. [Google Scholar] [CrossRef] [PubMed]
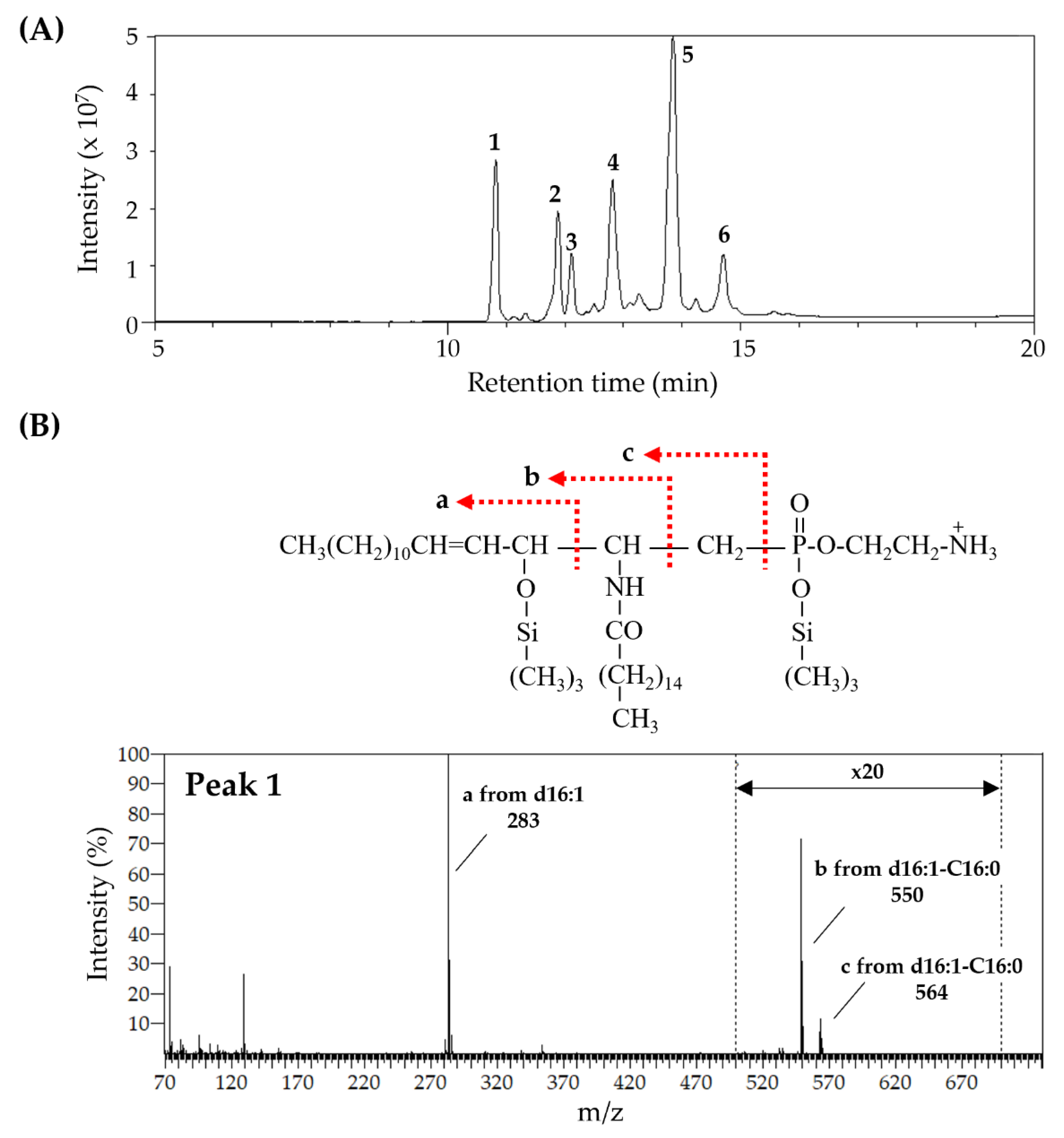

| Fatty Acid | CAEP | SPM | LCB | CAEP | SPM |
|---|---|---|---|---|---|
| mol% | mol% | ||||
| C16:0 | 34.2 | 80.2 | d16:1 | 84.2 | nd |
| C18:0 | 10.2 | 7.9 | d18:0 | nd | 6.2 |
| C20:1 | 12.0 | nd | d18:1 | 14.1 | 93.8 |
| C22:1 | 37.6 | nd | d19:2 | 1.7 | nd |
| C24:1 | 2.8 | 4.6 | d19:3 | trace | nd |
| Others | 3.3 | 7.3 | |||
| Control | DMH | DMH + CAEP | DMH + SPM | |
|---|---|---|---|---|
| Final body weight (g) | 22.33 ± 0.34 a | 20.37 ± 0.38 b | 20.73 ± 0.35 b | 20.76 ± 0.15 b |
| Body weight gain (g/9 weeks) | 3.92 ± 0.31 a | 2.05 ± 0.28 b | 1.56 ± 0.20 b | 1.83 ± 0.26 b |
| Feed intake (g/9 weeks) * | 166.04 ± 0.06 a | 165.05 ± 5.11 a | 174.97 ± 3.52 a | 178.09 ± 0.37 a |
| Liver weight (g) | 0.93 ± 0.03 a | 0.81 ± 0.03 b | 0.84 ± 0.03 ab | 0.86 ± 0.02 ab |
| Spleen weight (g) | 0.12 ± 0.01 a | 0.12 ± 0.00 a | 0.13 ± 0.01 a | 0.13 ± 0.01 a |
| Colon length (cm) | 10.75 ± 0.38 a | 11.36 ± 0.53 a | 11.31 ± 0.40 a | 10.80 ± 0.55 a |
| Dry feces (g/2 days) * | 0.28 ± 0.01 a | 0.33 ± 0.03 a | 0.36 ± 0.06 a | 0.37 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, S.; Yutani, W.; Sugimoto, M.; Miyashita, K.; Kinoshita, M. Role of Dietary Ceramide 2-Aminoethylphosphonate on Aberrant Crypt Foci Formation and Colon Inflammation in 1,2-Dimethylhydrazine-Treated Mice: A Comparison with the Role of Sphingomyelin. Metabolites 2025, 15, 147. https://doi.org/10.3390/metabo15030147
Yamashita S, Yutani W, Sugimoto M, Miyashita K, Kinoshita M. Role of Dietary Ceramide 2-Aminoethylphosphonate on Aberrant Crypt Foci Formation and Colon Inflammation in 1,2-Dimethylhydrazine-Treated Mice: A Comparison with the Role of Sphingomyelin. Metabolites. 2025; 15(3):147. https://doi.org/10.3390/metabo15030147
Chicago/Turabian StyleYamashita, Shinji, Wakaba Yutani, Maho Sugimoto, Kazuo Miyashita, and Mikio Kinoshita. 2025. "Role of Dietary Ceramide 2-Aminoethylphosphonate on Aberrant Crypt Foci Formation and Colon Inflammation in 1,2-Dimethylhydrazine-Treated Mice: A Comparison with the Role of Sphingomyelin" Metabolites 15, no. 3: 147. https://doi.org/10.3390/metabo15030147
APA StyleYamashita, S., Yutani, W., Sugimoto, M., Miyashita, K., & Kinoshita, M. (2025). Role of Dietary Ceramide 2-Aminoethylphosphonate on Aberrant Crypt Foci Formation and Colon Inflammation in 1,2-Dimethylhydrazine-Treated Mice: A Comparison with the Role of Sphingomyelin. Metabolites, 15(3), 147. https://doi.org/10.3390/metabo15030147







