Multi-Omics Analysis Reveals Distinct Lipid Remodelling and Mitochondrial Stress in SH-SY5Y Cells Modelling Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability and Functional Assays
2.2.1. Cell Viability Assay
2.2.2. TMRM Assay
2.2.3. Seahorse XF Cell Mito Stress Test
2.2.4. MDA Assay
2.3. Molecular and Imaging Analyses
2.3.1. Confocal Imaging
2.3.2. Western Blotting
2.4. Lipidomics, Bioinformatics, and Statistical Analysis
2.4.1. Lipidomic Analysis
2.4.2. RNA Sequencing (RNA-Seq)
2.4.3. Statistical Analysis
3. Results
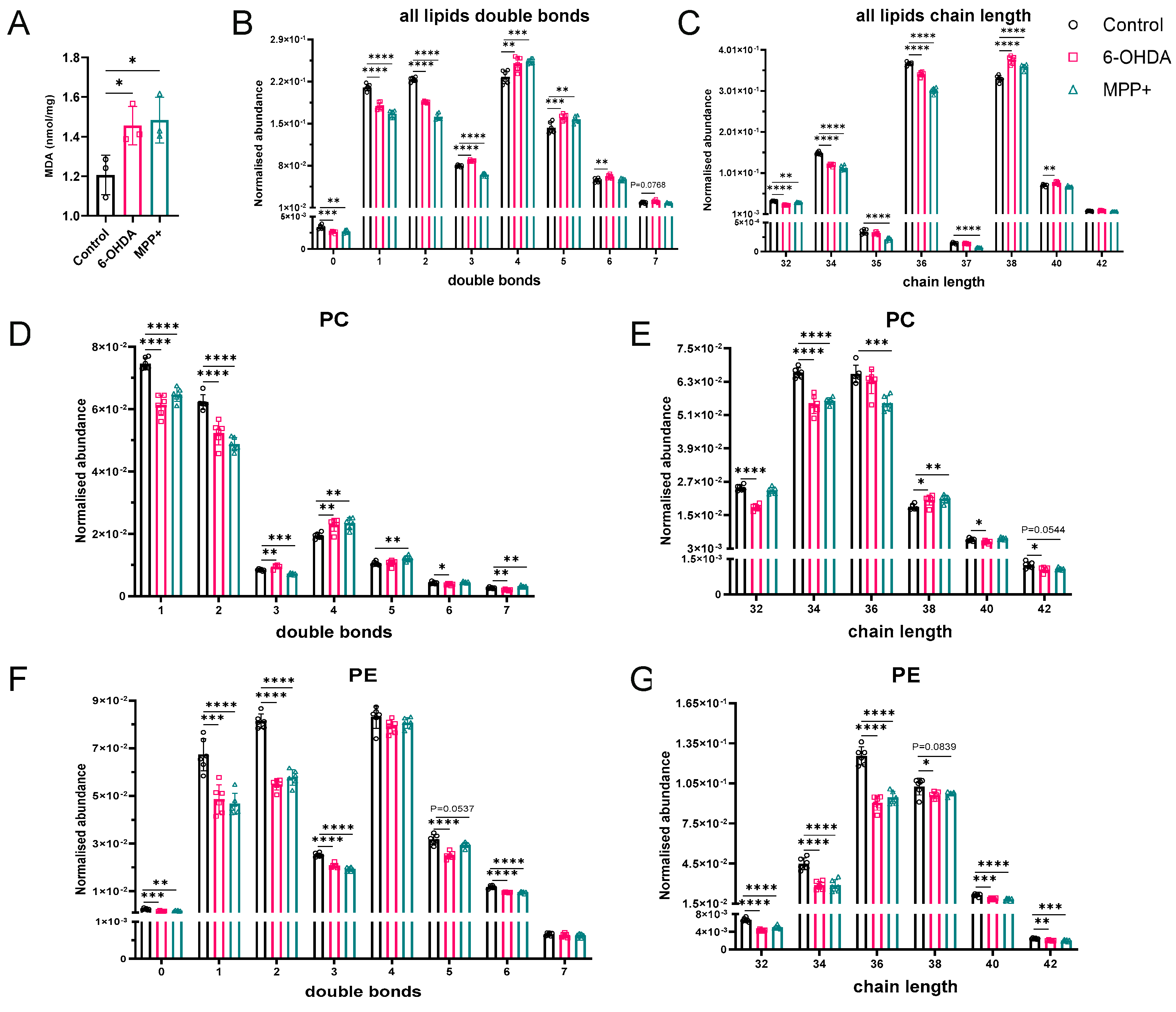
3.1. MPP+ Induces More Pronounced Mitochondrial Dysfunction than 6-OHDA
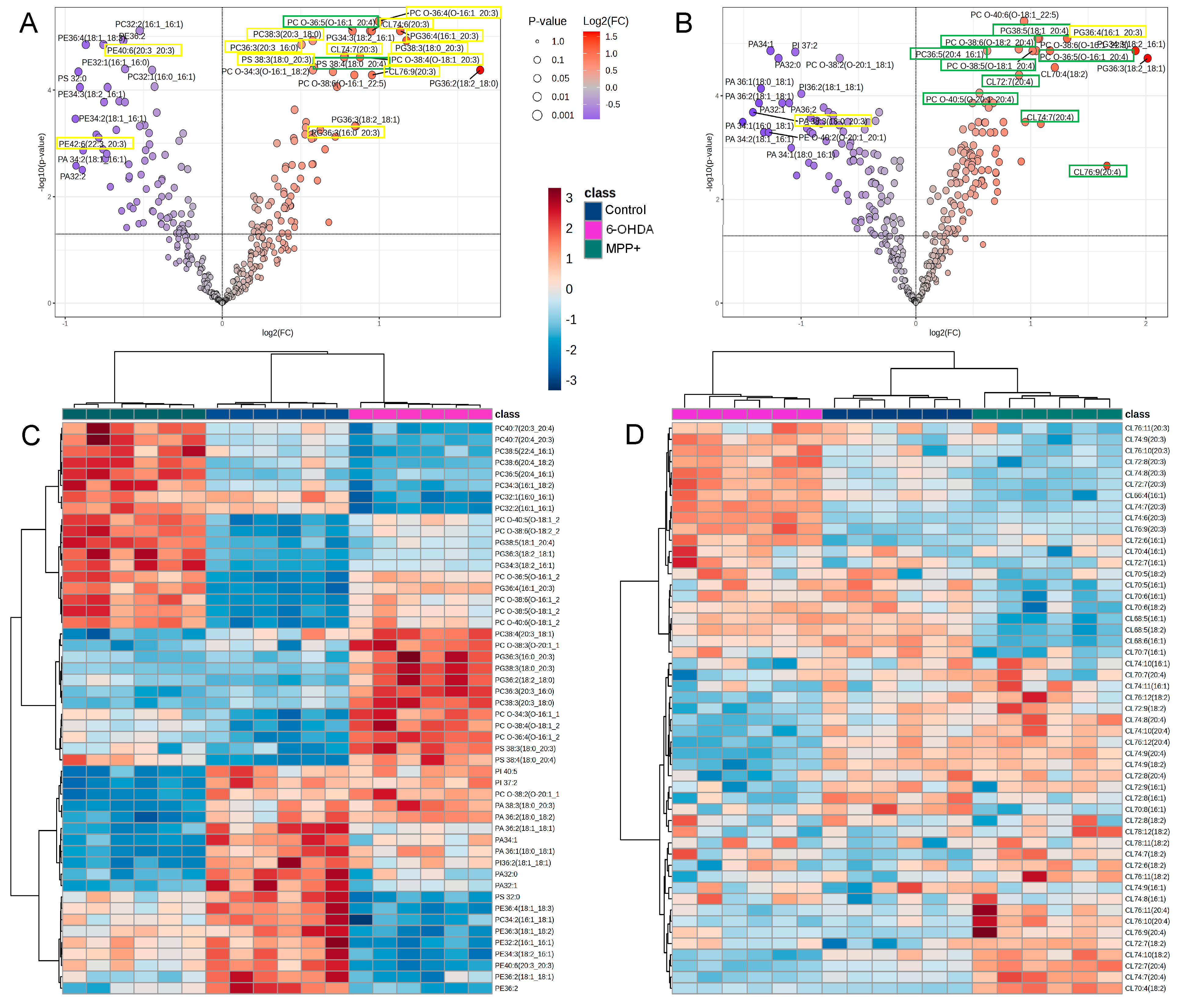
3.2. MPP+-Induced GPL Profiles Show Greater Concordance with PD Patient Brain than 6-OHDA, but Not with Serum
3.3. Acyl Chain Elongation and Unsaturation of Glycerophospholipids in 6-OHDA and MPP+ Models May Underlie Increased Susceptibility to Lipid Peroxidation
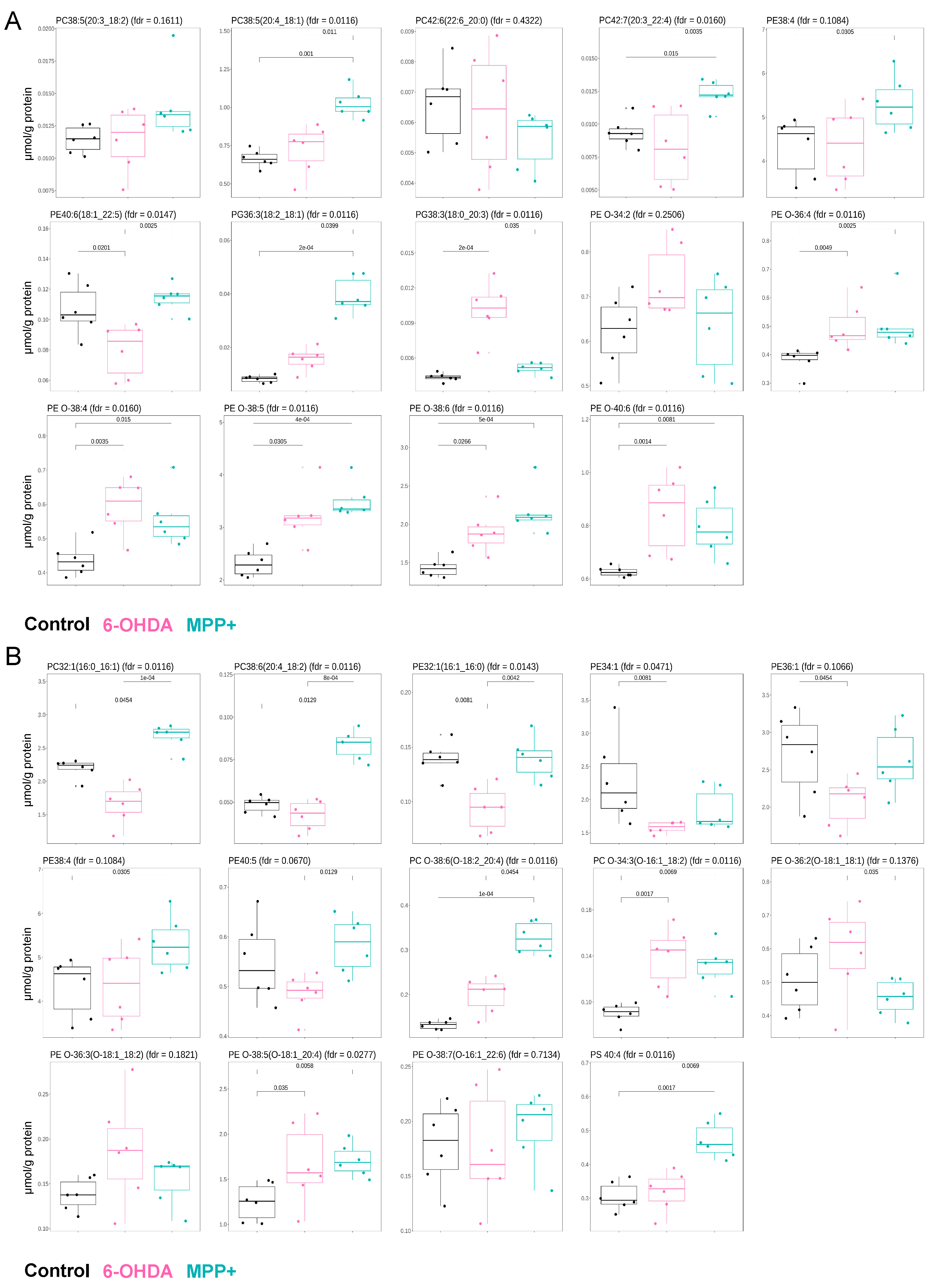
3.4. Commonly Used PD Models Induce Opposing GPL Remodelling Patterns
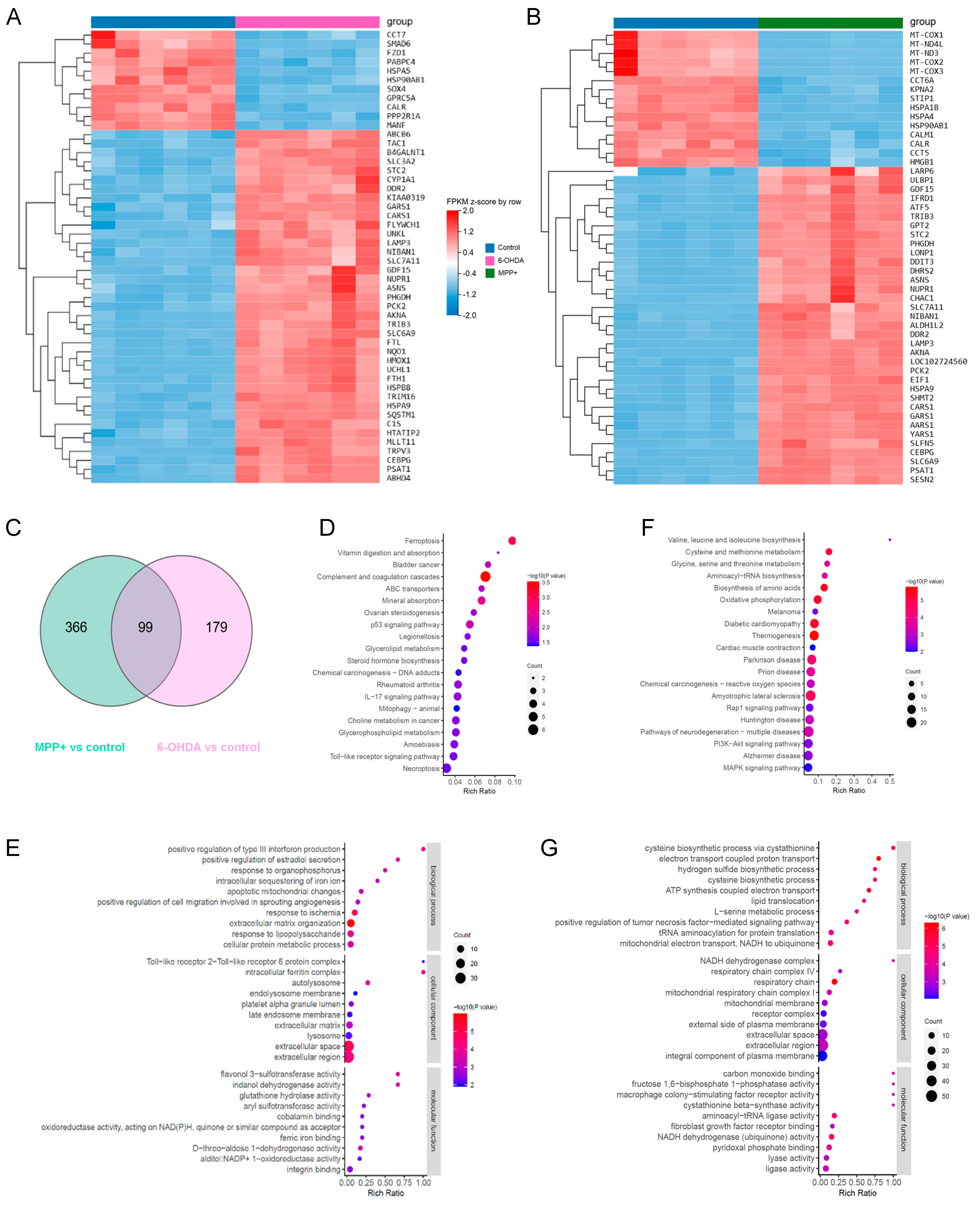
3.5. Transcriptomic Differences May Underlie Divergent GPL Remodelling in Different PD Models
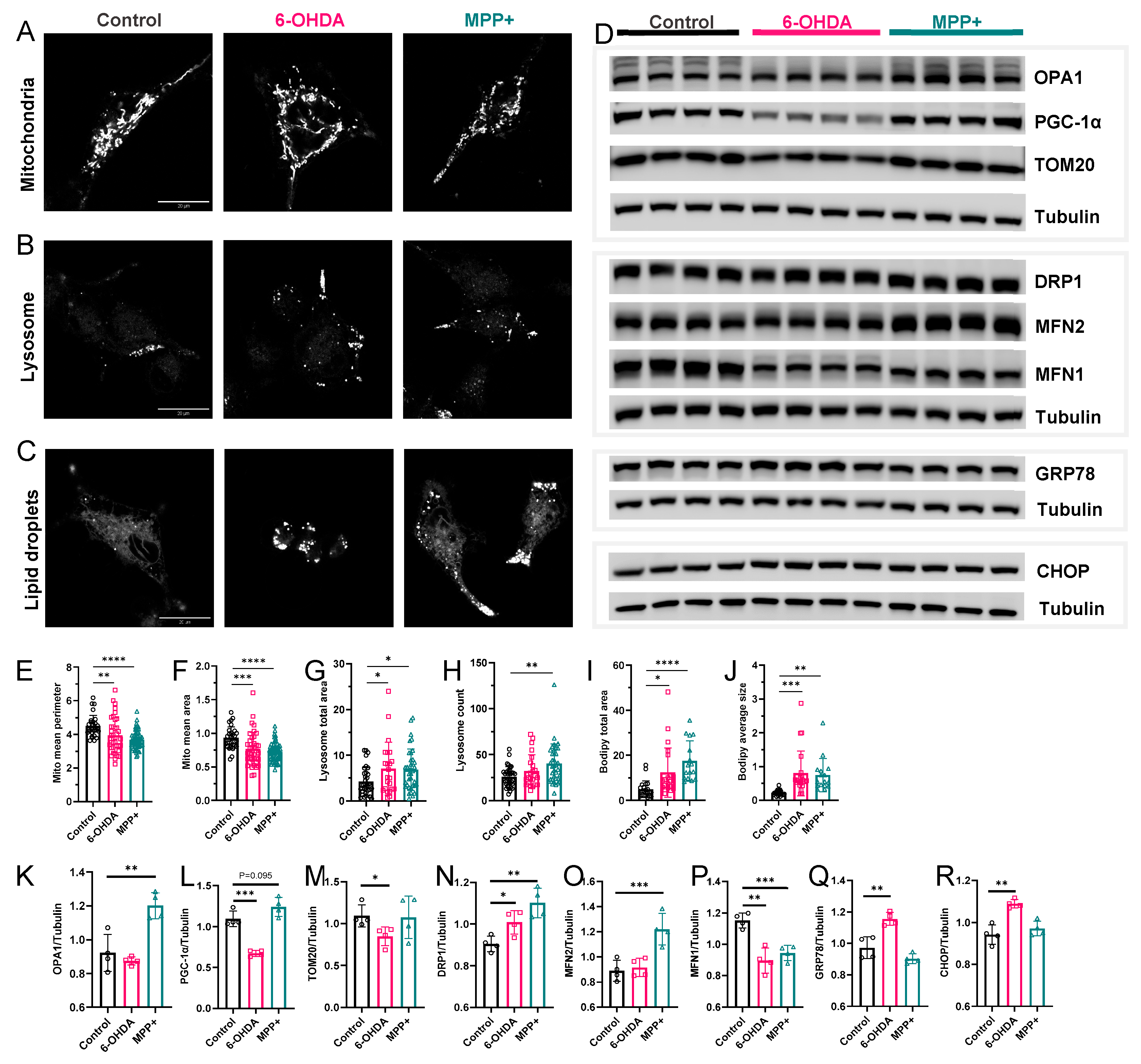
3.6. Organelle Stress Phenotypes Reveal Both Shared and Divergent Features in 6-OHDA and MPP+ Models
4. Discussion
4.1. GPL Signatures Reveal Model-Specific Acyl Chain Remodelling and Stronger Concordance of MPP+ with PD Brain Profiles
4.2. Integrated Transcriptomic and Organelle Stress Responses Support Lipid Remodelling Divergence
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of dopamine in the pathophysiology of Parkinson’s disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar]
- Hyon, J.-Y.; Lee, H.; Yun, S.; Han, E.H.; Chung, Y.-H. Comparative proteomics study of mitochondrial electron transport system modulation in SH-SY5Y cells following MPP+ versus 6-OHDA-induced neurodegeneration. J. Anal. Sci. Technol. 2023, 14, 1. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Qi, S.; Liu, Z.; Zhang, X.; Zheng, Y.; Andersen, J.P.; Zhang, W.; Strong, R.; Martinez, P.A.; et al. Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson’s diseases. Aging Cell 2019, 18, e12941. [Google Scholar] [CrossRef]
- Kaya, I.; Nilsson, A.; Luptáková, D.; He, Y.; Vallianatou, T.; Bjärterot, P.; Svenningsson, P.; Bezard, E.; Andrén, P.E. Spatial lipidomics reveals brain region-specific changes of sulfatides in an experimental MPTP Parkinson’s disease primate model. npj Park. Dis. 2023, 9, 118. [Google Scholar] [CrossRef]
- Xicoy, H.; Brouwers, J.F.; Kalnytska, O.; Wieringa, B.; Martens, G.J.M. Lipid Analysis of the 6-Hydroxydopamine-Treated SH-SY5Y Cell Model for Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 848–859. [Google Scholar] [CrossRef]
- Parrales, V.; Arcile, G.; Laserre, L.; Normant, S.; Le Goff, G.; Da Costa Noble, C.; Ouazzani, J.; Callizot, N.; Haik, S.; Rabhi, C.; et al. Neuroprotective Effect of Withaferin Derivatives toward MPP+ and 6-OHDA Toxicity to Dopaminergic Neurons. ACS Chem. Neurosci. 2025, 16, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.M.; Hung, S.Y. Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson’s Disease. Antioxidants 2020, 9, 1007. [Google Scholar] [CrossRef]
- Lu, X.; Kim-Han, J.S.; Harmon, S.; Sakiyama-Elbert, S.E.; O’Malley, K.L. The Parkinsonian mimetic, 6-OHDA, impairs axonal transport in dopaminergic axons. Mol. Neurodegener. 2014, 9, 17. [Google Scholar] [CrossRef]
- Mapa, M.S.T.; Le, V.Q.; Wimalasena, K. Characteristics of the mitochondrial and cellular uptake of MPP+, as probed by the fluorescent mimic, 4′I-MPP. PLoS ONE 2018, 13, e0197946. [Google Scholar] [CrossRef] [PubMed]
- Risiglione, P.; Leggio, L.; Cubisino, S.A.; Reina, S.; Paternò, G.; Marchetti, B.; Magrì, A.; Iraci, N.; Messina, A. High-Resolution Respirometry Reveals MPP+ Mitochondrial Toxicity Mechanism in a Cellular Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 7809. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Lee, J.; Darley-Usmar, V.M.; Zhang, J. Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS ONE 2012, 7, e44610. [Google Scholar] [CrossRef] [PubMed]
- Lotharius, J.; Dugan, L.L.; O’Malley, K.L. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J. Neurosci. 1999, 19, 1284–1293. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, F.; Yu, J.; Liang, Y.; Wu, Y. Investigating plasma lipid profiles in association with Parkinson’s disease risk. npj Park. Dis. 2025, 11, 99. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ashrafi, N.; Ashrafi, R.; Akyol, S.; Saiyed, N.; Kerševičiūtė, I.; Gabrielaite, M.; Gordevicius, J.; Graham, S.F. Lipid profiling of Parkinson’s disease brain highlights disruption in Lysophosphatidylcholines, and triacylglycerol metabolism. npj Park. Dis. 2025, 11, 159. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Nimer, R.M.; Rashed, M.; Wells, J.D.; Fiehn, O. Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease. Metabolites 2023, 13, 990. [Google Scholar] [CrossRef]
- Vos, M.; Klein, C.; Hicks, A.A. Role of Ceramides and Sphingolipids in Parkinson’s Disease. J. Mol. Biol. 2023, 435, 168000. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Pan, M.H.; Sun, J.; Wang, M.; Huang, Z.H.; Wang, G.; Wang, R.; Gong, H.B.; Huang, R.T.; Huang, F.; et al. Membrane phospholipid peroxidation promotes loss of dopaminergic neurons in psychological stress-induced Parkinson’s disease susceptibility. Aging Cell 2023, 22, e13970. [Google Scholar] [CrossRef]
- Pennington, E.R.; Funai, K.; Brown, D.A.; Shaikh, S.R. The role of cardiolipin concentration and acyl chain composition on mitochondrial inner membrane molecular organization and function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1039–1052. [Google Scholar] [CrossRef]
- Ji, J.; Kline, A.E.; Amoscato, A.; Samhan-Arias, A.K.; Sparvero, L.J.; Tyurin, V.A.; Tyurina, Y.Y.; Fink, B.; Manole, M.D.; Puccio, A.M.; et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 2012, 15, 1407–1413. [Google Scholar] [CrossRef]
- Lam, S.M.; Wang, Z.; Song, J.W.; Shi, Y.; Liu, W.Y.; Wan, L.Y.; Duan, K.; Chua, G.H.; Zhou, Y.; Wang, G.; et al. Non-invasive lipid panel of MASLD fibrosis transition underscores the role of lipoprotein sulfatides in hepatic immunomodulation. Cell Metab. 2025, 37, 69–86.e7. [Google Scholar] [CrossRef]
- Miao, H.; Li, B.; Wang, Z.; Mu, J.; Tian, Y.; Jiang, B.; Zhang, S.; Gong, X.; Shui, G.; Lam, S.M. Lipidome Atlas of the Developing Heart Uncovers Dynamic Membrane Lipid Attributes Underlying Cardiac Structural and Metabolic Maturation. Research 2022, 2022, 0006. [Google Scholar] [CrossRef]
- Lam, S.M.; Zhang, C.; Wang, Z.; Ni, Z.; Zhang, S.; Yang, S.; Huang, X.; Mo, L.; Li, J.; Lee, B.; et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 2021, 3, 909–922. [Google Scholar] [CrossRef]
- Mallick, K.; Paul, S.; Banerjee, S.; Banerjee, S. Lipid Droplets and Neurodegeneration. Neuroscience 2024, 549, 13–23. [Google Scholar] [CrossRef]
- Navarro-Romero, A.; Fernandez-Gonzalez, I.; Riera, J.; Montpeyo, M.; Albert-Bayo, M.; Lopez-Royo, T.; Castillo-Sanchez, P.; Carnicer-Caceres, C.; Arranz-Amo, J.A.; Castillo-Ribelles, L.; et al. Lysosomal lipid alterations caused by glucocerebrosidase deficiency promote lysosomal dysfunction, chaperone-mediated-autophagy deficiency, and alpha-synuclein pathology. npj Park. Dis. 2022, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Domingues, M.R.; Moreira, P.I.; Pereira, C.F. A Perspective on the Link between Mitochondria-Associated Membranes (MAMs) and Lipid Droplets Metabolism in Neurodegenerative Diseases. Biology 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Takai, T.; Hisatomi, H. Cell Death via Lipid Peroxidation and Protein Aggregation Diseases. Biology 2021, 10, 399. [Google Scholar] [CrossRef]
- Chen, Z.; Ho, I.L.; Soeung, M.; Yen, E.Y.; Liu, J.; Yan, L.; Rose, J.L.; Srinivasan, S.; Jiang, S.; Edward Chang, Q.; et al. Ether phospholipids are required for mitochondrial reactive oxygen species homeostasis. Nat. Commun. 2023, 14, 2194. [Google Scholar] [CrossRef]
- Naudí, A.; Jové, M.; Ayala, V.; Portero-Otín, M.; Barja, G.; Pamplona, R. Membrane lipid unsaturation as physiological adaptation to animal longevity. Front. Physiol. 2013, 4, 372. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging mechanisms of lipid peroxidation in regulated cell death and its physiological implications. Cell Death Dis. 2024, 15, 859. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Murawska, G.M.; Armando, A.M.; Dennis, E.A. Lipidomics of phospholipase A2 reveals exquisite specificity in macrophages. J. Lipid Res. 2024, 65, 100571. [Google Scholar] [CrossRef]
- Perez, M.A.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells. Dev. Cell 2020, 54, 447–454.e4. [Google Scholar] [CrossRef] [PubMed]
- Magrinelli, F.; Mehta, S.; Di Lazzaro, G.; Latorre, A.; Edwards, M.J.; Balint, B.; Basu, P.; Kobylecki, C.; Groppa, S.; Hegde, A.; et al. Dissecting the Phenotype and Genotype of PLA2G6-Related Parkinsonism. Mov. Disord. 2022, 37, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Chisaguano, A.M.; Montes, R.; Pérez-Berezo, T.; Castellote, A.I.; Guerendiain, M.; Bustamante, M.; Morales, E.; García-Esteban, R.; Sunyer, J.; Franch, À.; et al. Gene expression of desaturase (FADS1 and FADS2) and Elongase (ELOVL5) enzymes in peripheral blood: Association with polyunsaturated fatty acid levels and atopic eczema in 4-year-old children. PLoS ONE 2013, 8, e78245. [Google Scholar] [CrossRef]
- Xicoy, H.; Klemann, C.J.H.M.; De Witte, W.; Martens, M.B.; Martens, G.J.M.; Poelmans, G. Shared genetic etiology between Parkinson’s disease and blood levels of specific lipids. npj Park. Dis. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Clostio, A.J.; Houston, I.R.; Ruiz, J.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Ether lipid deficiency disrupts lipid homeostasis leading to ferroptosis sensitivity. PLoS Genet. 2022, 18, e1010436. [Google Scholar] [CrossRef]
- Sun, D.; Wang, L.; Wu, Y.; Yu, Y.; Yao, Y.; Yang, H.; Hao, C. Lipid metabolism in ferroptosis: Mechanistic insights and therapeutic potential. Front. Immunol. 2025, 16, 1545339. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhai, W.; Lu, X.; Wang, G. The Cross-Links of Endoplasmic Reticulum Stress, Autophagy, and Neurodegeneration in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 691881. [Google Scholar] [CrossRef]
- Di Maio, R.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; et al. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 2016, 8, 342ra78. [Google Scholar] [CrossRef] [PubMed]
- Franco-Iborra, S.; Cuadros, T.; Parent, A.; Romero-Gimenez, J.; Vila, M.; Perier, C. Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death Dis. 2018, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wang, M.; Song, W.M.; Shen, Q.; McKenzie, A.; Choi, I.; Zhou, X.; Pan, P.Y.; Yue, Z.; et al. The landscape of multiscale transcriptomic networks and key regulators in Parkinson’s disease. Nat. Commun. 2019, 10, 5234. [Google Scholar] [CrossRef]
- Cappelletti, C.; Henriksen, S.P.; Geut, H.; Rozemuller, A.J.; van de Berg, W.D.; Pihlstrøm, L.; Toft, M. Transcriptomic profiling of Parkinson’s disease brains reveals disease stage specific gene expression changes. Acta Neuropathol. 2023, 146, 227–244. [Google Scholar] [CrossRef]
- Gai, C.; Feng, W.D.; Qiang, T.Y.; Ma, H.J.; Chai, Y.; Zhang, S.J.; Guo, Z.Y.; Hu, J.H.; Sun, H.M. Da-Bu-Yin-Wan and Qian-Zheng-San Ameliorate Mitochondrial Dynamics in the Parkinson’s Disease Cell Model Induced by MPP+. Front. Pharmacol. 2019, 10, 372. [Google Scholar] [CrossRef]
- Ulecia-Morón, C.; Bris, Á.G.; MacDowell, K.S.; Madrigal, J.L.; García-Bueno, B.; Leza, J.C.; Caso, J.R. Chronic mild stress disrupts mitophagy and mitochondrial status in rat frontal cortex. J. Transl. Med. 2025, 23, 580. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ni, Z.; Wang, G.; Zhang, J.; Tan, Y.; Hong, E.; Wang, Y.; Chen, H.; Hou, H.; Hu, Q. Multi-Omics Analysis Reveals Distinct Lipid Remodelling and Mitochondrial Stress in SH-SY5Y Cells Modelling Parkinson’s Disease. Metabolites 2025, 15, 781. https://doi.org/10.3390/metabo15120781
Wang S, Ni Z, Wang G, Zhang J, Tan Y, Hong E, Wang Y, Chen H, Hou H, Hu Q. Multi-Omics Analysis Reveals Distinct Lipid Remodelling and Mitochondrial Stress in SH-SY5Y Cells Modelling Parkinson’s Disease. Metabolites. 2025; 15(12):781. https://doi.org/10.3390/metabo15120781
Chicago/Turabian StyleWang, Shu, Zhen Ni, Gaoge Wang, Jingzheng Zhang, Yunfu Tan, Enliang Hong, Yunting Wang, Huan Chen, Hongwei Hou, and Qingyuan Hu. 2025. "Multi-Omics Analysis Reveals Distinct Lipid Remodelling and Mitochondrial Stress in SH-SY5Y Cells Modelling Parkinson’s Disease" Metabolites 15, no. 12: 781. https://doi.org/10.3390/metabo15120781
APA StyleWang, S., Ni, Z., Wang, G., Zhang, J., Tan, Y., Hong, E., Wang, Y., Chen, H., Hou, H., & Hu, Q. (2025). Multi-Omics Analysis Reveals Distinct Lipid Remodelling and Mitochondrial Stress in SH-SY5Y Cells Modelling Parkinson’s Disease. Metabolites, 15(12), 781. https://doi.org/10.3390/metabo15120781






