Age-Related Changes in Caudate Glucose Metabolism: Insights from Normative Modeling Study in Healthy Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Acquisition and Preprocessing
2.3. ROI Correlation Analysis
2.4. Voxel-Wise Correlation Analysis
2.5. Normative Modeling Analysis
3. Results
3.1. ROI Correlation Analysis
3.2. Voxel-Wise Correlation Analysis
3.3. Normative Modeling Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dienel, G.A.; Hertz, L. Glucose and lactate metabolism during brain activation. J. Neurosci. Res. 2001, 66, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Lizarraga, A.; Caminiti, S.P.; Calhoun, V.D.; Eickhoff, S.B.; Habeck, C. Brain connectomics: Time for a molecular imaging perspective? Trends Cogn. Sci. 2023, 27, 353–366. [Google Scholar] [CrossRef]
- Deery, H.A.; Liang, E.X.; Siddiqui, M.N.; Murray, G.; Voigt, K.; Di Paolo, R.; Moran, C.; Egan, G.F.; Jamadar, S.D. Reconfiguration of metabolic connectivity in ageing. Commun. Biol. 2024, 7, 1600. [Google Scholar] [CrossRef]
- Pardo, J.V.; Lee, J.T.; Sheikh, S.A.; Surerus-Johnson, C.; Shah, H.; Munch, K.R.; Carlis, J.V.; Lewis, S.M.; Kuskowski, M.A.; Dysken, M.W. Where the brain grows old: Decline in anterior cingulate and medial prefrontal function with normal aging. NeuroImage 2007, 35, 1231–1237. [Google Scholar] [CrossRef]
- Malpetti, M.; Ballarini, T.; Presotto, L.; Garibotto, V.; Tettamanti, M.; Perani, D. Gender differences in healthy aging and Alzheimer’s Dementia: A 18F-FDG-PET study of brain and cognitive reserve. Hum. Brain Mapp. 2017, 38, 4212–4227. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, H.; Gazes, Y.; Stern, Y.; Miyata, Y.; Uchiyama, S. Characterizing the normative profile of F-FDG PET brain imaging: Sex difference, aging effect, and cognitive reserve. Psychiatry Res.-Neuroimaging 2014, 221, 78–85. [Google Scholar] [CrossRef]
- Kakimoto, A.; Ito, S.; Okada, H.; Nishizawa, S.; Minoshima, S.; Ouchi, Y. Age-Related Sex-Specific Changes in Brain Metabolism and Morphology. J. Nucl. Med. 2016, 57, 221–225. [Google Scholar] [CrossRef]
- Subtirelu, R.C.; Teichner, E.M.; Su, Y.; Al-Daoud, O.; Patel, M.; Patil, S.; Writer, M.; Werner, T.; Revheim, M.-E.; Høilund-Carlsen, P.F.; et al. Aging and Cerebral Glucose Metabolism: 18F-FDG-PET/CT Reveals Distinct Global and Regional Metabolic Changes in Healthy Patients. Life 2023, 13, 2044. [Google Scholar] [CrossRef]
- Shen, X.; Liu, H.; Hu, Z.; Hu, H.; Shi, P. The Relationship between Cerebral Glucose Metabolism and Age: Report of a Large Brain PET Data Set. PLoS ONE 2012, 7, e51517. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Varma, V.R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C.W.; Egan, J.M.; Ferrucci, L.; Troncoso, J.; et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 318–329. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, X.; Liu, X.; Liao, J.; Lin, J.; Zhu, C.; Meng, X.; Xie, D.; Chao, D.; Fenoy, A.J.; et al. Low Cerebral Glucose Metabolism: A Potential Predictor for the Severity of Vascular Parkinsonism and Parkinson’s Disease. Aging Dis. 2015, 6, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Zhong, C.J. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: Implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef]
- Deery, H.A.; Di Paolo, R.; Moran, C.; Egan, G.F.; Jamadar, S.D. Lower brain glucose metabolism in normal ageing is predominantly frontal and temporal: A systematic review and pooled effect size and activation likelihood estimates meta-analyses. Hum. Brain Mapp. 2023, 44, 1251–1277. [Google Scholar] [CrossRef]
- Cools, R.; D’Esposito, M. Inverted-U–Shaped Dopamine Actions on Human Working Memory and Cognitive Control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. The primate basal ganglia: Parallel and integrative networks. J. Chem. Neuroanat. 2004, 26, 317–330. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, M.R.; O’Shea, J.; Mars, R.B.; Rushworth, M.F.; Cools, R. Controlling Human Striatal Cognitive Function via the Frontal Cortex. J. Neurosci. 2012, 32, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic control of the striatum for high-level cognition. Curr. Opin. Neurobiol. 2011, 21, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson’s Disease. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Scherman, D.; Desnos, C.; Darchen, F.; Pollak, P.; Javoy-Agid, F.; Agid, Y. Striatal dopamine deficiency in parkinson’s disease: Role of aging. Ann. Neurol. 1989, 26, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Rodríguez-Oroz, M.C.; Benitez-Temino, B.; Blesa, F.J.; Guridi, J.; Marin, C.; Rodriguez, M. Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov. Disord. 2008, 23, S548–S559. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.; Bertoux, M.; Hornberger, M. Beyond and below the cortex: The contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. J. Neurol. Neurosurg. Psychiatry 2014, 85, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Sahlberg, N.; Ruottinen, H.; Nagren, K.; Lehikoinen, P. Striatal uptake of the dopamine reuptake ligand [C]β-CFT is reduced in Alzheimer’s disease assessed by positron emission tomography. Neurology 1998, 50, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Pievani, M.; Bocchetta, M.; Boccardi, M.; Cavedo, E.; Bonetti, M.; Thompson, P.M.; Frisoni, G.B. Striatal morphology in early-onset and late-onset Alzheimer’s disease: A preliminary study. Neurobiol. Aging 2013, 34, 1728–1739. [Google Scholar] [CrossRef]
- Kang, S.; Jeon, S.; Lee, Y.G.; Ye, B.S. Striatal dopamine transporter uptake, parkinsonism and cognition in Alzheimer’s disease. Eur. J. Neurol. 2023, 30, 3105–3113. [Google Scholar] [CrossRef]
- Arber, S.; Costa, R.M. Networking brainstem and basal ganglia circuits for movement. Nat. Rev. Neurosci. 2022, 23, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J.; Williams, L.; Jones, D.T.; Benarroch, E.E. Caudate nucleus as a component of networks controlling behavior. Neurology 2017, 89, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.; Kia, S.M.; Wolfers, T.; Fraza, C.; Zabihi, M.; Dinga, R.; Berthet, P.; Worker, A.; Verdi, S.; Ruhe, H.G.; et al. The normative modeling framework for computational psychiatry. Nat. Protoc. 2022, 17, 1711–1734. [Google Scholar] [CrossRef]
- Verdi, S.; Rutherford, S.; Fraza, C.; Tosun, D.; Altmann, A.; Raket, L.L. Personalizing progressive changes to brain structure in Alzheimer’s disease using normative modeling. Alzheimers Dement. 2024, 20, 6998–7012. [Google Scholar] [CrossRef]
- Kato, T.; Inui, Y.; Nakamura, A.; Ito, K. Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res. Rev. 2016, 30, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, Y.; Liu, Y.; Chen, Z.; Zhai, H.; Zhuang, M.; Zhang, N.; Jiang, Y.; Gao, Y.; Feng, H.; et al. Population-specific brain [18F]-FDG PET templates of Chinese subjects for statistical parametric mapping. Sci. Data 2021, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Shi, L.; Chen, N.; Luo, Y.; Wang, X.; Liu, K.; Mok, V.C.; Chu, W.C.; Wang, D.; Li, K. Construction of brain atlases based on a multi-center MRI dataset of 2020 Chinese adults. Sci. Rep. 2015, 5, 18216. [Google Scholar] [CrossRef] [PubMed]
- Rios, G.; Tobar, F. Compositionally-warped Gaussian processes. Neural Netw. 2019, 118, 235–246. [Google Scholar] [CrossRef]
- Rutherford, S.; Fraza, C.; Dinga, R.; Kia, S.M.; Wolfers, T.; Zabihi, M.; Berthet, P.; Worker, A.; Verdi, S.; Andrews, D.; et al. Charting brain growth and aging at high spatial precision. eLife 2022, 11, 72904. [Google Scholar] [CrossRef] [PubMed]
- Fraza, C.J.; Dinga, R.; Beckmann, C.F.; Marquand, A.F. Warped Bayesian linear regression for normative modelling of big data. NeuroImage 2021, 245, 118715. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; Kang, K.; Vandekar, S.; Rogers, B.P.; Heckers, S.; Woodward, N.D. Lifespan development of thalamic nuclei and characterizing thalamic nuclei abnormalities in schizophrenia using normative modeling. Neuropsychopharmacology 2024, 49, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Erixon-Lindroth, N.; Farde, L.; Wahlin, T.-B.R.; Sovago, J.; Halldin, C.; Bäckman, L. The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res. Neuroimaging 2005, 138, 1–12. [Google Scholar] [CrossRef]
- Brickman, A.M.; Buchsbaum, M.S.; Shihabuddin, L.; Hazlett, E.A.; Borod, J.C.; Mohs, R.C. Striatal size, glucose metabolic rate, and verbal learning in normal aging. Cogn. Brain Res. 2003, 17, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The Role of the Dorsal Striatum in Reward and Decision-Making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef]
- Bäckman, L.; Ginovart, N.; Dixon, R.A.; Wahlin TB, R.; Wahlin, Å.; Halldin, C.; Farde, L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am. J. Psychiatry 2000, 157, 635–637. [Google Scholar] [CrossRef]
- Raz, N.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Gunning-Dixon, F.; Acker, J.D. Differential Aging of the Human Striatum: Longitudinal Evidence. AJNR Am. J. Neuroradiol. 2003, 24, 1849–1856. [Google Scholar] [PubMed]
- Berry, A.S.; Shah, V.D.; Baker, S.L.; Vogel, J.W.; O’Neil, J.P.; Janabi, M.; Schwimmer, H.D.; Marks, S.M.; Jagust, W.J. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J. Neurosci. 2016, 36, 12559–12569. [Google Scholar] [CrossRef]
- Volkow, N.D.; Gur, R.C.; Wang, G.J.; Fowler, J.S.; Moberg, P.J.; Ding, Y.S.; Hitzemann, R.; Smith, G.; Logan, J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry 1998, 155, 344–349. [Google Scholar]
- Cropley, V.L.; Fujita, M.; Innis, R.B.; Nathan, P.J. Molecular Imaging of the Dopaminergic System and its Association with Human Cognitive Function. Biol. Psychiatry 2006, 59, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Remy, P.; Samson, Y. The role of dopamine in cognition: Evidence from functional imaging studies. Curr. Opin. Neurol. 2003, 16, S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 2006, 30, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Benningfield, M.M.; Blackford, J.U.; Ellsworth, M.E.; Samanez-Larkin, G.R.; Martin, P.R.; Cowan, R.L.; Zald, D.H. Caudate responses to reward anticipation associated with delay discounting behavior in healthy youth. Dev. Cogn. Neurosci. 2014, 7, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Friedman, H.R.; Davachi, L.; Goldman-Rakic, P.S. Differential Activation of the Caudate Nucleus in Primates Performing Spatial and Nonspatial Working Memory Tasks. J. Neurosci. 1997, 17, 3870–3882. [Google Scholar] [CrossRef]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Blangero, J.; Sanghera, M.K.; Pessoa, L.; Fox, P.M.; Uecker, A.; Friehs, G.; Young, K.A.; et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage 2012, 60, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Parkes, L.; Fulcher, B.D.; Yücel, M.; Fornito, A. Transcriptional signatures of connectomic subregions of the human striatum. Genes Brain Behav. 2017, 16, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Tullo, S.; Patel, R.; Devenyi, G.A.; Salaciak, A.; Bedford, S.A.; Farzin, S.; Wlodarski, N.; Tardif, C.L.; the PREVENT-AD Research Group; Breitner, J.C.S.; et al. MR-based age-related effects on the striatum, globus pallidus, and thalamus in healthy individuals across the adult lifespan. Hum. Brain Mapp. 2019, 40, 5269–5288. [Google Scholar] [CrossRef]
- Serbruyns, L.; Leunissen, I.; Huysmans, T.; Cuypers, K.; Meesen, R.L.; van Ruitenbeek, P.; Sijbers, J.; Swinnen, S.P. Subcortical volumetric changes across the adult lifespan: Subregional thalamic atrophy accounts for age-related sensorimotor performance declines. Cortex 2015, 65, 128–138. [Google Scholar] [CrossRef]
- Boisgontier, M.P.; van Ruitenbeek, P.; Leunissen, I.; Chalavi, S.; Sunaert, S.; Levin, O.; Swinnen, S.P. Nucleus accumbens and caudate atrophy predicts longer action selection times in young and old adults. Hum. Brain Mapp. 2016, 37, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Hörtnagl, H.; Pifl, C.; Hörtnagl, E.; Reiner, A.; Sperk, G. Distinct gradients of various neurotransmitter markers in caudate nucleus and putamen of the human brain. J. Neurochem. 2020, 152, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A.; Whitwell, J.L.; Parisi, J.E.; Petersen, R.C.; Boeve, B.F.; Jr, C.R.J.; Dickson, D.W. Caudate atrophy on MRI is a characteristic feature of FTLD-FUS. Eur. J. Neurol. 2010, 17, 969–975. [Google Scholar] [CrossRef]
- Lee, Y.; Byun, S.; Na, S.J. Behavioral Variant Frontotemporal Dementia With the Dominantly Affected Caudate Nucleus in 18F-FP-CIT PET/CT. Clin. Nucl. Med. 2024, 49, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Albin, R.L.; Müller, M.L.T.M.; Petrou, M.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.H.; Frey, K.A. Frequency of Cholinergic and Caudate Nucleus Dopaminergic Deficits Across the Predemented Cognitive Spectrum of Parkinson Disease and Evidence of Interaction Effects. JAMA Neurol. 2015, 72, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.K.; Ho, A.J.; Hua, X.; Saharan, P.S.; Toga, A.W.; Jack, C.R., Jr.; Weiner, M.W.; Thompson, P.M.; Alzheimer’s Disease Neuroimaging Initiative. 3D maps localize caudate nucleus atrophy in 400 Alzheimer’s disease, mild cognitive impairment, and healthy elderly subjects. Neurobiol. Aging 2010, 31, 1312–1325. [Google Scholar] [CrossRef]
- Feng, B.; Cao, J.; Yu, Y.; Yang, H.; Jiang, Y.; Liu, Y.; Wang, R.; Zhao, Q. Gender-Related Differences in Regional Cerebral Glucose Metabolism in Normal Aging Brain. Front. Aging Neurosci. 2022, 14, 809767. [Google Scholar] [CrossRef]
- Jiang, J.; Sheng, C.; Chen, G.; Liu, C.; Jin, S.; Li, L.; Jiang, X.; Han, Y.; Weiner, M.W.; Aisen, P.; et al. Glucose metabolism patterns: A potential index to characterize brain ageing and predict high conversion risk into cognitive impairment. GeroScience 2022, 44, 2319–2336. [Google Scholar] [CrossRef] [PubMed]
- Verdi, S.; Marquand, A.F.; Schott, J.M.; Cole, J.H. Beyond the average patient: How neuroimaging models can address heterogeneity in dementia. Brain 2021, 144, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.; Tso, I.F.; Taylor, S.; Duval, E.; Beckmann, C.; Ruhe, H.; Marquand, A. Lifespan Normative Modeling of Internalizing & Psychotic Disorders. Biol. Psychiatry 2021, 89, S189. [Google Scholar] [CrossRef]
- Galiano, A.; Mengual, E.; De Eulate, R.G.; Galdeano, I.; Vidorreta, M.; Recio, M.; Riverol, M.; Zubieta, J.L.; Fernández-Seara, M.A. Coupling of cerebral blood flow and functional connectivity is decreased in healthy aging. Brain Imaging Behav. 2020, 14, 436–450. [Google Scholar] [CrossRef] [PubMed]

| Gender/Age | 20–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–81 |
|---|---|---|---|---|---|---|
| Male | 2 | 14 | 18 | 18 | 20 | 5 |
| Female | 0 | 9 | 16 | 11 | 3 | 0 |
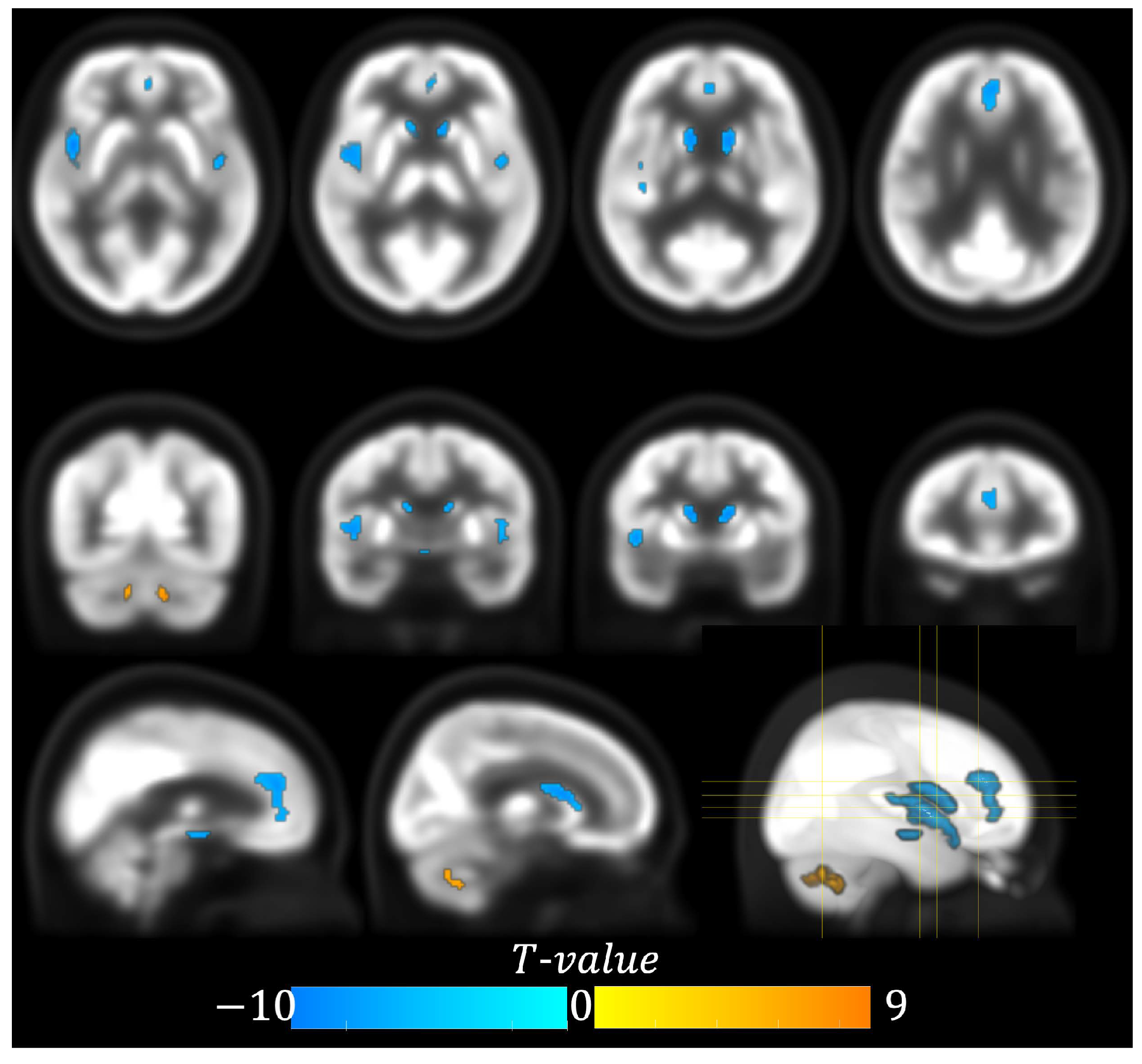
| Cluster | Anatomical Region | Cluster Size (#Voxel) | Peak Coordinates (x, y, z) Chinese2020 Space | Peak t-Value (df = 112) |
|---|---|---|---|---|
| 1 | Left Cerebellum 7b | 81 | −10, −60, −38 | 9.14 |
| 2 | Right Cerebellum 9 | 52 | 8, −46, −40 | 7.18 |
| 3 | Right Insula | 303 | 42, 8, −4 | −9.91 |
| 4 | Left Caudate | 159 | −12, 10, 10 | −9.23 |
| 5 | Right Caudate | 125 | 10, 12, 8 | −8.71 |
| 6 | Anterior Cingulate Gyrus | 215 | −2, 40, 16 | −7.43 |
| 7 | Left Insula | 72 | −42, −2, −4 | −7.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Y.; Xia, Q.; Yu, Q.; Wei, L.; Wu, G.-R. Age-Related Changes in Caudate Glucose Metabolism: Insights from Normative Modeling Study in Healthy Subjects. Metabolites 2025, 15, 67. https://doi.org/10.3390/metabo15020067
Zhang Z, Li Y, Xia Q, Yu Q, Wei L, Wu G-R. Age-Related Changes in Caudate Glucose Metabolism: Insights from Normative Modeling Study in Healthy Subjects. Metabolites. 2025; 15(2):67. https://doi.org/10.3390/metabo15020067
Chicago/Turabian StyleZhang, Zijing, Yuchen Li, Qi Xia, Qing Yu, Luqing Wei, and Guo-Rong Wu. 2025. "Age-Related Changes in Caudate Glucose Metabolism: Insights from Normative Modeling Study in Healthy Subjects" Metabolites 15, no. 2: 67. https://doi.org/10.3390/metabo15020067
APA StyleZhang, Z., Li, Y., Xia, Q., Yu, Q., Wei, L., & Wu, G.-R. (2025). Age-Related Changes in Caudate Glucose Metabolism: Insights from Normative Modeling Study in Healthy Subjects. Metabolites, 15(2), 67. https://doi.org/10.3390/metabo15020067







