Serum Calprotectin is Associated with Overweight and Laboratory Markers of Glucose Metabolism in Apparently Healthy Young Adults—A Cross-Sectional Descriptive Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Anthropometric and Blood Pressure Measurements
2.3. Blood Samples Collection and Laboratory Tests
2.4. Definitions of Cardiometabolic Risk Factors
2.5. Statistical Analysis
3. Results
3.1. Clinical and Biochemical Characteristics of the Study Participants
3.2. Correlation Analysis
3.3. Relationship Between Serum Calprotectin Concentration and Risk Factors for Metabolic Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes |
| CVD | cardiovascular disease |
| CRP | C-reactive protein |
| IL-6 | interleukin 6 |
| IL-1 | interleukin 1 |
| IBD | inflammatory bowel disease |
| VAT | visceral adipose tissue |
| BMI | body mass index |
| FPG | fasting plasma glucose |
| HbA1c | glycated hemoglobin |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| WC | waist circumference |
| WHR | waist-hip ratio |
| hs-CRP | high sensitivity C-reactive protein |
| ELISA | enzyme-linked immunosorbent assay |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| LDL-C | low-density lipoprotein cholesterol |
| non-HDL-C | non-high-density lipoprotein cholesterol |
| HDL-C | high-density lipoprotein cholesterol |
| TC | total cholesterol |
| TG | triglycerides |
| RYGB | Roux-en-Y gastric bypass |
| IL-8 | interleukin 8 |
| VFA | visceral fat area |
| SFA | subcutaneous fat area |
| EAT | epicardial adipose tissue |
| CAD | coronary artery disease |
| IMT | intima media thickness |
| ASCVD | atherosclerotic cardiovascular disease |
References
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Vasques-Nóvoa, F.; Neves, J.S.; Zannad, F.; Leite-Moreira, A. Comparison of interleukin-6 and high-sensitivity C-reactive protein for cardiovascular risk assessment: Findings from the MESA study. Atherosclerosis 2024, 390, 117461. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef]
- Dajti, E.; Frazzoni, L.; Iascone, V.; Secco, M.; Vestito, A.; Fuccio, L.; Eusebi, L.H.; Fusaroli, P.; Rizzello, F.; Calabrese, C.; et al. Systematic review with meta-analysis: Diagnostic performance of faecal calprotectin in distinguishing inflammatory bowel disease from irritable bowel syndrome in adults. Aliment. Pharmacol. Ther. 2023, 58, 1120–1131. [Google Scholar] [CrossRef]
- Shi, J.-T.; Chen, N.; Xu, J.; Goyal, H.; Wu, Z.-Q.; Zhang, J.-X.; Xu, H.-G. Diagnostic Accuracy of Fecal Calprotectin for Predicting Relapse in Inflammatory Bowel Disease: A Meta-Analysis. J. Clin. Med. 2023, 12, 1206. [Google Scholar] [CrossRef]
- Manfredi, M.; Van Hoovels, L.; Benucci, M.; De Luca, R.; Coccia, C.; Bernardini, P.; Russo, E.; Amedei, A.; Guiducci, S.; Grossi, V.; et al. Circulating Calprotectin (cCLP) in autoimmune diseases. Autoimmun. Rev. 2023, 22, 103295. [Google Scholar] [CrossRef]
- Wirtz, T.H.; Buendgens, L.; Weiskirchen, R.; Loosen, S.H.; Haehnsen, N.; Puengel, T.; Abu Jhaisha, S.; Brozat, J.F.; Hohlstein, P.; Koek, G.; et al. Association of Serum Calprotectin Concentrations with Mortality in Critically Ill and Septic Patients. Diagnostics 2020, 10, 990. [Google Scholar] [CrossRef]
- Kruzliak, P.; Novák, J.; Novák, M.; Fodor, G.J. Role of calprotectin in cardiometabolic diseases. Cytokine Growth Factor Rev. 2014, 25, 67–75. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Fernández-Real, J.M.; Salvador, J.; et al. Increased levels of calprotectin in obesity are related to macrophage content: Impact on inflammation and effect of weight loss. Mol. Med. 2011, 17, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, R.; Kishida, K.; Nakatsuji, H.; Nakagawa, T.; Funahashi, T.; Shimomura, I. High circulating levels of S100A8/A9 complex (calprotectin) in male Japanese with abdominal adiposity and dysregulated expression of S100A8 and S100A9 in adipose tissues of obese mice. Biochem. Biophys. Res. Commun. 2012, 419, 782–789. [Google Scholar] [CrossRef]
- Zuo, Y.; NaveenKumar, S.K.; Navaz, S.; Liang, W.; Sugur, K.; Kmetova, K.; Ayers, C.R.; Kluge, L.; Chong, E.; Shah, A.M.; et al. Epidemiological and Translational Study of Calprotectin and Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2025, 10, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Ling, C.; Sun, Q.; Harb, R.; Ashmaig, M.; Warnick, R.; Sethi, A.; Fleming, J.K.; Otvos, J.D.; Meeusen, J.W.; et al. A New Equation for Calculation of Low-Density Lipoprotein Cholesterol in Patients With Normolipidemia and/or Hypertriglyceridemia. JAMA Cardiol. 2020, 5, 540–548. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar] [CrossRef]
- Abbott Laboratories. Architect Insulin [Package Insert]; ref. 8K41-28; Abbott Laboratories: Lake Bluff, IL, USA, 2015. [Google Scholar]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Abbott Laboratories. Multigent CRP Vario [Package Insert]; ref. 6K26-30; Abbott Laboratories: Lake Bluff, IL, USA, 2015. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Biasucci, L.M.; CDC; AHA. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Clinical use of inflammatory markers in patients with cardiovascular diseases: A background paper. Circulation 2004, 110, e560–e567. [Google Scholar] [CrossRef]
- Pedersen, L.; Nybo, M.; Poulsen, M.K.; Henriksen, J.E.; Dahl, J.; Rasmussen, L.M. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc. Disord. 2014, 14, 196. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Bourgonje, M.F.; Sokooti, S.; la Bastide-van Gemert, S.; Nilsen, T.; Hidden, C.; Gansevoort, R.T.; Mulder, D.J.; Hillebrands, J.L.; Bakker, S.J.L.; et al. Plasma Calprotectin and New-onset Type 2 Diabetes in the General Population: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2024, 110, e150–e159. [Google Scholar] [CrossRef]
- Ortega, F.J.; Sabater, M.; Moreno-Navarrete, J.M.; Pueyo, N.; Botas, P.; Delgado, E.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur. J. Endocrinol. 2012, 167, 569–578. [Google Scholar] [CrossRef]
- Grand, A.; Rochette, E.; Dutheil, F.; Gozal, D.; Calcaterra, V.; Berni Canani, R.; Cobanoglu, N.; Derikx, J.P.M.; Terrin, G.; Pereira, B.; et al. Body Mass Index and Calprotectin Blood Level Correlation in Healthy Children: An Individual Patient Data Meta-Analysis. J. Clin. Med. 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; De Amici, M.; Leonard, M.M.; De Silvestri, A.; Pelizzo, G.; Buttari, N.; Michev, A.; Leggio, M.; Larizza, D.; Cena, H. Serum Calprotectin Level in Children: Marker of Obesity and its Metabolic Complications. Ann. Nutr. Metab. 2018, 73, 177–183. [Google Scholar] [CrossRef]
- Yu, J.Y.; Choi, W.J.; Lee, H.S.; Lee, J.W. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine 2019, 98, e14740. [Google Scholar] [CrossRef]
- Pereira-Manfro, W.F.; Lima, G.R.; Nogueira Neto, J.F.; Portugal, M.R.C.; Milagres, L.G.; Bezerra, F.F.; Faerstein, E.; Koury, J.C. Association between visceral/subcutaneous adipose tissue ratio and plasma inflammatory markers and score for cardiovascular risk prediction in a Brazilian cohort: Pró-Saúde Study. Braz. J. Med. Biol. Res. 2021, 54, e11521. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Duan, H.; Han, D.; He, B.; Xie, X.J.; Lu, L.; Jiang, J.; Li, R.H. Epicardial fat in patients with metabolic syndrome: A systematic review and meta-analysis. Eur. J. Radiol. 2023, 167, 111056. [Google Scholar] [CrossRef]
- Kuleta, K.; Krauz, K.; Żmuda, J.; Momot, K.; Zarębiński, M.; Poprawa, I.; Wojciechowska, M. Pharmacological and Non-Pharmacological Interventions in Diabetes Mellitus: Effects on Epicardial Adipose Tissue. Int. J. Mol. Sci. 2025, 26, 9271. [Google Scholar] [CrossRef] [PubMed]
- Sedaia, E.S.; Revenco, V.; Ochisor, V. The role of metabolic syndrome, visceral obesity and insulin resistance in right and left ventricular hypertrophy. Atherosclerosis 2022, 355, 129. [Google Scholar] [CrossRef]
- Ionita, M.G.; Vink, A.; Dijke, I.E.; Laman, J.D.; Peeters, W.; van der Kraak, P.H.; Moll, F.L.; de Vries, J.P.; Pasterkamp, G.; de Kleijn, D.P. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.H.; Jian, W.X.; Li, H.L.; Hou, L.; Wei, Y.D.; Li, W.M.; Xu, Y.W. Increased serum myeloid-related protein 8/14 level is associated with atherosclerosis in type 2 diabetic patients. Cardiovasc. Diabetol. 2011, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.; Sundström, J.; Lind, L.; Larsson, A. Serum calprotectin levels in elderly males and females without bacterial or viral infections. Clin. Biochem. 2014, 47, 1065–1068. [Google Scholar] [CrossRef]
- Gursoy, A.Y.; Caglar, G.S.; Kiseli, M.; Pabuccu, E.; Candar, T.; Demirtas, S. CRP at early follicular phase of menstrual cycle can cause misinterpretation for cardiovascular risk assessment. Interv. Med. Appl. Sci. 2015, 7, 143–146. [Google Scholar] [CrossRef]
- Masama, C.; Jarkas, D.A.; Thaw, E.; Daneshmend, A.Z.B.; Franklyn, S.I.; Beaurepaire, C.; McQuaid, R.J. Hormone contraceptive use in young women: Altered mood states, neuroendocrine and inflammatory biomarkers. Horm. Behav. 2022, 144, 105229. [Google Scholar] [CrossRef]
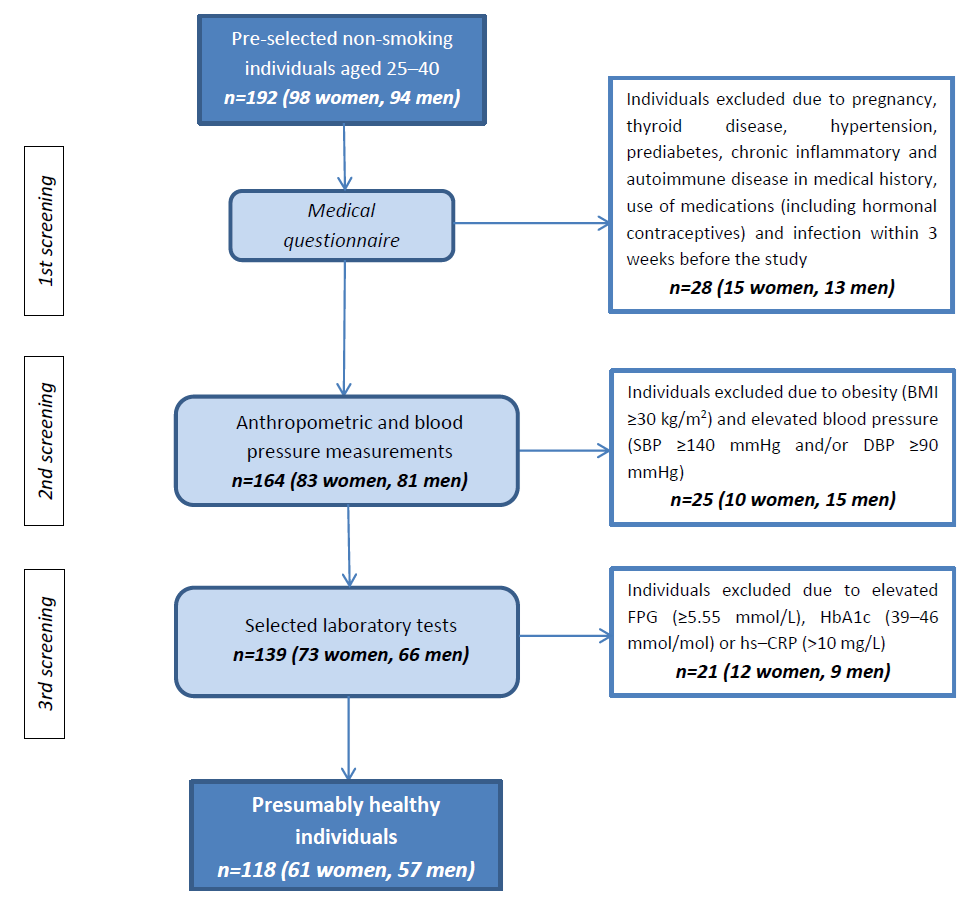

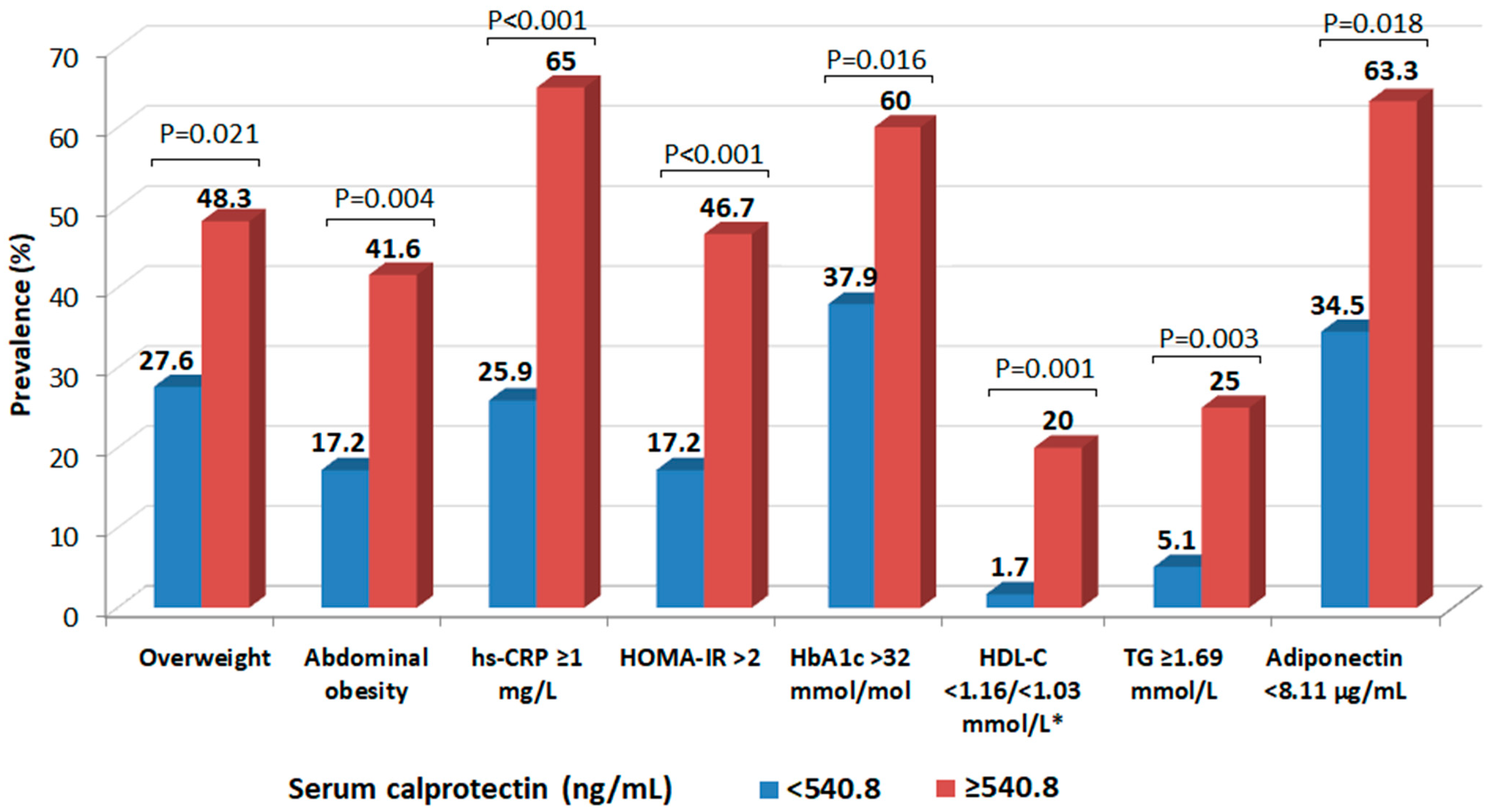
| Variables | All (n = 118) | Women (n = 61) | Men (n = 57) | p * | Normal Weight (n = 90) | Overweight (n = 28) | p ** |
|---|---|---|---|---|---|---|---|
| Age (years) | 30.5 (26–34) | 31 (26–36) | 30 (27–33) | 0.901 | 29 (26–33) | 33 (28–36) | 0.001 |
| BMI (kg/m2) | 23.8 (21.1–25.9) | 21.6 (20.3–23.8) | 24.5 (22.9–26.7) | <0.001 | 21.3 (20.1–23.4) | 26.8 (25.7–28.1) | <0.001 |
| Overweight (n; [%]) | 28; [23.7] | 10; [16.4] | 18; [31.6] | 0.052 | --- | --- | --- |
| WC (cm) | 82.5 (72–92) | 72 (69–79) | 91 (87–96) | <0.001 | 73 (69–82) | 92 (89–97) | <0.001 |
| WHR | 0.83 (0.76–0.88) | 0.77 (0.74–0.79) | 0.88 (0.86–0.91) | <0.001 | 0.77 (0.74–0.84) | 0.88 (0.84–0.92) | <0.001 |
| Abdominal obesity (n; [%]) | 32; [27.1] | 13; [21.3] | 19; [33.3] | 0.143 | 12; [13.3] | 20 [71.4] | <0.001 |
| SBP (mmHg) | 116 (104–128) | 110 (102–119) | 126 (115–135) | <0.001 | 118 (111–128) | 128 (123–135) | <0.001 |
| DBP (mmHg) | 77 (68–84) | 75 (66–82) | 82 (73–88) | <0.001 | 79 (75–85) | 82 (78–87) | 0.017 |
| Variables | All (n = 118) | Women (n = 61) | Men (n = 57) | p * | Normal Weight (n = 90) | Overweight (n = 28) | p ** |
|---|---|---|---|---|---|---|---|
| FPG (mmol/L) | 5.00 (4.83–5.33) | 4.94 (4.72–5.16) | 5.22 (5.00–5.44) | <0.001 | 5.06 (4.78–5.28) | 5.17 (4.89–5.39) | 0.001 |
| HbA1c (mmol/mol) | 32 (30–34) | 32 (29–33) | 33 (31–36) | <0.001 | 32 (30–34) | 33 (30–36) | 0.423 |
| Insulin (µU/mL) | 7.30 (5.37–10.01) | 6.44 (5.03–8.14) | 8.65 (6.27–11.21) | 0.001 | 6.82 (4.68–8.77) | 7.89 (5.95–11.06) | 0.011 |
| HOMA-IR | 1.67 (1.20–2.25) | 1.46 (1.13–1.79) | 1.90 (1.41–2.54) | <0.001 | 1.48 (1.11–1.94) | 1.86 (1.35–2.50) | 0.008 |
| hs-CRP (mg/L) | 0.65 (0.40–1.50) | 0.50 (0.30–1.80) | 0.75 (0.40–1.40) | 0.374 | 0.50 (0.30–1.40) | 1.00 (0.55–1.70) | 0.007 |
| TC (mmol/L) | 4.91 (4.40–5.40) | 4.96 (4.40–5.30) | 4.89 (4.40–5.40) | 0.821 | 4.86 (4.14–5.20) | 4.99 (4.42–5.71) | 0.043 |
| LDL-C (mmol/L) | 2.97 (2.59–3.49) | 2.87 (2.40–3.21) | 3.13 (2.61–3.67) | 0.062 | 2.87 (2.35–3.21) | 3.23 (2.66–3.83) | 0.004 |
| HDL-C (mmol/L) | 1.40 (1.16–1.60) | 1.55 (1.34–1.71) | 1.21 (1.11–1.42) | <0.001 | 1.47 (1.34–1.68) | 1.19 (1.09–1.42) | <0.001 |
| non-HDL-C (mmol/L) | 3.46 (3.00–4.03) | 3.36 (2.84–3.77) | 3.75 (3.23–4.16) | 0.010 | 3.36 (2.71–3.77) | 3.88 (3.26–4.39) | <0.001 |
| TG (mmol/L) | 0.92 (0.70–1.31) | 0.81 (0.63–1.08) | 1.02 (0.83–1.77) | <0.001 | 0.87 (0.70–1.12) | 1.41 (0.98–1.79) | <0.001 |
| Calprotectin (ng/mL) | 540.8 (451.4–634.0) | 528.6 (436.2–608.8) | 593.6 (483.6–689.0) | 0.016 | 496.0 (436.2–595.2) | 604.3 (500.6–814.5) | <0.001 |
| Adiponectin (µg/mL) | 8.11 (6.45–9.71) | 8.99 (7.48–10.45) | 7.01 (5.36–8.43) | <0.001 | 8.77 (7.15–10.37) | 6.83 (5.63–8.46) | <0.001 |
| Variables | All (n = 118) | Women (n = 61) | Men (n = 57) | p * | |||
|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | Women vs. Men | |
| Age | 0.06 | 0.535 | 0.13 | 0.310 | 0.02 | 0.892 | 0.559 |
| BMI | 0.44 | <0.001 | 0.48 | <0.001 | 0.37 | 0.004 | 0.478 |
| WC | 0.38 | <0.001 | 0.46 | <0.001 | 0.18 | 0.183 | 0.098 |
| WHR | 0.32 | <0.001 | 0.38 | <0.001 | 0.12 | 0.356 | 0.142 |
| SBP | 0.21 | 0.021 | 0.03 | 0.824 | 0.22 | 0.086 | 0.308 |
| DBP | 0.06 | 0.538 | 0.03 | 0.828 | 0.08 | 0.557 | 0.791 |
| FPG | 0.15 | 0.094 | 0.18 | 0.161 | 0.01 | 0.109 | 0.365 |
| HbA1c | 0.29 | 0.001 | 0.14 | 0.271 | 0.33 | 0.010 | 0.288 |
| Insulin | 0.29 | 0.001 | 0.28 | 0.023 | 0.20 | 0.130 | 0.654 |
| HOMA-IR | 0.30 | <0.001 | 0.30 | 0.017 | 0.15 | 0.253 | 0.404 |
| hs-CRP | 0.48 | <0.001 | 0.58 | <0.001 | 0.39 | 0.002 | 0.188 |
| TC | 0.11 | 0.289 | 0.08 | 0.537 | 0.07 | 0.615 | 0.958 |
| LDL-C | 0.05 | 0.603 | 0.06 | 0.609 | 0.09 | 0.517 | 0.873 |
| HDL-C | −0.29 | <0.001 | −0.13 | 0.293 | −0.32 | 0.012 | 0.290 |
| Non-HDL-C | 0.21 | 0.017 | 0.21 | 0.099 | 0.17 | 0.195 | 0.827 |
| TG | 0.34 | <0.001 | 0.24 | 0.061 | 0.36 | 0.004 | 0.486 |
| Adiponectin | −0.24 | 0.008 | −0.13 | 0.294 | −0.20 | 0.135 | 0.704 |
| Risk Factors | Unadjusted | Adjusted for Sex and BMI | Adjusted for Sex, BMI and CRP | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Overweight (BMI > 25 kg/m2) | 2.529 (1.194–5.357) | 0.015 | --- | --- | 1.787 (0.732–4.364) ** | 0.203 |
| Abdominal obesity (WC ≥ 80/≥94 cm, F/M) | 3.217 (1.409–7.348) | 0.006 | 1.518 (0.549–4.192) | 0.421 | 1.274 (0.452–3.590) | 0.647 |
| hs-CRP ≥ 1 mg/L | 5.000 (2.307–10.835) | <0.001 | 4.788 (2.111–10.864) | <0.001 | --- | --- |
| HbA1c > 32 mmol/mol * | 2.166 (1.018–4.608) | 0.021 | 2.484 (1.089–5.668) | 0.013 | 3.000 (1.256–7.169) | 0.011 |
| HOMA-IR > 2 | 4.394 (1.892–10.204) | <0.001 | 3.641 (1.465–9.052) | 0.005 | 3.233 (1.273–8.211) | 0.014 |
| TG ≥ 1.69 mmol/L | 1.609 (1.184–2.401) | 0.010 | 1.323 (1.017–1.583) | 0.047 | 1.255 (0.801–1.323) | 0.099 |
| HDL-C < 1.16/<1.03 mmol/L (F/M) | 1.127 (1.079–1.412) | 0.031 | 1.310 (0.934–1.542) | 0.114 | 1.064 (0.853–1.862) | 0.314 |
| Adiponectin < 8.11 µg/mL * | 1.647 (0.803–3.377) | 0.174 | 1.213 (0.526–2.799) | 0.650 | 0.975 (0.409–2.324) | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, K.; Stefańska, A.; Kuligowska-Prusińska, M.; Krintus, M. Serum Calprotectin is Associated with Overweight and Laboratory Markers of Glucose Metabolism in Apparently Healthy Young Adults—A Cross-Sectional Descriptive Study. Metabolites 2025, 15, 756. https://doi.org/10.3390/metabo15120756
Bergmann K, Stefańska A, Kuligowska-Prusińska M, Krintus M. Serum Calprotectin is Associated with Overweight and Laboratory Markers of Glucose Metabolism in Apparently Healthy Young Adults—A Cross-Sectional Descriptive Study. Metabolites. 2025; 15(12):756. https://doi.org/10.3390/metabo15120756
Chicago/Turabian StyleBergmann, Katarzyna, Anna Stefańska, Magdalena Kuligowska-Prusińska, and Magdalena Krintus. 2025. "Serum Calprotectin is Associated with Overweight and Laboratory Markers of Glucose Metabolism in Apparently Healthy Young Adults—A Cross-Sectional Descriptive Study" Metabolites 15, no. 12: 756. https://doi.org/10.3390/metabo15120756
APA StyleBergmann, K., Stefańska, A., Kuligowska-Prusińska, M., & Krintus, M. (2025). Serum Calprotectin is Associated with Overweight and Laboratory Markers of Glucose Metabolism in Apparently Healthy Young Adults—A Cross-Sectional Descriptive Study. Metabolites, 15(12), 756. https://doi.org/10.3390/metabo15120756






