Oregano Young Plants Cultured at Low Temperature Reveal an Enhanced Healing Effect of Their Extracts: Anatomical, Physiological and Cytotoxicity Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Exposure Setup
2.2. Microscopy
2.3. Histochemistry
2.4. Pigments Protocol
2.5. Protocol for MDA (Malondialdehyde) and H2O2 Determination in Plant Tissues
2.6. Determination of Total Phenolic Content
2.7. Extraction of Plant Material
2.8. Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry (UHPLC–HRMS/MS) Analysis
2.9. Indicator Microorganisms and Antimicrobial Bioassays
2.10. Cell Lines’ Bioassays
2.11. ROS Measurements on Cell Cultures
2.12. Cell Death Measurements
2.13. Data Preprocessing and Statistical Analysis
3. Results
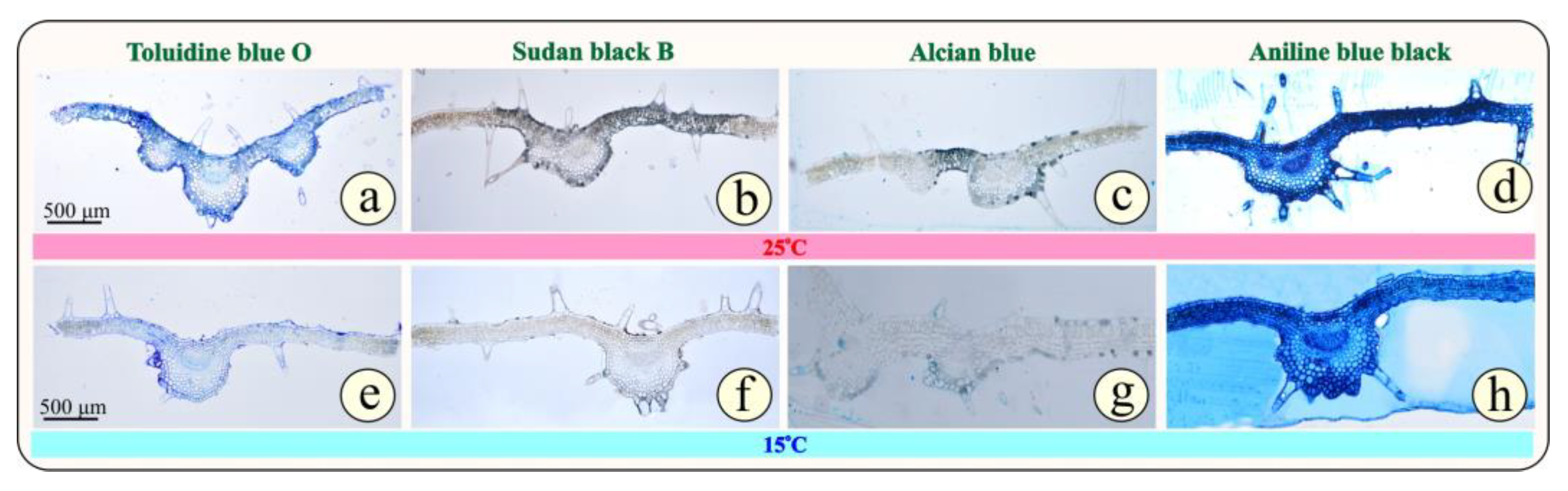
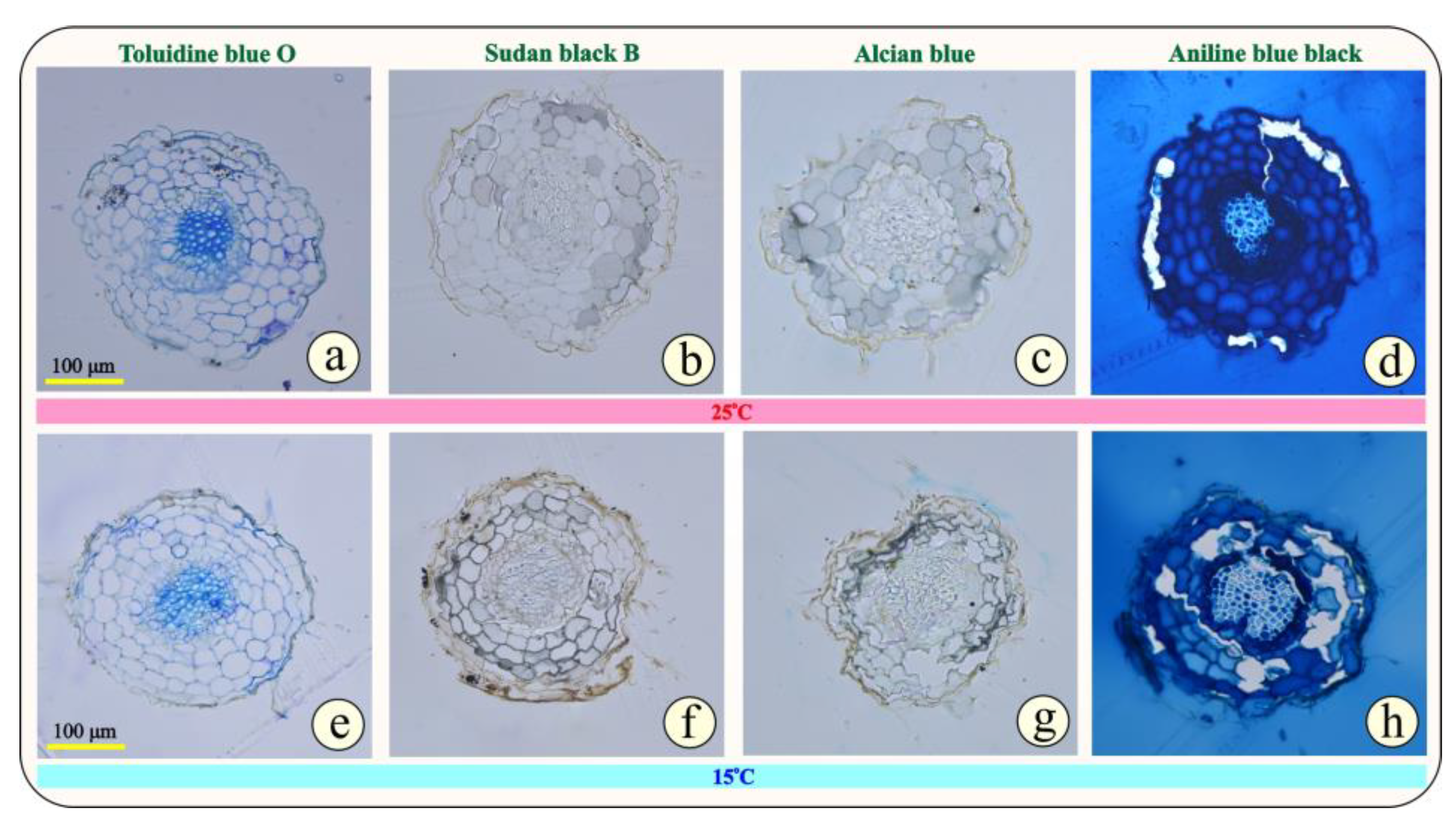
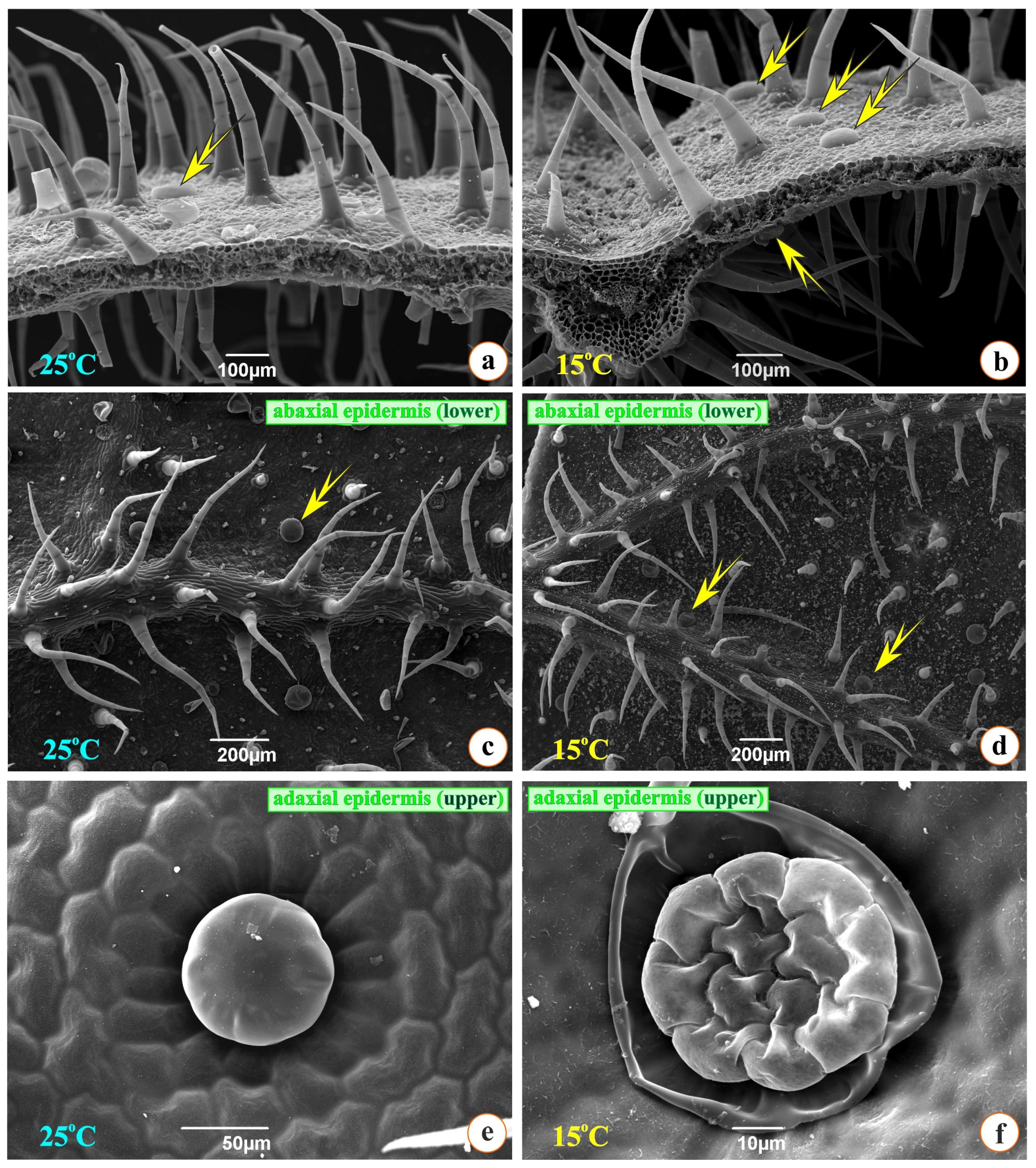
3.1. Anatomical Changes Under Cold Stress
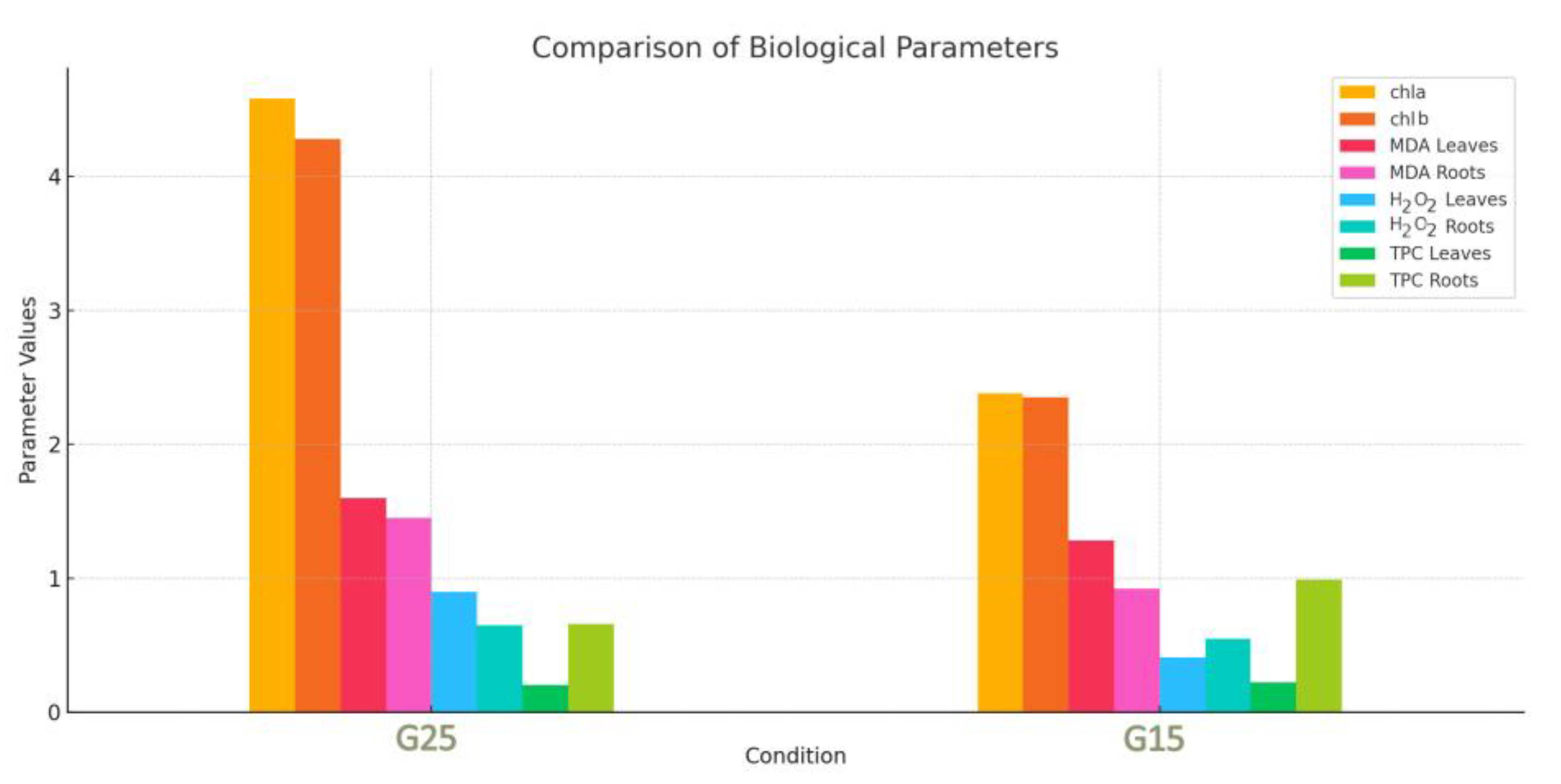
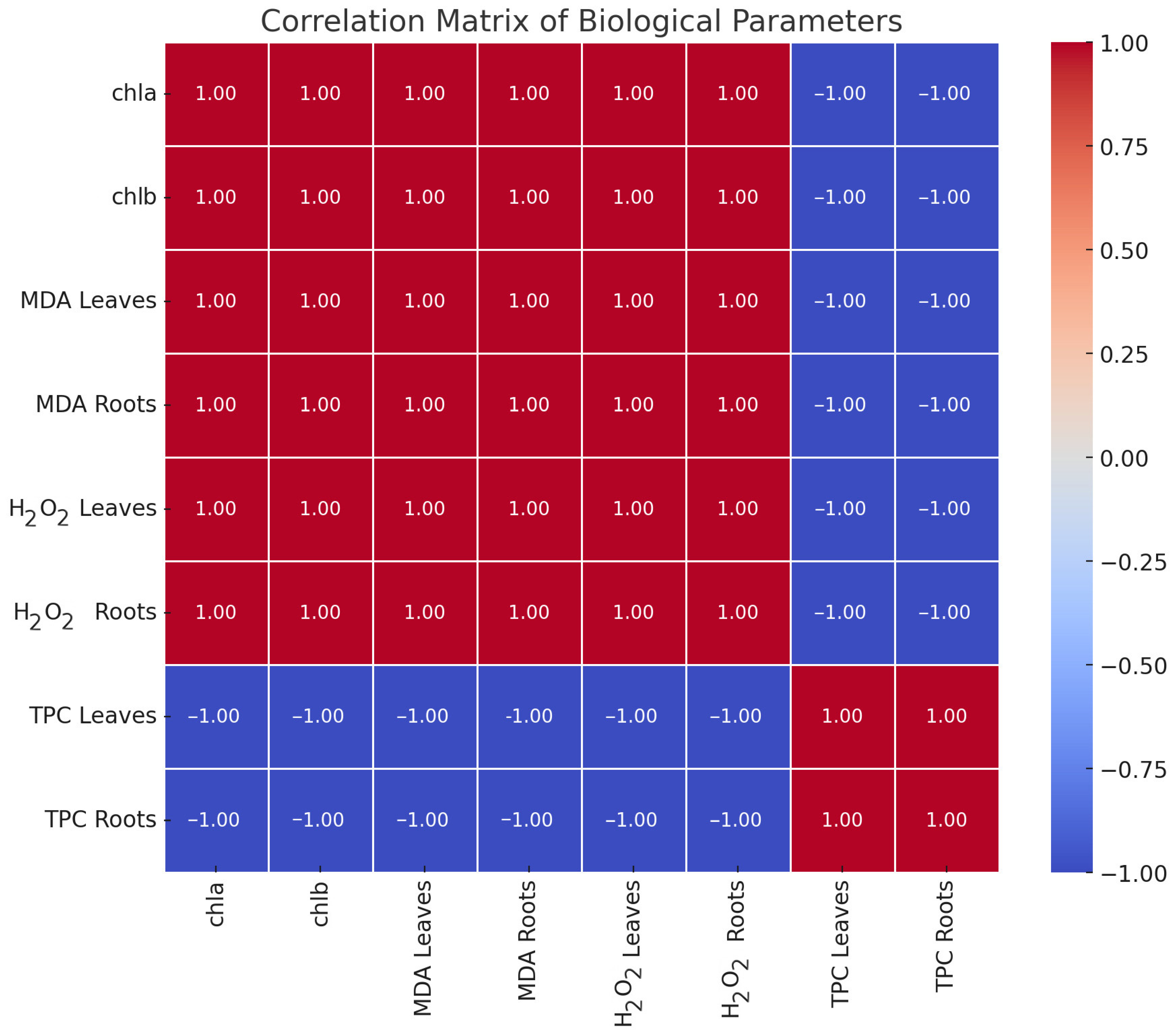
3.2. Physiology
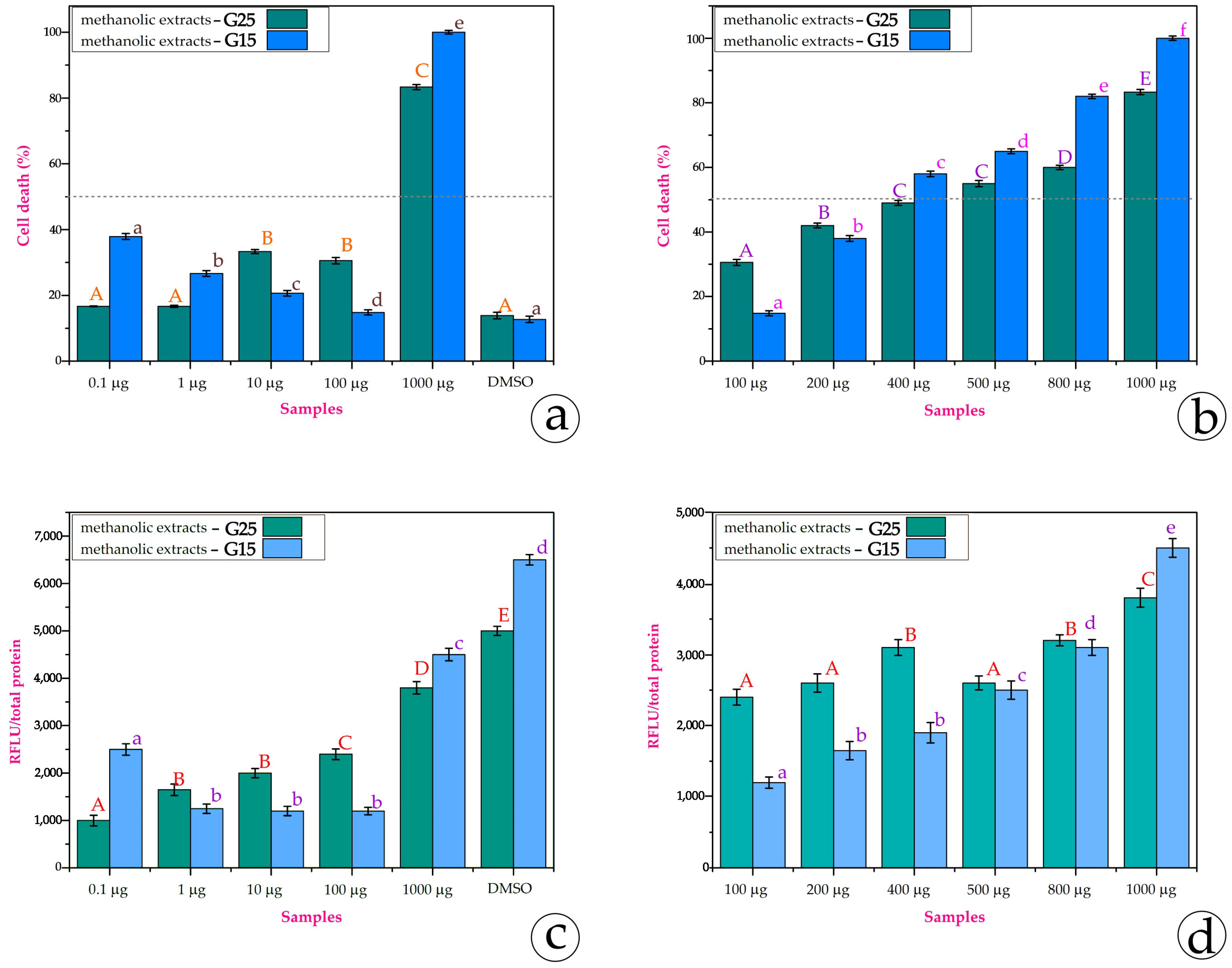
3.3. Cytotoxicity Assays
3.4. LC-HRMS/MS Analyses of Methanolic Extracts
4. Discussion
4.1. Anatomy and Microscopy
4.2. MDA, TPC, H2O2, and Photosynthetic Pigments
4.3. Bioassays
4.4. LC-HRMS/MS Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. Cluj. Napoca 2019, 47, 545–552. [Google Scholar] [CrossRef]
- Singletary, K. Oregano: Overview of the Literature on Health Benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Kintzios, S.E. Oregano. In Handbook of Herbs and Spices, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; Volume 2, pp. 417–436. [Google Scholar] [CrossRef]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M.A. Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. and Subspecies. Iran. J. Pharm. Res. 2021, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An Update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Ashfaqullah, S.; Tamta, S.; Scholar, R. Studies on High Value Medicinal Plant-Origanum vulgare L.: A Review. Int. J. Res. Anal. Rev. 2019, 6, 39–44. Available online: https://ijrar.org/papers/IJRAR19J1826.pdf (accessed on 18 January 2025).
- Soares, A.R.; Marchiosi, R.; Siqueira-Soares, R.C.; Barbosa de Lima, R.; Dantas dos Santos, W.; Ferrarese-Filho, O. The role of L-DOPA in plants. Plant Signal. Behav. 2014, 9, e28275. [Google Scholar] [CrossRef] [PubMed]
- Christodoulakis, N.S. An anatomical study of seasonal dimorphism in the leaves of Phlomis fruticosa. Ann. Bot. 1989, 63, 389–394. [Google Scholar] [CrossRef]
- Christodoulakis, N.S.; Bazos, J. Leaf Anatomy of three Seasonally Dimorphic. Subshrubs. Acta Oecol. 1990, 11, 291–296. [Google Scholar]
- Christodoulakis, N.S.; Fasseas, C. Air pollution effects on the leaf structure of Laurus nobilis, an injury resistant species. Bull. Environ. Contam. Toxicol. 1990, 44, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Mitrakos, K. A theory for Mediterranean plant life. Acta Oecol. Oec. Plant. 1980, 1, 245–252. [Google Scholar]
- Lattanzio, V.; Di Venere, D.; Linsalata, V.; Bertolini, P.; Ippolito, A.; Salerno, M. Low temperature metabolism of apple phenolics and quiescence of Phlyctaena vagabonda. J. Agric. Food Chem. 2001, 49, 5817–5821. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Ahlawat, Y.K.; Singh, M.; Manorama, K.; Lakra, N.; Zaid, A.; Zulfiqar, F. Plant phenolics: Neglected secondary metabolites in plant stress tolerance. Rev. Bras. Bot. 2023, 47, 703–721. [Google Scholar] [CrossRef]
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation–what is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Griffith, M.; Yaish, M.W.F. Antifreeze proteins in over wintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Elavarthi, S.; Martin, B. Spectrophotometric Assays for Antioxidant Enzymes in Plants. In Plant Stress Tolerance. Methods in Molecular Biology; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 273–281. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An Overview of Plant Phenolics and Their Involvement in Abiotic Stress Tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- OBrien, T.P.; McCully, M.E. The Study of Plant Structure Principles and Selected Methods. Termarcarphi Pty. Ltd., Melbourne. Scientific Research Publishing. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1453666 (accessed on 29 December 2024).
- Christodoulakis, N.S.; Kotsironi, K.; Tsafantakis, N.; Stefi, A.L.; Fokialakis, N. Leaf structure and phytochemical analysis of Aristolochia baetica, a traditionally used pharmaceutical plant. J. Herbs Spices Med. Plants 2019, 25, 88–103. [Google Scholar] [CrossRef]
- Bronner, R. Simultaneous Demonstration of Lipids and Starch in Plant Tissues. Stain. Technol. 1975, 50, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mowry, R.W. Alcian blue techniques for the histochemical study of acidic carbohydrates. J. Histochem. Cytochem. 1956, 4, 407–411. [Google Scholar]
- Fisher, D.B. Protein Staining of Ribboned Epon Sections for Light Microscopy. Histochemie 1968, 16, 92–96. [Google Scholar] [CrossRef]
- Gechev, T.; Mehterov, N.; Denev, I.; Hille, J. A Simple and Powerful Approach for Isolation of Arabidopsis Mutants with Increased Tolerance to H2O2-Induced Cell Death. Methods Enzymol. 2013, 527, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. Available online: https://www.researchgate.net/publication/313724134 (accessed on 18 January 2025). [CrossRef]
- Taulavuori, E.; Hellström, E.K.; Taulavuori, K.; Laine, K. Comparison of Two Methods Used to Analyse Lipid Peroxidation from Vaccinium myrtillus (L.) during Snow Removal, Reacclimation and Cold Acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Enhanced Reactive Oxygen Species Scavenging by Overproduction of Superoxide Dismutase and Catalase Delays Postharvest Physiological Deterioration of Cassava Storage Roots. Plant Physiol. 2013, 161, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 73–99. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2597356 (accessed on 18 January 2025).
- Erhonyota, C.; Edo, G.I.; Onoharigho, F.O.; Erhonyota, C.; Edo, G.I.; Onoharigho, F.O. Comparison of Poison Plate and Agar Well Diffusion Method Determining the Antifungal Activity of Protein Fractions. AcEcS 2023, 43, 684–689. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Comşa, Ş.; Cimpean, A.M.; Raica, M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer. Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Stefi, A.L.; Margaritis, L.H.; Skouroliakou, A.S.; Vassilacopoulou, D. Mobile phone electromagnetic radiation affects Amyloid Precursor Protein and α-synuclein metabolism in SH-SY5Y cells. Pathophysiology 2019, 26, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Christensen, M.E.; Waterhouse, N.J. Measuring Cell Death by Trypan Blue Uptake and Light Microscopy. Cold Spring Harb. Protoc. 2016, 2016, 643–646. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. SciKit-Learn: Machine Learning in Python. arXiv 2012, arXiv:1201.0490. [Google Scholar]
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical Composition and in Vitro Antimicrobial and Antioxidant Activities of Some Medicinal Plants. Food Chem. 2013, 136, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Dina, E.; Vontzalidou, A.; Cheilari, A.; Bagatzounis, P.; Agapidou, E.; Giannenas, I.; Grigoriadou, K.; Aligiannis, N. Sustainable use of Greek Herbs By-Products, as an alternative source of biologically active ingredients for innovative products. Front. Nutr. 2022, 9, 867666. [Google Scholar] [CrossRef]
- Gök, H.N.; Luca, S.V.; Ay, S.T.; Komsta, Ł.; Salmas, R.E.; Orhan, I.E.; Skalicka-Woźniak, K. Profiling the Annual Change of the Neurobiological and Antioxidant Effects of Five Origanum Species in Correlation with Their Phytochemical Composition. Food Chem. 2022, 368, 130775. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-Based Metabolite Profiling of Methanolic Extracts from the Medicinal and Aromatic Species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Al-Tameme, H.J.; Hameed, I.H.; Idan, S.A.; Hadi, M.Y.H. Biochemical analysis of Origanum vulgare seeds by fourier-transform infrared (FT-IR) spectroscopy and gas chromatography-mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 2015, 7, 221–237. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Mikropoulou, E.V.; Mitakou, S.; Halabalaki, M.; Kalpoutzakis, E. A GC-MS and LC-HRMS Perspective on the Chemotaxonomic Investigation of the Natural Hybrid Origanum × Lirium and Its Parents, O. vulgare Subsp. hirtum and O. scabrum. Phytochem. Anal. 2023, 34, 289–300. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Karioti, A.; Bergonzi, M.C.; Pescitelli, G.; Di Bari, L.; Skaltsa, H. Polar Constituents from the Aerial Parts of Origanum vulgare L. ssp. Hirtum Growing Wild in Greece. J. Agric. Food Chem. 2006, 54, 5388–5392. [Google Scholar] [CrossRef]
- Matsuura, H.; Chiji, H.; Asakawa, C.; Amano, M.; Yoshihara, T.; Mizutani, J. DPPH radical scavengers from dried leaves of oregano (Origanum vulgare). Biosci. Biotechnol. Biochem. 2003, 67, 2311–2316. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS Analysis and Biological Properties of Origanum vulgare Subsp. Viridulum Obtained by Different Extraction Methods. Ind. Crops Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- Liu, A.H.; Guo, H.; Ye, M.; Lin, Y.H.; Sun, J.H.; Xu, M.; Guo, D.A. Detection, Characterization and Identification of Phenolic Acids in Danshen Using High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 2007, 1161, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Ruiz-Medina, A.; Zengin, G.; Ak, G.; Jugreet, S.; Mahomoodally, M.F.; Emre, G.; Orlando, G.; Libero, M.L.; Nilofar; et al. New Biological and Chemical Evidences of Two Lamiaceae Species (Thymbra Capitata and Thymus Sipyleus Subsp. Rosulans): In Vitro, In Silico and Ex Vivo Approaches. Molecules 2022, 27, 9029. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of hairy root cultures of Salvia bulleyana Diels for production of polyphenolic compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, O.A.; Drioiche, A.; Remok, F.; Saidi, S.; Imache, A.E.; Makhoukhi, F.E.; Alsfouk, B.A.; Zair, T. Identification of compounds from Origanum compactum and Origanum elongatum using HPLC/UV-ESI-MS and comparative analysis of their antioxidant, antimicrobial, anticoagulant, and antidiabetic properties. Saudi Pharm. J. 2024, 32, 102184. [Google Scholar] [CrossRef]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Phenolic Compounds from Origanum vulgare and Their Antioxidant and Antiviral Activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Voynikov, Y.; Gevrenova, R.; Balabanova, V.A. Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS. Molecules 2024, 29, 204. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.Y.; Sun, Y.P.; Wang, Z.B.; Kuang, H.X. Moslae Herba: Botany, Traditional Uses, Phytochemistry, and Pharmacology. Molecules 2024, 29, 1716. [Google Scholar] [CrossRef]
- Picos-Salas, M.A.; Heredia, J.B.; Leyva-López, N.; Ambriz-Pérez, D.L.; Gutiérrez-Grijalva, E.P. Extraction Processes Affect the Composition and Bioavailability of Flavones from Lamiaceae Plants: A Comprehensive Review. Processes 2021, 9, 1675. [Google Scholar] [CrossRef]
- Onyebuchi, C.; Kavaz, D. Chitosan And N, N, N-Trimethyl Chitosan Nanoparticle Encapsulation Of Ocimum Gratissimum Essential Oil: Optimised Synthesis, In Vitro Release And Bioactivity. Int. J. Nanomed. 2019, 14, 7707–7727. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Wiśniewski, R. Determination of triterpenoids, carotenoids, chlorophylls, and antioxidant capacity in Allium ursinum L. at different times of harvesting and anatomical parts. Eur. Food Res. Technol. 2018, 244, 1269–1280. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Statti, G.A.; Conforti, F. Origanum spp.: An Update of Their Chemical and Biological Profiles. Phytochem. Rev. 2018, 17, 873–888. [Google Scholar] [CrossRef]
- Szczalba, M.; Kaffkova, K.; Kalisz, A.; Kopta, T.; Pokluda, R.; Sekara, A. Combined effect of chilling and light stress on the metabolic profile of Origanum vulgare L. in the juvenile stage. Fresenius Environ. Bull. 2019, 28, 3981–3990. Available online: https://www.researchgate.net/publication/332632062 (accessed on 18 January 2025).
- Sulaiman, H.Y.; Liu, B.; Abiola, Y.O.; Kaurilind, E.; Niinemets, Ü. Impact of Heat Priming on Heat Shock Responses in Origanum vulgare: Enhanced Foliage Photosynthetic Tolerance and Biphasic Emissions of Volatiles. Plant Physiol. Biochem. 2023, 196, 567–579. [Google Scholar] [CrossRef]
- Stefi, A.L.; Vassilacopoulou, D.; Routsi, E.; Stathopoulos, P.; Argyropoulou, A.; Skaltsounis, A.L.; Christodoulakis, N.S. The Combined Environmental Stress on the Leaves of Olea europaea L. and the Relief Mechanism Through Biosynthesis of Certain Secondary Metabolites. J. Plant Growth Regul. 2021, 40, 1044–1059. [Google Scholar] [CrossRef]
- dos Santos, H.T.; Sermarini, R.A.; Moreno-Pizani, M.A.; Marques, P.A.A. Effects of Irrigation Management and Seasonal Stages on Essential Oil Content and Biomass of Origanum vulgare L. Not. Sci. Biol. 2020, 12, 42–56. [Google Scholar] [CrossRef]
- Castro, M.D.M.; Demarco, D. Phenolic compounds produced by secretory structures in plants: A brief review. Nat. Prod. Commun. 2008, 3, 1273–1284. [Google Scholar]
- Gang, D.R.; Wang, J.; Dudareva, N.; Nam, K.H.; Simon, J.E.; Lewinsohn, E.; Pichersky, E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001, 125, 539–555. [Google Scholar] [CrossRef]
- Li, S.; Tosens, T.; Harley, P.C.; Jiang, Y.; Kanagendran, A.; Grosberg, M.; Jaamets, K.; Niinemets, Ü. Glandular trichomes as a barrier against atmospheric oxidative stress: Relationships with ozone uptake, leaf damage, and emission of LOX products across a diverse set of species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol agents and secondary metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 419–441. [Google Scholar]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Chalker-Scott, L.; Fuchigami, L.H. The Role of Phenolic Compounds in Plant Stress Responses. In Low Temperature Stress Physiology in Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–79. [Google Scholar] [CrossRef]
- Kofidis, G.; Bosabalidis, A.M. Seasonal Distribution of Phenolics in Leaves of Aromatic Plants (Origanum vulgare L., Mentha spicata L., Clinopodium vulgare L.) and Their Ecophysiological Implications. Biol. Lett. 2013, 49, 65–72. [Google Scholar] [CrossRef][Green Version]
- Christodoulakis, N.S.; Kogia, D.; Mavroeidi, D.; Fasseas, C. Anatomical and histochemical investigation of the leaf of Teucrium polium, a pharmaceutical sub-shrub of the Greek phryganic formations. J. Biol. Res. 2010, 14, 199–209. Available online: https://api.semanticscholar.org/CorpusID:55013901 (accessed on 18 January 2025).
- Węglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Baczek, K. The Quality of Greek Oregano (O. vulgare L. Subsp. Hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. Subsp. vulgare) Cultivated in the Temperate Climate of Central Europe. Foods 2020, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Moradipour, A.; Dariushnejad, H.; Ahmadizadeh, C.; Lashgarian, H.E. Dietary flavonoid carvacrol triggers the apoptosis of human breast cancer MCF-7 cells via the p53/Bax/Bcl-2 axis. Med. Oncol. 2022, 40, 46. [Google Scholar] [CrossRef]
- Cui, Z.W.; Xie, Z.X.; Wang, B.F.; Zhong, Z.H.; Chen, X.Y.; Sun, Y.H.; Sun, Q.F.; Yang, G.Y.; Bian, L.G. Carvacrol protects neuroblastoma SH-SY5Y cells against Fe(2+)-induced apoptosis by suppressing activation of MAPK/JNK-NF-κB signaling pathway. Acta Pharmacol. Sin. 2015, 36, 1426–1436. [Google Scholar] [CrossRef]
- Razak, N.A.; Abu, N.; Ho, W.Y.; Zamberi, N.R.; Tan, S.W.; Alitheen, N.B.; Long, K.; Yeap, S.K. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci. Rep. 2019, 9, 1514. [Google Scholar] [CrossRef]
- Berrington, D.; Lall, N. Anticancer Activity of Certain Herbs and Spices on the Cervical Epithelial Carcinoma (HeLa) Cell Line. Evid. Based Complement. Altern. Med 2012, 2012, 564927. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, J.; Campos-Xolalpa, N.; Serrano, R.; Pérez, C.; Pérez, S. Essential oils and monoterpenes as potential anti-cancer agents. In Frontiers in Clinical Drug Research. Anti-Cancer Agents (Print)/Frontiers in Clinical Drug Research. Anti-Cancer Agents; Atta-Ur-Rahman, Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; pp. 1–36. ISBN 978-1-68108-776-4. [Google Scholar] [CrossRef]
- Singh, P.; Kaushik, U.; Sharma, D. Overview on Phytochemistry and Pharmacological Activity of the Leaves of Origanum vulgare. Philipp. J. Sci. 2024, 153, 1757–1775. [Google Scholar]
- Gezici, S.; Turkmen, M.; Karahan, F. Exploring the Anti-Cancer Properties of Essential Oils from Some Lamiaceae Species against Human Cancer Cells with Multivariate Analysis. S. Afr. J. Bot. 2024, 166, 287–296. [Google Scholar] [CrossRef]
- Abdollahi-Ghehi, H.; Sonboli, A.; Ebrahimi, S.N.; Esmaeili, M.A.; Mirjalili, M.H. Triterpenic Acid Content and Cytotoxicity of Some Salvia Species from Iran. Nat. Prod. Commun. 2019, 14, 1934578X19842722. [Google Scholar] [CrossRef]
- Bitgen, N.; Baran, M.; Önder, G.Ö.; Suna, P.A.; Gürbüz, P.; Yay, A. Effect of Melissa officinalis L. on Human Breast Cancer Cell Line via Apoptosis and Autophagy. Cukurova Med. J. 2022, 47, 765–775. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food. Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Shiade, S.R.G.; Zand-Silakhoor, A.; Fathi, A.; Rahimi, R.; Minkina, T.; Rajput, V.D.; Zulfiqar, U.; Chaudhary, T. Plant metabolites and signaling pathways in response to biotic and abiotic stresses: Exploring bio stimulant applications. Plant Stress 2024, 12, 100454. [Google Scholar] [CrossRef]
- Sarac, N.; Ugur, A. Antimicrobial Activities and Usage in Folkloric Medicine of Some Lamiaceae Species Growing in Mugla, Turkey. EurAsi. J. BioSci. 2007, 4, 28–37. Available online: https://www.researchgate.net/publication/238763319 (accessed on 18 January 2025).
- Bloem, E.; Haneklaus, S.; Kleinwächter, M.; Paulsen, J.; Schnug, E.; Selmar, D. Stress-induced changes of bioactive compounds in Tropaeolum majus L. Ind. Crops Prod. 2014, 60, 349–359. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Robbins, J.; Fisher, C.; Moltz, A.; Martin, S. Elimination of Listeria monocytogenes Biofilms by Ozone, Chlorine, and Hydrogen Peroxide. J. Food Prot. 2005, 68, 494–498. [Google Scholar] [CrossRef]
- Chen, J.; Teng, J.; Ma, L.; Tong, H.; Ren, B.; Wang, L.; Li, W. Flavonoids Isolated from the Flowers of Limonium Bicolor and Their in Vitro Antitumor Evaluation. Pharmacogn. Mag. 2017, 13, 222–225. [Google Scholar] [CrossRef]
- Stanoevaa, J.P.; Stefova, M.; Andonovska, K.B.; Stafilova, T. LC/DAD/MS and ICP-AES Assay and Correlations between Phenolic Compounds and Toxic Metals in Endemic Thymus alsarensis from the Thallium Enriched Allchar Locality. Nat. Prod. Commun. 2017, 12, 167–170. [Google Scholar] [CrossRef]
- Lahlou, R.A.; Samba, N.; Soeiro, P.; Alves, G.; Gonçalves, A.C.; Silva, L.R.; Silvestre, S.; Rodilla, J.; Ismael, M.I. Thymus Hirtus Willd. Ssp. Algeriensis Boiss. and Reut: A Comprehensive Review on Phytochemistry, Bioactivities, and Health-Enhancing Effects. Foods 2022, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Pereira-Caro, G.; Moreno-Rojas, J.; Romano, A. Nutrient Deficiency-Induced Stress Improves Skincare Effects and Phytochemical Content of Green Extracts from Lamiaceae In Vitro Cultures. Horticulturae 2024, 10, 947. [Google Scholar] [CrossRef]
- Leng, X.; Kan, H.; Wu, Q.; Li, C.; Zheng, Y.; Peng, G. Inhibitory Effect of Salvia Miltiorrhiza Extract and Its Active Components on Cervical Intraepithelial Neoplastic Cells. Molecules 2022, 27, 1582. [Google Scholar] [CrossRef]
- Zeng, G.; Xiao, H.; Liu, J.; Liang, X. Identification of Phenolic Constituents in Radix Salvia Miltiorrhizae by Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Z.R.; Yang, X.H.; Xu, Y.; Ran, N.J.; Liu, M.J.; Jin, S.G.; Jia, H.N.; Zhang, Y. Monomethyl Lithospermate Alleviates Ischemic Stroke Injury in Middle Cerebral Artery Occlusion Mice in Vivo and Protects Oxygen Glucose Deprivation/Reoxygenation Induced SHSY-5Y Cells in Vitro via Activation of PI3K/Akt Signaling. Front. Pharmacol. 2022, 13, 1024439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.W.; Lau, K.M.; Hon, P.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Zhang, L.; Murray, F.; Yokouchi, H.; Zambon, A.C. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol. 2012, 204, 277–287. [Google Scholar] [CrossRef]
- Tzitiridou, P.; Zoi, V.; Papagrigoriou, T.; Lazari, D.; Sioka, C.; Alexiou, G.A.; Kyritsis, A.P. Antineoplastic Activity of 9″-Lithospermic Acid Methyl Ester in Glioblastoma Cells. Int. J. Mol. Sci. 2024, 25, 2094. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Khedr, A.H.A.; Abbas, M.A.; Abdel Wahid, A.A.; Quick, W.P.; Abogadallah, G.M. Proline Induces the Expression of Salt-stress-responsive Proteins and May Improve the Adaptation of Pancratium maritimum L. to Salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef] [PubMed]






| No | Indicator Strains | Accession Number | Culture Media | Incubation Temperature | Incubation Time |
|---|---|---|---|---|---|
| 1 | Bacillus subtilis | DSM10 | Luria–Bertani (LB) agar/broth | 30 °C | 24 h |
| 2 | Escherichia coli | DSM6897 | Luria–Bertani (LB) agar/broth | 37 °C | 24 h |
| 3 | Pseudomonas aeruginosa | DSM50071 | Luria–Bertani (LB) agar/broth | 30 °C | 24 h |
| 4 | Candida albicans | DSM1386 | YPD agar/broth | 37 °C | 48 h |
| 5 | Staphylococcus aureus | DSM346 | Luria–Bertani (LB) agar/broth | 37 °C | 24 h |
| 6 | Saccharomyces cerevisiae | DSM1333 | YPD agar/broth | 30 °C | 48 h |
| 7 | Xanthomonas campestris pv. campestris | 1656 BPIC | Luria–Bertani (LB) agar/broth | 30 °C | 24 h |
| 8 | Pseudomonas syrigae pv. syringae | - | Luria–Bertani (LB) agar/broth | 30 °C | 24 h |
| 9 | Erwinia amylovora | 842 BPIC | Luria–Bertani (LB) agar/broth | 30 °C | 48 h |
| Treatment | Biomass (g) of Above Ground Parts | Biomass (g) of Roots |
|---|---|---|
| Origanum vulgare G25 | 2.1 ± 0.1 * | 0.9 ± 0.1 |
| Origanum vulgare G15 | 2.7 ± 0.2 * | 1.2 ± 0.3 |
| Treatment | Number of Stomata/mm2—Abaxial | Number of Secretory Trichomes/mm2—Abaxial | Number of Secretory Trichomes/mm2—Adaxial |
|---|---|---|---|
| Origanum vulgare G25 | 325 ± 108 * | 125 ± 30 * | 69 ± 18 |
| Origanum vulgare G15 | 207 ± 66 * | 62 ± 42 * | 54 ± 28 |
| ID | Rt (min) | Elemental Composition | Experimental m/z [M − H]− | Experimental m/z[M + H]+ | RDBeq. Values | Δm (ppm) | Annotated Compound | HRMS/MS Ions | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.90 | C24H42O21 | 705.1843 [M + K]+ | 3.5 | −1.07 | Stachyose | 543.1321 (100) 705.1849 (82) | - | |
| 2 | 0.91 | C12H22O11 | 341.1083 | 2.5 | −1.75 | Bis-hexose | 59.0138 (100) 89.0243 (69) 71.0138 (51) 101.0243 (26) | [42] | |
| 3 | 0.92 | C7H12O6 | 191.0559 | 2.5 | −1.22 | Quinic acid | 191.0559 (100) 85.0294 (19) 93.0345 (6) 127.0399 (5) | [43,44] | |
| 4 | 0.92 | C18H32O16 | 503.1612 | 3.5 | Tris-hexose | 59.0138 (100) 89.0243 (81) 71.0138 (70) 221.0665 (54) 101.0243 (54) | [45] | ||
| 5 | 0.92 | C5H9NO2 | 116.0705 | 1.5 | −0.48 | L-proline | 70.0651 (100) 116.0706 (42) | - | |
| 6 | 4.67 | C9H8O4 | 179.0348 | 6.5 | −0.99 | Caffeic acid | 135.0450 (100) 179.0348 (17) | [46] | |
| 7 | 4.77 | C12H18SO7 | 305.0698 | 4.5 | −0.93 | 12-Sulfojasmonate | 96.96 (100) 305.0698 (37) 59.0138 (35) 225.1131 (34) 79.9573 (22) | - | |
| 8 | 4.93 | C27H30O15 | 593.1505 | 13.5 | −1.25 | Vicenin 2 | 353.0662 (100) 383.9767 (53) 473.1087 (52) 593.1505 (49) 297.0764 (27) | [46,47] | |
| 9 | 4.97 | C18H28O9 | 387.1653 | 5.5 | −1.84 | 12-Hydroxyjasmonic acid hexoside | 59.0138 (100) 387.1658 (41) 207.1025 (18) 89.0244 (16) 71.0138 (14) | [46] | |
| 10 | 5.48 | C12H18O4 | 225.1131 | 4.5 | −0.80 | 12-Hydroxyjasmonic acid | 59.0138 (100) 225.1134 (10) 97.0657 (5) | [44] | |
| 11 | 5.74 | C21H20O10 | 433.1127 | 11.5 | −0.44 | Isovitexin | 283.0601 (100) 313.0707 (92) 337.0709 (35) 379.0812 (20) 397.0916 (19) | [44] | |
| 12 | 5.78 | C20H22O11 | 437.1085 | 10.5 | −1.09 | Oreganol | 153.0192 (100) 109.0295 (27) | [48] | |
| 13 | 6.04 | C15H12O7 | 303.0508 | 10.5 | −0.91 | Taxifolin | 125.0243 (100) 285.0403 (31) 175.0397 (23) 57.0345 (19) 177.0192 (16) | [43,44] | |
| 14 | 6.43 | C29H32O16 | 635.1609 | 14.5 | 0.45 | Apigenin 7-O-hexosyl-acetyl-hexoside | 269.0453 (100) 268.0374 (11) | [49] | |
| 15 | 6.53 | C36H32O16 | 719.1606 | 21.5 | −1.63 | Sagerinic acid isomer | 161.0249 (100) 321.0394 (46) | [46] | |
| 16 | 6.54 | C18H16O8 | 359.0764 | 11.5 | −2.30 | Rosmarinic acid | 161.0244 (100) 197.0454 (24) 72.9930 (19) 178.0349 (18) 135.0451 (13) | [46] | |
| 17 | 6.56 | C26H22O10 | 493.1133 | 16.5 | −1.56 | Salvianolic acid A isomer | 295.0609 (100) 109.0294 (97) 185.0242 (50) 159.0450 (25) 135.0450 (25) | [46] | |
| 18 | 6.60 | C30H34O17 | 667.1866 | 13.5 | −0.44 | Diosmetin diglucoside I | 301.0706 (100) 286.0474 (15) 153.0184 (4) 203.0336 (3) | - | |
| 19 | 6.75 | C27H22O12 | 537.1031 | 17.5 | −1.64 | Lithospermic acid | 185.0244 (100) 109.0294 (70) 295.0611 (67) 135.0451 (39) 197.0453 (28) | [46] | |
| 20 | 6.77 | C36H30O16 | 717.1449 | 22.5 | −1.69 | Salvianolic acid B | 321.0402 (100) 229.0506 (30) 295.0609 (26) 109.0294 (19) 185.0242 (17) | [50] | |
| 21 | 7.01 | C28H30O15 | 605.1507 | 14.5 | −0.82 | Apigenin 7-O-pentosyl-acetyl-hexoside | 269.0453 (100) 268.0376 (17) | [46] | |
| 22 | 7.17 | C29H32O16 | 635.1613 | 14.5 | −0.79 | Diosmetin diglycoside II | 299.0558 (100) 284.0323 (80) 593.1501 (8) 575.1384 (4) | - | |
| 23 | 7.28 | C28H24O12 | 551.1188 | 17.5 | −1.20 | Monomethyl lithospermate | 321.0402 (100) 109.0294 (37) 293.0451 (32) 231.0298 (21) 277.0499 (16) | [51] | |
| 24 | 7.32 | C27H28O14 | 575.1403 | 14.5 | −0.64 | Apigenin diglycoside I | 515.1189 (100) 269.0452 (97) 268.0374 (83) 65.0032 (12) | - | |
| 25 | 7.38 | C15H12O6 | 287.0559 | 10.5 | −0.60 | Eriodictyol | 135.0451 (100) 151.0035 (76) | [46] | |
| 26 | 7.41 | C31H34O17 | 677.1717 | 15.5 | −0.93 | Apigenin diglycoside II | 269.0453 (100) 268.0376 (10) | ||
| 27 | 7.42 | C15H10O6 | 285.0402 | 11.5 | −0.95 | Luteolin | 285.0403 (100) 133.0294 (11) | [46] | |
| 28 | 7.46 | C15H10O7 | 301.0351 | 11.5 | −0.83 | Quercetin | 151.0036 (100) 301.0354 (65) 178.9985 (38) 65.0032 (21) 121.0293 (20) | [33] | |
| 29 | 7.49 | C28H30O15 | 605.1507 | 14.5 | −0.72 | Diosmetin 7-O-pentosyl-acetyl-pentoside | 299.0558 (100) 284.0322 (69) | [30] | |
| 30 | 7.50 | C30H34O16 | 649.1770 | 14.5 | −0.63 | Acacetin diglycoside I | 283.0609 (100) 268.0375 (50) | ||
| 31 | 7.53 | C27H30O14 | 579.1710 | 12.5 | 0.33 | Acacetin 7-O-pentosyl-hexoside | 285.0759 (100) 153.0182 (6) 315.0865 (6) | [49] | |
| 32 | 7.62 | C16H12O6 | 299.0559 | 11.5 | −0.68 | Hispidulin | 284.0323 (100) 299.0558 (45) 65.0032 (15) 136.9879 (14) | [46] | |
| 33 | 7.79 | C35H28O14 | 671.1392 | 22.5 | −0.55 | Salvianolic acid B decarboxylated isomer I | 321.0404 (100) 339.0508 (77) 293.0450 (44) 295.0609 (43) 109.0295 (27) | - | |
| 34 | 7.82 | C17H14O7 | 329.0665 | 11.5 | −0.54 | Thymusin | 314.0429 (100) 299.0197 (56) 271.0246 (41) 329.0663 (23) 65.0033 (15) | [43,46] | |
| 35 | 7.92 | C35H28O14 | 671.1402 | 22.5 | −0.64 | Salvianolic acid B decarboxylated isomer II | 321.0402 (100) 339.0509 (53) 295.0611 (36) 293.0452 (34) 185.0241 (23) | - | |
| 36 | 7.93 | C17H14O6 | 313.0714 | 11.5 | −1.12 | Salvianolic acid F isomer I | 161.0243 (100) 133.0294 (14) 151.0399 (9) | [52,53] | |
| 37 | 7.92 | C26H28O13 | 547.1453 | 13.5 | −0.71 | Acacetin diglycoside II | 283.0609 (100) 268.0376 (40) 65.0033 (6) | - | |
| 38 | 7.93 | C29H32O15 | 619.1663 | 14.5 | −0.84 | Acacetin 7-O-acetyl-hexosyl-pentoside | 283.0609 (100) 268.0376 (40) | [47,50] | |
| 39 | 8.09 | C15H10O5 | 269.0453 | 11.5 | −0.85 | Apigenin | 269.0453 (100) 117.0344 (19) 151.0037 (12) | [46] | |
| 40 | 8.12 | C15H12O5 | 271.0612 | 10.5 | −0.82 | Naringenin | 151.0035 (100) 119.0502 (74) 271.0611 (59) 114.9340 (58) 65.0033 (30) | [46] | |
| 41 | 8.14 | C29H32O15 | 619.1663 | 14.5 | −0.94 | Acacetin 7-O-pentosyl-acetyl-hexoside | 283.0609 (100) 268.0376 (40) | [54] | |
| 42 | 8.22 | C18H32O5 | 327.2173 | 3.5 | −1.35 | Trihydroxyoctadecadienoic acid | 327.2173 (100) 211.1337 (96) 229.1443 (60) 171.1024 (24) 221.1180 (15) | [42,55] | |
| 43 | 8.23 | C15H10O6 | 285.0401 | 11.5 | −1.26 | Kaempferol | 285.0403 (100) 65.0033 (3) | [46] | |
| 44 | 8.30 | C17H14O6 | 313.0714 | 11.5 | −1.22 | Salvianolic acid F isomer II | 161.0243 (100) 133.0294 (13) 151.0399 (7) | [52,53] | |
| 45 | 8.50 | C32H36O17 | 691.1873 | 15.5 | −0.96 | Acacetin diglycoside III | 283.0609 (100) 268.0374 (44) | - | |
| 46 | 8.52 | C28H30O14 | 589.1558 | 14.5 | −0.77 | Acacetin 7-O-acetyl-pentosyl-pentoside | 283.0610 (100) 268.0375 (33) | [56] | |
| 47 | 8.63 | C18H34O5 | 329.2332 | 2.5 | −0.49 | Trihydroxyoctadecenoic acid isomer I | 329.2331 (100) 211.1338 (57) 171.1025 (50) 229.1444 (31) 139.1127 (21) | [42,55] | |
| 48 | 8.95 | C31H34O16 | 661.1769 | 15.5 | −0.71 | Acacetin 7-O-acetyl-pentosyl-acetyl-hexoside | 283.0608 (100) 268.0373 (31) | [56] | |
| 49 | 9.99 | C16H12O5 | 285.0756 | 10.5 | −0.55 | Acacetin | 285.0760 (100) 270.0528 (6) 242.0581 (5) | [46] | |
| 50 | 10.04 | C18H16O7 | 345.0971 | 10.5 | −0.66 | Navadensin | 345.0972 (100) 315.0503 (45) 312.0631 (39) 71.0128 (22) 240.0784 (13) | [57] | |
| 51 | 11.07 | C21H36O4 | 353.2687 | 3.5 | 0.29 | 1-Monolinolenin | 81.07 (100) 67.0543 (88) 261.222 (78) 95.0856 (60) 93.0699 (48) 79.0544 (45) | [58] | |
| 52 | 11.07 | C33H56O14 | 699.3563 [M + Na]+ | 5.5 | −1.52 | DGMG (18:3)—Lipid | 699.3565 (100) 537.3035 (16) 700.3599 (6) 347.0955 (1) | - | |
| 53 | 11.24 | C27H48NO7P | 518.3242 | 3.5 | 0.20 | LysoPC (18:3) isomer I | 184.0735 (100) 104.1071 (76) 86.0965 (34) 98.9843 (22) 125.0000 (14) 60.0808 (13) | - | |
| 54 | 11.87 | C18H28O2 | 277.2163 [M−3H2O + H]+ | 4.5 | 0.04 | Trihydroxyoctadecenoic acid isomer II | 93.07 (100) 79.0543 (67) 121.1012 (66) 135.117 (55) 107.0857 (52) 81.07 (40) | - | |
| 55 | 11.96 | C26H50NO7P | 520.3398 | 2.5 | 0.15 | LysoPC (18:3) isomer II | 184.0735 (100) 104.107 (74) 86.0965 (30) 98.9842 (20) 124.9998 (16) 71.0730 (14) | - | |
| 56 | 11.97 | C18H28O2 | 277.2162 [M−3H2O + H]+ | 4.5 | 0.15 | Trihydroxyoctadecenoic acid isomer III | 93.0700 (100) 79.0543 (80) 135.1170 (64) 107.0856 (63) 121.1012 (51) 67.0543 (37) | - | |
| 57 | 12.16 | C21H36O4 | 353.2687 | 3.5 | 0.12 | 1-Monolinolenin isomer | 261.2213 (100) 81.0699 (86) 67.0543 (85) 95.0856 (77) 93.0700 (49)121.1013 (44) 109.1012 (43) 107.0856 (42) | - | |
| 58 | 12.29 | C31H58O14 | 677.3722 | 2.5 | 0.46 | DGMG (16:0)—Lipid | 677.3722 (100) 515.3192 (12) 678.3778 (6) 167.4701 (2) | - | |
| 59 | 12.51 | C24H50NO7P | 496.3399 | 0.5 | 0.34 | LysoPC (16:0) | 184.0735 (100) 104.1071 (70) 86.0965 (25) 98.9843 (18) 124.9999 (11) 71.0730 (11) | - | |
| 60 | 12.88 | C26H52NO7P | 522.3553 | 1.5 | −0.13 | LysoPC (18:1) | 184.0735 (100) 104.1070 (82) 86.0965 (31) 98.9843 (22) 124.9998 (13) 60.0808 (10) | - | |
| 61 | 14.89 | C30H48O3 | 439.3572 [M + H–H2O]+ | 7.5 | 0.41 | Oleanolic acid | 95.0856 (100) 137.1326 (86) 81.0699 (67) 123.1169 (49) 109.1013 (44) 67.0543 (22) 393.3518 (20) 55.0543 (19) | [46] | |
| 62 | 15.25 | C30H48O3 | 439.3569 [M + H–H2O]+ | 7.5 | −0.42 | Ursolic acid | 203.1796 (100) 189.1640 (74) 191.1796 (65) 95.0856 (57) 91.0543 (53) 109.1013 (40) 119.0856 (39) 121.1012 (38) | [46] | |
| 63 | 15.77 | C35H36N4O6 | 609.2707 | 19.5 | −0.12 | Epoxypheophorbide a | 609.2713 (100) 591.2606 (69) 531.2393 (62) 559.2344 (42) 515.2444 (22) 475.2132 (22) 476.2206 (17) 477.2289 (17) | - | |
| 64 | 16.06 | C35H36N4O5 | 593.2756 | 19.5 | −0.46 | Pheophorbide a | 593.2758 (100) 533.2549 (19) 594.2791 (10) 460.2258 (7) 461.2336 (6) 505.2226 (5) 476.2206 (17) 477.2289 (17) | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefi, A.L.; Chalkiadaki, M.; Dimitriou, K.; Mitsigiorgi, K.; Gkikas, D.; Papageorgiou, D.; Ntroumpogianni, G.C.; Vassilacopoulou, D.; Halabalaki, M.; Christodoulakis, N.S. Oregano Young Plants Cultured at Low Temperature Reveal an Enhanced Healing Effect of Their Extracts: Anatomical, Physiological and Cytotoxicity Approach. Metabolites 2025, 15, 103. https://doi.org/10.3390/metabo15020103
Stefi AL, Chalkiadaki M, Dimitriou K, Mitsigiorgi K, Gkikas D, Papageorgiou D, Ntroumpogianni GC, Vassilacopoulou D, Halabalaki M, Christodoulakis NS. Oregano Young Plants Cultured at Low Temperature Reveal an Enhanced Healing Effect of Their Extracts: Anatomical, Physiological and Cytotoxicity Approach. Metabolites. 2025; 15(2):103. https://doi.org/10.3390/metabo15020103
Chicago/Turabian StyleStefi, Aikaterina L., Maria Chalkiadaki, Katerina Dimitriou, Konstantina Mitsigiorgi, Dimitrios Gkikas, Danae Papageorgiou, Georgia C. Ntroumpogianni, Dido Vassilacopoulou, Maria Halabalaki, and Nikolaos S. Christodoulakis. 2025. "Oregano Young Plants Cultured at Low Temperature Reveal an Enhanced Healing Effect of Their Extracts: Anatomical, Physiological and Cytotoxicity Approach" Metabolites 15, no. 2: 103. https://doi.org/10.3390/metabo15020103
APA StyleStefi, A. L., Chalkiadaki, M., Dimitriou, K., Mitsigiorgi, K., Gkikas, D., Papageorgiou, D., Ntroumpogianni, G. C., Vassilacopoulou, D., Halabalaki, M., & Christodoulakis, N. S. (2025). Oregano Young Plants Cultured at Low Temperature Reveal an Enhanced Healing Effect of Their Extracts: Anatomical, Physiological and Cytotoxicity Approach. Metabolites, 15(2), 103. https://doi.org/10.3390/metabo15020103






