Metabolomic Profiling of the Striatum in Shank3 Knockout ASD Rats: Effects of Early Swimming Regulation
Abstract
1. Introduction
2. Materials and Methods
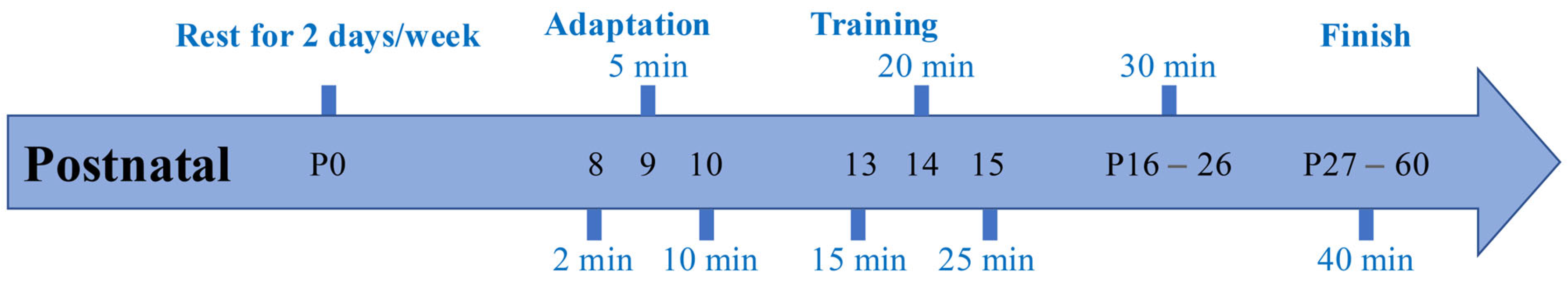
2.1. Experimental Animals and Grouping
2.2. Early Swimming Exercise Program
2.3. Striatal Metabolite Analysis
2.3.1. Chemicals
2.3.2. Metabolite Extraction
2.3.3. Chromatographic Conditions
2.3.4. Mass Spectrometric Conditions
2.3.5. Data Analysis
3. Results
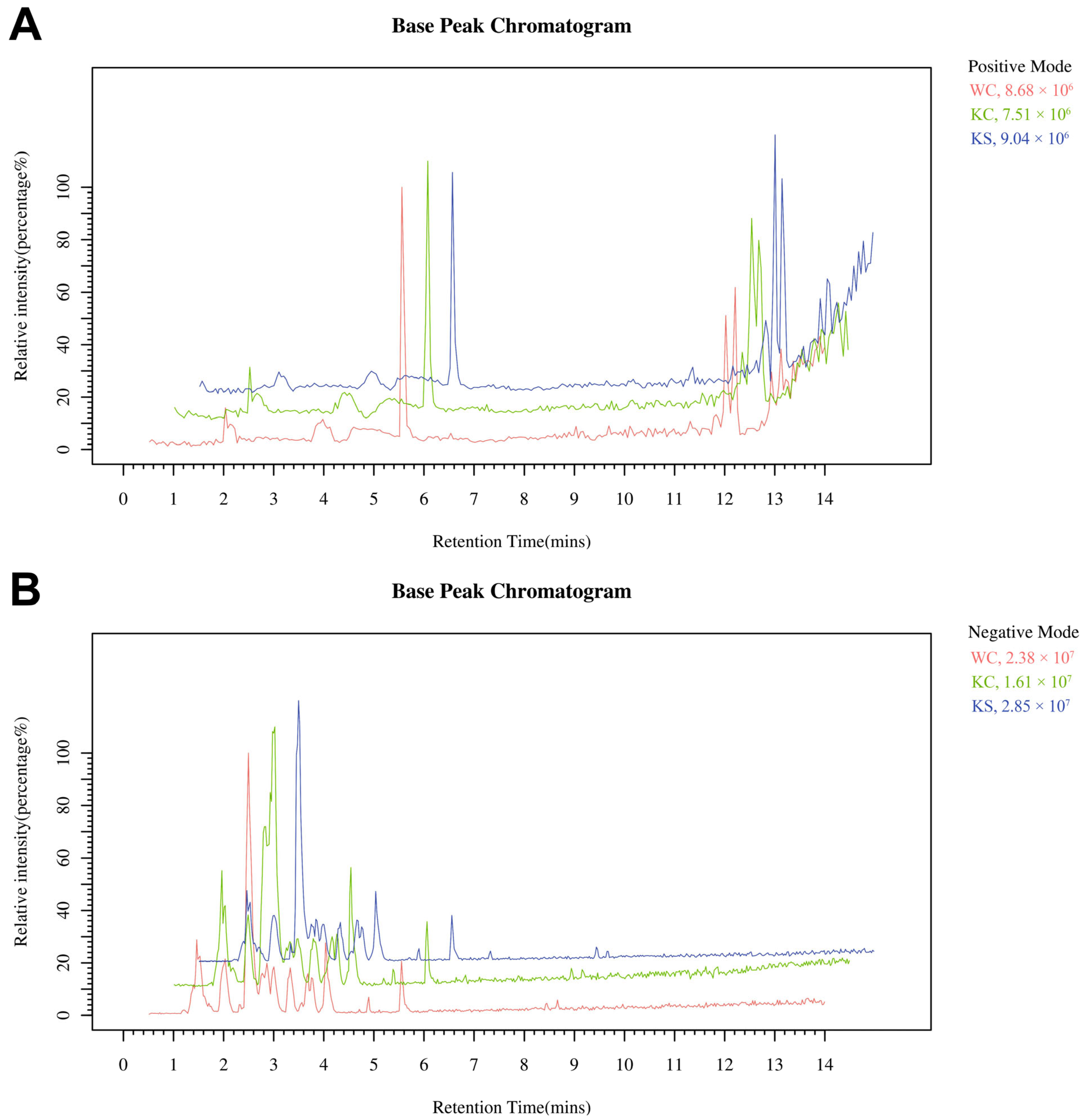
3.1. LC-MS Analysis of the Striatal Metabolome
3.2. PLS-DA
3.3. Differential Metabolite Screening
3.4. Metabolic Network Analysis of the Differential Metabolites
4. Discussion
4.1. Metabolomic Analysis of the Striatum in Shank3 Knockout Rats
4.2. Metabolomic Changes in the Striatum of Rats After Early Swimming Interventions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knopf, A. Autism prevalence increases from 1 in 60 to 1 in 54: CDC. Brown Univ. Child Adolesc. Behav. Lett. 2020, 36, 4. [Google Scholar] [CrossRef]
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Zhao, M.; Chen, S. The Effects of Structured Physical Activity Program on Social Interaction and Communication for Children with Autism. BioMed Res. Int. 2018, 2018, 1825046. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, X.; Su, J.; Liu, N. Prevalence of autism spectrum disorder in mainland china over the past 6 years: A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 404. [Google Scholar] [CrossRef]
- Shahat, A.R.S.; Greco, G. The Economic Costs of Childhood Disability: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 3531. [Google Scholar] [CrossRef]
- Qiu, S.; Qiu, Y.; Li, Y.; Cong, X. Genetics of autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Transl. Psychiatry 2022, 12, 249. [Google Scholar] [CrossRef]
- Ioannidis, V.; Pandey, R.; Bauer, H.F.; Schön, M.; Bockmann, J.; Boeckers, T.M.; Lutz, A.K. Disrupted extracellular matrix and cell cycle genes in autism-associated Shank3 deficiency are targeted by lithium. Mol. Psychiatry 2024, 29, 704–717. [Google Scholar] [CrossRef]
- Monteiro, P.; Feng, G.P. SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017, 18, 147–157. [Google Scholar] [CrossRef]
- Xu, D.; Meng, Y.; An, S.; Meng, W.; Li, H.; Zhang, W.; Xue, Y.; Lan, X.; Wang, X.; Li, M.; et al. Swimming exercise is a promising early intervention for autism-like behavior in Shank3 deletion rats. CNS Neurosci. Ther. 2023, 29, 78–90. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, D.; Zhang, W.; Meng, W.; Lan, X.; Wang, X.; Li, M.; Zhang, X.; Zhao, Y.; Yang, H.; et al. Effect of Early Swimming on the Behavior and Striatal Transcriptome of the Shank3 Knockout Rat Model of Autism. Neuropsychiatr. Dis. Treat. 2022, 18, 681–694. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, C.J.; Shin, M.S.; Lim, B.V. Treadmill exercise ameliorates memory impairment through ERK-Akt-CREB-BDNF signaling pathway in cerebral ischemia gerbils. J. Exerc. Rehabil. 2020, 16, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fragala-Pinkham, M.; Haley, S.M.; O’Neil, M.E. Group aquatic aerobic exercise for children with disabilities. Dev. Med. Child Neurol. 2008, 50, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y. The efficacy of an aquatic program on physical fitness and aquatic skills in children with and without autism spectrum disorders. Res. Autism Spectr. Disord. 2011, 5, 657–665. [Google Scholar] [CrossRef]
- Ennis, E. The effects of a physical therapy-directed aquatic program on children with autism spectrum disorders. J. Aquat. Phys. Ther. 2011, 19, 4–10. [Google Scholar]
- Kim, H.; Woo, R.S.; Yang, E.J.; Kim, H.B.; Jo, E.H.; Lee, S.; Im, H.; Kim, S.; Kim, H.S. Transcriptomic analysis in the striatum reveals the involvement of Nurr1 in the social behavior of prenatally valproic acid-exposed male mice. Transl. Psychiatry 2022, 12, 324. [Google Scholar] [CrossRef]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef]
- Roberts, B.M.; Lopes, E.F.; Cragg, S.J. Axonal modulation of striatal dopamine release by local γ-aminobutyric acid (GABA) signalling. Cells 2021, 10, 709. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Connelly, J.; Lindon, J.C.; Holmes, E. Metabonomics: A platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002, 1, 153–161. [Google Scholar] [CrossRef]
- Song, T.J.; Lan, X.Y.; Wei, M.P.; Zhai, F.J.; Boeckers, T.M.; Wang, J.N.; Yuan, S.; Jin, M.Y.; Xie, Y.F.; Dang, W.W.; et al. Altered Behaviors and Impaired Synaptic Function in a Novel Rat Model With a Complete Shank3 Deletion. Front. Cell. Neurosci. 2019, 13, 111. [Google Scholar] [CrossRef]
- de Santana Muniz, G.; da Silva, A.M.A.; Cavalcante, T.C.F.; da Silva França, A.K.; Ferraz, K.M.; do Nascimento, E. Early physical activity minimizes the adverse effects of a low-energy diet on growth and development parameters. Nutr. Neurosci. 2013, 16, 113–124. [Google Scholar] [CrossRef]
- Meng, W.; Xu, D.; Meng, Y.; Zhang, W.; Xue, Y.; Zhen, Z.; Gao, Y. Changes in the urinary proteome in rats with regular swimming exercise. PeerJ 2021, 9, e12406. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-M.; Bailey, M.E.; Cobb, S.R. Rett syndrome: From bed to bench. Pediatr. Neonatol. 2011, 52, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Saywell, V.; Villard, L.; Cozzone, P.J.; Lutz, N.W. Metabolic fingerprints of altered brain growth, osmoregulation and neurotransmission in a Rett syndrome model. PLoS ONE 2007, 2, e157. [Google Scholar] [CrossRef]
- Penagarikano, O.; Mulle, J.G.; Warren, S.T. The pathophysiology of fragile x syndrome. Annu. Rev. Genom. Hum. Genet. 2007, 8, 109–129. [Google Scholar] [CrossRef]
- Mientjes, E.; Nieuwenhuizen, I.; Kirkpatrick, L.; Zu, T.; Hoogeveen-Westerveld, M.; Severijnen, L.; Rifé, M.; Willemsen, R.; Nelson, D.; Oostra, B. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol. Dis. 2006, 21, 549–555. [Google Scholar] [CrossRef]
- Davidovic, L.; Navratil, V.; Bonaccorso, C.M.; Catania, M.V.; Bardoni, B.; Dumas, M.-E. A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model. Genome Res. 2011, 21, 2190–2202. [Google Scholar] [CrossRef]
- Bechara, E.G.; Didiot, M.C.; Melko, M.; Davidovic, L.; Bensaid, M.; Martin, P.; Castets, M.; Pognonec, P.; Khandjian, E.W.; Moine, H. A novel function for fragile X mental retardation protein in translational activation. PLoS Biol. 2009, 7, e1000016. [Google Scholar] [CrossRef]
- de Diego-Otero, Y.; Romero-Zerbo, Y.; Bekay, R.; Decara, J.; Sanchez, L.; Fonseca, F.R.-d.; Arco-Herrera, I.d. α-tocopherol protects against oxidative stress in the fragile X knockout mouse: An experimental therapeutic approach for the Fmr1 deficiency. Neuropsychopharmacology 2009, 34, 1011–1026. [Google Scholar] [CrossRef]
- Dickinson, A.; Bruyns-Haylett, M.; Jones, M.; Milne, E. Increased peak gamma frequency in individuals with higher levels of autistic traits. Eur. J. Neurosci. 2015, 41, 1095–1101. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ko, T.H.; Jin, C.; Zhang, Y.; Kang, H.R.; Ma, R.; Li, H.; Choi, J.I.; Han, K. The emerging roles of Shank3 in cardiac function and dysfunction. Front. Cell Dev. Biol. 2023, 11, 1191369. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, L.; Zhao, L.; Fei, X.; Wang, Z.; Liu, T.; Tang, Y.; Li, D.; Gong, H.; Luo, Y.; et al. Puerarin attenuates valproate-induced features of ASD in male mice via regulating Slc7a11-dependent ferroptosis. Neuropsychopharmacology 2024, 49, 497–507. [Google Scholar] [CrossRef]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder-Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef]
- Sigwalt, A.R.; Budde, H.; Helmich, I.; Glaser, V.; Ghisoni, K.; Lanza, S.; Cadore, E.L.; Lhullier, F.L.R.; de Bem, A.F.; Hohl, A.; et al. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience 2011, 192, 661–674. [Google Scholar] [CrossRef]
- Boracı, H.; Kirazlı, Ö.; Gülhan, R.; Yıldız Sercan, D.; Şehirli, Ü.S. Neuroprotective effect of regular swimming exercise on calretinin-positive striatal neurons of Parkinsonian rats. Anat. Sci. Int. 2020, 95, 429–439. [Google Scholar] [CrossRef]
- Gyorkos, A.M.; McCullough, M.J.; Spitsbergen, J.M. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience 2014, 257, 111–118. [Google Scholar] [CrossRef]
- Liu, W.; Xue, X.; Xia, J.; Liu, J.; Qi, Z. Swimming exercise reverses CUMS-induced changes in depression-like behaviors and hippocampal plasticity-related proteins. J. Affect. Disord. 2018, 227, 126–135. [Google Scholar] [CrossRef]
- Ko, I.-G.; Kim, S.-E.; Kim, T.-W.; Ji, E.-S.; Shin, M.-S.; Kim, C.-J.; Hong, M.-H.; Bahn, G.H. Swimming exercise alleviates the symptoms of attention-deficit hyperactivity disorder in spontaneous hypertensive rats. Mol. Med. Rep. 2013, 8, 393–400. [Google Scholar] [CrossRef]
- Leite, H.R.; Mourão, F.A.G.; Drumond, L.E.; Ferreira-Vieira, T.H.; Bernardes, D.; Silva, J.F.; Lemos, V.S.; Moraes, M.F.D.; Pereira, G.S.; Carvalho-Tavares, J.; et al. Swim training attenuates oxidative damage and promotes neuroprotection in cerebral cortical slices submitted to oxygen glucose deprivation. J. Neurochem. 2012, 123, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Jr, A.S.; Castro, A.A.; Moreira, E.L.; Glaser, V.; Santos, A.R.; Tasca, C.I.; Latini, A.; Prediger, R.D. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 2011, 132, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Devi, S.A.; Ravikiran, T. Differential expression of the cerebral cortex proteome in physically trained adult rats. Brain Res. Bull. 2014, 104, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Harmon, T.C.; McLean, D.L.; Raman, I.M. Integration of Swimming-Related Synaptic Excitation and Inhibition by olig2(+) Eurydendroid Neurons in Larval Zebrafish Cerebellum. J. Neurosci. 2020, 40, 3063–3074. [Google Scholar] [CrossRef]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Lee, H.; Hong, S.H.; Park, C.; Park, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Hwang, H.J.; et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef]



| No. | Metabolites | m/z | RT (s) | Formula | KEGG | Type | Fold Change | ||
|---|---|---|---|---|---|---|---|---|---|
| KC/WC | KS/KC | KS/WC | |||||||
| 1 | Taurine | 124.01 | 89.03 | C2H7NO3S | C00245 | [M−H]− | 0.92 * | 1.23 * | 1.13 * |
| 2 | Pyroglutamic acid | 128.04 | 177.84 | C5H7NO3 | C01879 | [M−H]− | 0.88 | 1.57 * | 1.39 * |
| 3 | (E)-Glutaconate | 129.02 | 103.77 | C5H6O4 | C02214 | [M−H]− | 0.31 * | 4.53 * | 1.43 * |
| 4 | Creatine | 130.06 | 99.11 | C4H9N3O2 | C00300 | [M−H]− | 0.88 * | 1.29 * | 1.14 * |
| 5 | L-Malic acid | 133.01 | 119.43 | C4H6O5 | C00149 | [M−H]− | 0.84 * | 1.62 * | 1.36 * |
| 6 | Threonic acid | 135.03 | 97.76 | C4H8O5 | C01620 | [M−H]− | 0.74 * | 2.15 * | 1.59 * |
| 7 | L-Glutamine | 145.06 | 88.52 | C5H10N2O3 | C00064 | [M−H]− | 0.86 * | 1.58 * | 1.36 * |
| 8 | L-Glutamic acid | 146.05 | 92.43 | C5H9NO4 | C00025 | [M−H]− | 0.86 * | 1.53 * | 1.32 * |
| 9 | 3-Hydroxymethylglutaric acid | 161.05 | 239.48 | C6H10O5 | C03761 | [M−H]− | 0.78 * | 2.66 * | 2.08 * |
| 10 | Myo-Inositol | 161.05 | 556.35 | C6H12O6 | C00137 | [M−H2O−H]− | 0.61 * | 1.25 | 0.76 * |
| 11 | L-Phenylalanine | 166.09 | 280.18 | C9H11NO2 | C00079 | [M+H]+ | 1.06 | 2.16 * | 2.29 * |
| 12 | (2R)-2-Hydroxy-3-propanoate | 166.98 | 105.63 | C3H7O7P | C00197 | [M−H2O−H]− | 1.05 | 2.04 * | 2.14 * |
| 13 | N-Acetyl-L-aspartic acid | 174.04 | 151.21 | C6H9NO5 | C01042 | [M−H]− | 1.41 * | 1.95 * | 2.76 * |
| 14 | Alpha-D-Glucose | 179.06 | 763.79 | C6H12O6 | C00267 | [M−H]− | 0.46 * | 1.31 | 0.61 * |
| 15 | L-Iditol | 181.07 | 665.47 | C6H14O6 | C01507 | [M−H]− | 0.76 * | 1.21 | 0.92 |
| 16 | (S)-beta-Tyrosine | 181.07 | 808.76 | C9H11NO3 | C21308 | [M]− | 0.29 * | 1.20 | 0.35 * |
| 17 | N-Acetylglutamic acid | 188.06 | 200.43 | C7H11NO5 | C00624 | [M−H]− | 0.84 | 2.28 * | 1.92 * |
| 18 | Citric acid | 191.02 | 167.31 | C6H8O7 | C00158 | [M−H]− | 0.99 | 1.85 * | 1.82 * |
| 19 | Sebacic acid | 201.11 | 565.53 | C10H18O4 | C08277 | [M−H]− | 0.96 | 7.66 * | 7.32 * |
| 20 | Pantothenic acid | 218.10 | 294.11 | C9H17NO5 | C00864 | [M−H]− | 0.59 * | 1.64 * | 0.97 |
| 21 | Gamma-Glutamylcysteine | 248.96 | 81.79 | C8H14N2O5S | C00669 | [M−H]− | 0.84 * | 1.18 | 0.98 |
| 22 | N2-gamma-Glutamylglutamine | 274.10 | 101.94 | C10H17N3O6 | C05283 | [M−H]− | 1.10 | 1.77 * | 1.95 * |
| 23 | Dehydroepiandrosterone | 288.29 | 724.49 | C19H28O2 | C01227 | [M]+ | 1.89 * | 0.51 * | 0.96 |
| 24 | Oxidized glutathione | 611.14 | 219.81 | C20H32N6O12S2 | C00127 | [M−H]− | 2.20 * | 2.05 * | 4.52 * |
| 25 | Theophylline | 179.06 | 643.65 | C7H8N4O2 | C07130 | [M−H]− | 0.88 | 0.85 | 0.74 * |
| 26 | Hypoxanthine | 135.03 | 170.48 | C5H4N4O | C00262 | [M−H]− | 1.24 | 1.29 | 1.60 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Hu, Y.; Xue, Y.; Zhen, Z. Metabolomic Profiling of the Striatum in Shank3 Knockout ASD Rats: Effects of Early Swimming Regulation. Metabolites 2025, 15, 134. https://doi.org/10.3390/metabo15020134
Meng Y, Hu Y, Xue Y, Zhen Z. Metabolomic Profiling of the Striatum in Shank3 Knockout ASD Rats: Effects of Early Swimming Regulation. Metabolites. 2025; 15(2):134. https://doi.org/10.3390/metabo15020134
Chicago/Turabian StyleMeng, Yunchen, Yiling Hu, Yaqi Xue, and Zhiping Zhen. 2025. "Metabolomic Profiling of the Striatum in Shank3 Knockout ASD Rats: Effects of Early Swimming Regulation" Metabolites 15, no. 2: 134. https://doi.org/10.3390/metabo15020134
APA StyleMeng, Y., Hu, Y., Xue, Y., & Zhen, Z. (2025). Metabolomic Profiling of the Striatum in Shank3 Knockout ASD Rats: Effects of Early Swimming Regulation. Metabolites, 15(2), 134. https://doi.org/10.3390/metabo15020134






