The Crosstalk Between Brain Energy Metabolism and Neuropathic Pain: Mechanisms and Therapeutic Implications
Abstract
1. Introduction
2. Literature Search Methods
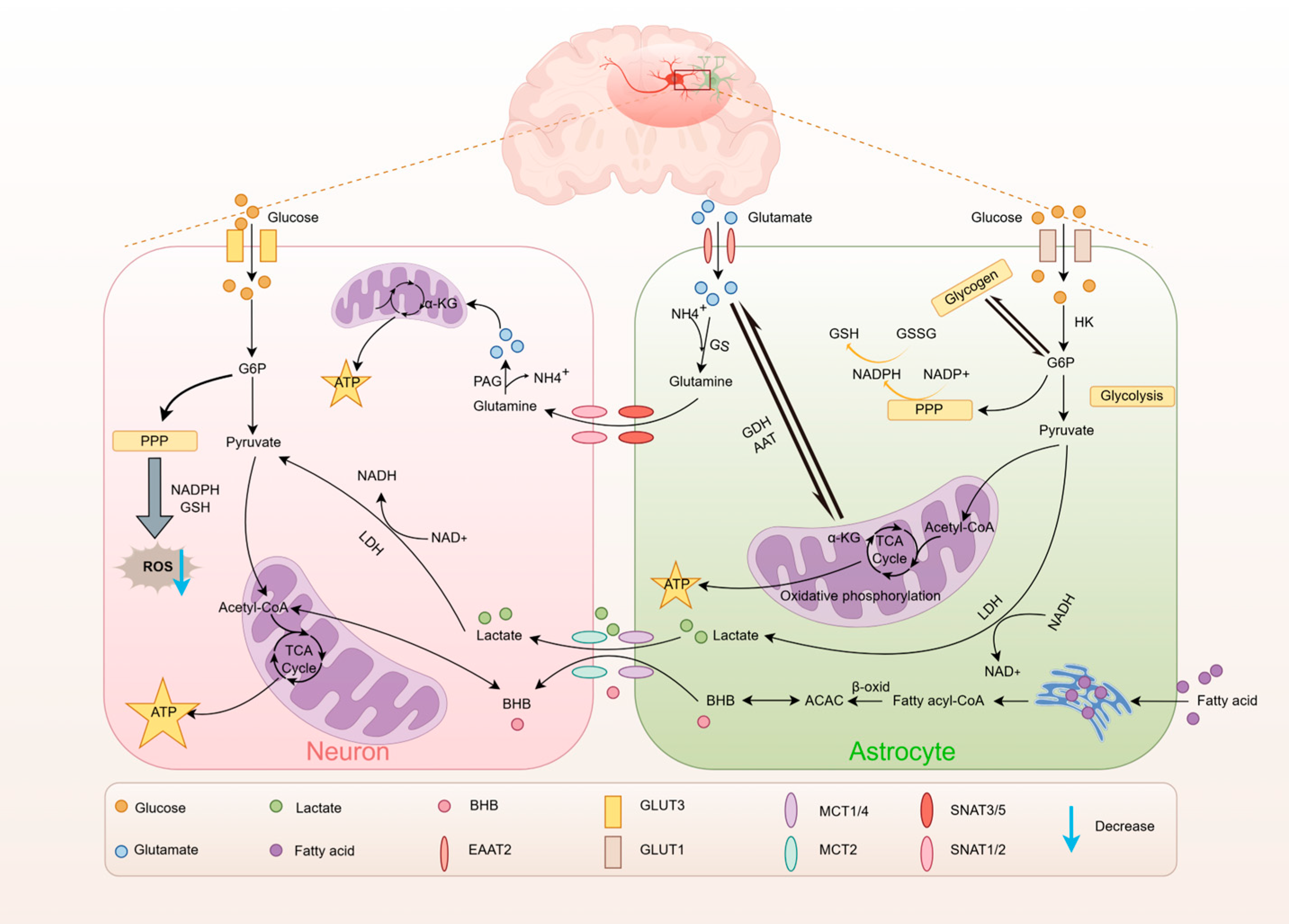
3. Brain Energy Metabolic Characteristics
3.1. Glucose Metabolism
3.2. Lactate Metabolism
3.3. Lipid Metabolism
3.4. Amino Acid Metabolism
4. Imbalance in Brain Energy Metabolism and Neuropathic Pain
4.1. Neuropathic Pain and Impaired Glucose Metabolism
4.2. Neuropathic Pain and Impaired Lactate Metabolism
4.3. Neuropathic Pain and Impaired Lipid Metabolism
4.4. Neuropathic Pain and Impaired Glutamate Metabolism
4.5. Neuropathic Pain and Microglial Metabolism
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Attal, N.; Lanteri-Minet, M.; Laurent, B.; Fermanian, J.; Bouhassira, D. The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain 2011, 152, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Backonja, M.; Rowbotham, M.C.; Allen, R.R.; Argoff, C.R.; Bennett, G.J.; Bushnell, M.C.; Farrar, J.T.; Galer, B.S.; Haythornthwaite, J.A.; et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch. Neurol. 2003, 60, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Ke, Y.; Jiang, Y.; Liu, Y.; Liu, Z. Links Between Cellular Energy Metabolism and Pain Sensation. Anesth. Analg. 2025, 140, 616–627. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Huo, B.B.; Zheng, M.X.; Hua, X.Y.; Shen, H.; Lu, Y.C.; Jiang, D.L.; Shan, C.L.; Xu, J.G. Evaluation of Neuropathic Pain in a Rat Model of Total Brachial Plexus Avulsion from Behavior to Brain Metabolism. Pain Physician 2019, 22, E215–E224. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.J.; Millecamps, M.; Aliaga, A.; Seminowicz, D.A.; Low, L.A.; Bedell, B.J.; Stone, L.S.; Schweinhardt, P.; Bushnell, M.C. Metabolic brain activity suggestive of persistent pain in a rat model of neuropathic pain. Neuroimage 2014, 91, 344–352. [Google Scholar] [CrossRef]
- Huo, B.B.; Zheng, M.X.; Hua, X.Y.; Shen, J.; Wu, J.J.; Xu, J.G. Metabolic Brain Network Analysis with (18)F-FDG PET in a Rat Model of Neuropathic Pain. Front. Neurol. 2021, 12, 566119. [Google Scholar] [CrossRef]
- Hua, T.; Kong, E.; Zhang, H.; Lu, J.; Huang, K.; Ding, R.; Wang, H.; Li, J.; Han, C.; Yuan, H. PRMT6 deficiency or inhibition alleviates neuropathic pain by decreasing glycolysis and inflammation in microglia. Brain Behav. Immun. 2024, 118, 101–114. [Google Scholar] [CrossRef]
- Kong, E.; Li, Y.; Ma, P.; Zhang, Y.; Ding, R.; Hua, T.; Yang, M.; Yuan, H. Lyn-mediated glycolysis enhancement of microglia contributes to neuropathic pain through facilitating IRF5 nuclear translocation in spinal dorsal horn. J. Cell. Mol. Med. 2023, 27, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ishikura, K.I.; Kume, K.; Ohsawa, M. Astrocyte-neuron lactate shuttle sensitizes nociceptive transmission in the spinal cord. Glia 2019, 67, 27–36. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.K.; Ha, H.T.T.; Nguyen, T.H.; Nguyen, L.N. The role of SLC transporters for brain health and disease. Cell. Mol. Life Sci. 2021, 79, 20. [Google Scholar] [CrossRef]
- Kreft, M.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A. Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro 2012, 4, AN20120007. [Google Scholar] [CrossRef]
- Simpson, I.A.; Dwyer, D.; Malide, D.; Moley, K.H.; Travis, A.; Vannucci, S.J. The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E242–E253. [Google Scholar] [CrossRef]
- Devraj, K.; Klinger, M.E.; Myers, R.L.; Mokashi, A.; Hawkins, R.A.; Simpson, I.A. GLUT-1 glucose transporters in the blood-brain barrier: Differential phosphorylation. J. Neurosci. Res. 2011, 89, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflugers Arch. 2020, 472, 1299–1343. [Google Scholar] [CrossRef]
- Takahashi, S. Neuroprotective Function of High Glycolytic Activity in Astrocytes: Common Roles in Stroke and Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 6568. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Wender, R.; Brown, A.M.; Fern, R.; Swanson, R.A.; Farrell, K.; Ransom, B.R. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci. 2000, 20, 6804–6810. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, O.W.; Fontes, J.D.; Carlson, G.M. The regulation of glycogenolysis in the brain. J. Biol. Chem. 2018, 293, 7099–7107. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Li, H.; Guglielmetti, C.; Sei, Y.J.; Zilberter, M.; Le Page, L.M.; Shields, L.; Yang, J.; Nguyen, K.; Tiret, B.; Gao, X.; et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 2023, 42, 112335. [Google Scholar] [CrossRef]
- Flores-Ponce, X.; Velasco, I. Dopaminergic neuron metabolism: Relevance for understanding Parkinson’s disease. Metabolomics 2024, 20, 116. [Google Scholar] [CrossRef]
- Wyss, M.T.; Jolivet, R.; Buck, A.; Magistretti, P.J.; Weber, B. In vivo evidence for lactate as a neuronal energy source. J. Neurosci. 2011, 31, 7477–7485. [Google Scholar] [CrossRef]
- Xue, X.; Liu, B.; Hu, J.; Bian, X.; Lou, S. The potential mechanisms of lactate in mediating exercise-enhanced cognitive function: A dual role as an energy supply substrate and a signaling molecule. Nutr. Metab. 2022, 19, 52. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Alberini, C.M.; Cruz, E.; Descalzi, G.; Bessières, B.; Gao, V. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 2018, 66, 1244–1262. [Google Scholar] [CrossRef]
- Rouach, N.; Koulakoff, A.; Abudara, V.; Willecke, K.; Giaume, C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 2008, 322, 1551–1555. [Google Scholar] [CrossRef]
- Hertz, L.; Peng, L.; Dienel, G.A. Energy metabolism in astrocytes: High rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow. Metab. 2007, 27, 219–249. [Google Scholar] [CrossRef]
- Falkowska, A.; Gutowska, I.; Goschorska, M.; Nowacki, P.; Chlubek, D.; Baranowska-Bosiacka, I. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. Int. J. Mol. Sci. 2015, 16, 25959–25981. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, C.; Levin, B.E. Fatty acid-induced astrocyte ketone production and the control of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1186–R1192. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Haller, R.G.; Walton, M.E. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 2003, 23, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, C.; Liu, Y. Navigating the metabolic maze: Anomalies in fatty acid and cholesterol processes in Alzheimer’s astrocytes. Alzheimers Res. Ther. 2024, 16, 63. [Google Scholar] [CrossRef]
- Panov, A.; Orynbayeva, Z.; Vavilin, V.; Lyakhovich, V. Fatty acids in energy metabolism of the central nervous system. Biomed. Res. Int. 2014, 2014, 472459. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Qi, Y.B.; Gao, Y.N.; Chen, W.G.; Zhou, T.; Zang, Y.; Li, J. Astrocyte metabolism and signaling pathways in the CNS. Front. Neurosci. 2023, 17, 1217451. [Google Scholar] [CrossRef]
- McKenna, M.C. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem. Res. 2012, 37, 2613–2626. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T.; Mitala, C.M.; Kundu, S.; Verma, A.; Elkind, J.A.; Nissim, I.; Cohen, A.S. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc. Natl. Acad. Sci. USA 2010, 107, 366–371. [Google Scholar] [CrossRef]
- Yu, Y.; Herman, P.; Rothman, D.L.; Agarwal, D.; Hyder, F. Evaluating the gray and white matter energy budgets of human brain function. J. Cereb. Blood Flow. Metab. 2018, 38, 1339–1353. [Google Scholar] [CrossRef]
- Gao, K.; Cheung-Hoi Yu, A. Glutamate, a Key for Astrocytes to Participate in Brain Function and Diseases. Neurochem. Res. 2025, 50, 166. [Google Scholar] [CrossRef]
- Norenberg, M.D.; Martinez-Hernandez, A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979, 161, 303–310. [Google Scholar] [CrossRef]
- Tani, H.; Dulla, C.G.; Farzampour, Z.; Taylor-Weiner, A.; Huguenard, J.R.; Reimer, R.J. A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 2014, 81, 888–900. [Google Scholar] [CrossRef]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; McNair, L.F.; Schousboe, A.; Waagepetersen, H.S. Specificity of exogenous acetate and glutamate as astrocyte substrates examined in acute brain slices from female mice using methionine sulfoximine (MSO) to inhibit glutamine synthesis. J. Neurosci. Res. 2017, 95, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Stridh, M.H.; McNair, L.F.; Sonnewald, U.; Waagepetersen, H.S.; Schousboe, A. Glutamate oxidation in astrocytes: Roles of glutamate dehydrogenase and aminotransferases. J. Neurosci. Res. 2016, 94, 1561–1571. [Google Scholar] [CrossRef]
- Pardo, B.; Rodrigues, T.B.; Contreras, L.; Garzón, M.; Llorente-Folch, I.; Kobayashi, K.; Saheki, T.; Cerdan, S.; Satrústegui, J. Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J. Cereb. Blood Flow. Metab. 2011, 31, 90–101. [Google Scholar] [CrossRef]
- Sokoloff, L.; Reivich, M.; Kennedy, C.; Des Rosiers, M.H.; Patlak, C.S.; Pettigrew, K.D.; Sakurada, O.; Shinohara, M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977, 28, 897–916. [Google Scholar] [CrossRef]
- Chen, F.Y.; Tao, W.; Cheng, X.; Wang, H.Y.; Hu, Y.S.; Zhang, X.H.; Li, Y.J. Brain glucose metabolic changes associated with chronic spontaneous pain due to brachial plexus avulsion: A preliminary positron emission tomography study. Chin. Med. J. 2008, 121, 1096–1100. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, N.Y.; Kim, J.Y.; An, Y.S.; Kim, Y.W. Changes in the Brain Metabolism Associated with Central Post-Stroke Pain in Hemorrhagic Pontine Stroke: An (18)F-FDG-PET Study of the Brain. Brain Sci. 2022, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Huang, Y.H.; Chao, T.H.; Lin, W.Y.; Sun, W.Z.; Yen, C.T. Gabapentin reverses central hypersensitivity and suppresses medial prefrontal cortical glucose metabolism in rats with neuropathic pain. Mol. Pain. 2014, 10, 63. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, C.; Zhong, Q.; Wang, L.; Gui, Z.; Zhu, J.; Manyande, A.; Xu, F.; Wang, J.; Zhang, Z. Influence of Cerebral Glucose Metabolism by Chronic Pain-Mediated Cognitive Impairment in Adolescent Rats. Mol. Neurobiol. 2022, 59, 3635–3648. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.; Oh, J.H.; Jung, H.H.; Kim, Y.B.; Cho, Z.H.; Chang, J.W. Longitudinal FDG microPET imaging of neuropathic pain: Does cerebellar activity correlate with neuropathic pain development in a rat model? Acta Neurochir. 2015, 157, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Rigor, B.M. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: Further in vitro validation. J. Neurochem. 1997, 69, 423–426. [Google Scholar] [CrossRef]
- Jha, M.K.; Song, G.J.; Lee, M.G.; Jeoung, N.H.; Go, Y.; Harris, R.A.; Park, D.H.; Kook, H.; Lee, I.K.; Suk, K. Metabolic Connection of Inflammatory Pain: Pivotal Role of a Pyruvate Dehydrogenase Kinase-Pyruvate Dehydrogenase-Lactic Acid Axis. J. Neurosci. 2015, 35, 14353–14369. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Z. Pyruvate kinase M2 at a glance. J. Cell Sci. 2015, 128, 1655–1660. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Fan, B.; Xu, X.; Chen, Y.; Lu, R.; Xu, Z.; Liu, X. PKM2 is involved in neuropathic pain by regulating ERK and STAT3 activation in rat spinal cord. J. Headache Pain. 2018, 19, 7. [Google Scholar] [CrossRef]
- Luo, W.; Semenza, G.L. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget 2011, 2, 551–556. [Google Scholar] [CrossRef]
- Kong, E.; Li, Y.; Deng, M.; Hua, T.; Yang, M.; Li, J.; Feng, X.; Yuan, H. Glycometabolism Reprogramming of Glial Cells in Central Nervous System: Novel Target for Neuropathic Pain. Front. Immunol. 2022, 13, 861290. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1155–1163. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Deng, M.; Fang, Y.; Chu, Y.; Ding, R.; Fan, X.; Wei, H.; Song, H.; Jiang, G.; Zhang, H.; Han, C.; et al. SIRT3 is required for the protective function of ketogenic diet on neural inflammation and neuropathic pain. Int. J. Biol. Sci. 2025, 21, 3011–3029. [Google Scholar] [CrossRef] [PubMed]
- Boccella, S.; Guida, F.; De Logu, F.; De Gregorio, D.; Mazzitelli, M.; Belardo, C.; Iannotta, M.; Serra, N.; Nassini, R.; de Novellis, V.; et al. Ketones and pain: Unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain. FASEB J. 2019, 33, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Ludman, T.; Melemedjian, O.K. Bortezomib-induced aerobic glycolysis contributes to chemotherapy-induced painful peripheral neuropathy. Mol. Pain. 2019, 15, 1744806919837429. [Google Scholar] [CrossRef]
- Cooper, M.A.; McCoin, C.; Pei, D.; Thyfault, J.P.; Koestler, D.; Wright, D.E. Reduced mitochondrial reactive oxygen species production in peripheral nerves of mice fed a ketogenic diet. Exp. Physiol. 2018, 103, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, K.; Riegel, A.; Nair, S.; Kalivas, P.W. Extracellular glutamate: Functional compartments operate in different concentration ranges. Front. Syst. Neurosci. 2011, 5, 94. [Google Scholar] [CrossRef]
- Nilsson, P.; Hillered, L.; Pontén, U.; Ungerstedt, U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow. Metab. 1990, 10, 631–637. [Google Scholar] [CrossRef]
- Hung, K.L.; Wang, S.J.; Wang, Y.C.; Chiang, T.R.; Wang, C.C. Upregulation of presynaptic proteins and protein kinases associated with enhanced glutamate release from axonal terminals (synaptosomes) of the medial prefrontal cortex in rats with neuropathic pain. Pain 2014, 155, 377–387. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, H.; Jeon, S.Y.; Kwon, J.M.; Lee, W.J.; Jang, J.H.; Lee, D.; Lee, Y.; Kang, D.H. Peripheral and Central Metabolites Affecting Depression, Anxiety, Suicidal Ideation, and Anger in Complex Regional Pain Syndrome Patients Using a Magnetic Resonance Spectroscopy: A Pilot Study. Psychiatry Investig. 2018, 15, 891–899. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, S.; Yu, X.; Li, L.; Yang, M.; Liang, S.; Liu, W.; Tao, J. Altered thalamic neurotransmitters metabolism and functional connectivity during the development of chronic constriction injury induced neuropathic pain. Biol. Res. 2020, 53, 36. [Google Scholar] [CrossRef]
- Malik, A.R.; Willnow, T.E. Excitatory Amino Acid Transporters in Physiology and Disorders of the Central Nervous System. Int. J. Mol. Sci. 2019, 20, 5671. [Google Scholar] [CrossRef] [PubMed]
- Suchak, S.K.; Baloyianni, N.V.; Perkinton, M.S.; Williams, R.J.; Meldrum, B.S.; Rattray, M. The ‘glial’ glutamate transporter, EAAT2 (Glt-1) accounts for high affinity glutamate uptake into adult rodent nerve endings. J. Neurochem. 2003, 84, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, A.L.; Robinson, M.B. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem. Int. 2007, 51, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.H.; Song, X.K.; Zhang, J.L.; Zhang, J.L.; Rong, P.J.; Wang, J.Y. Involvement of the astroglial glutamate-glutamine cycle in the analgesic effects of electroacupuncture in a rat model of chronic neuropathic pain. Acupunct. Med. 2025, 43, 14–25. [Google Scholar] [CrossRef]
- Zeng, J.; Cui, L.Y.; Feng, Y.; Ding, M.X. Electroacupuncture relieves neuropathic pain via upregulation of glutamate transporters in the spinal cord of rats. Neurosci. Lett. 2016, 620, 38–42. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, R.; Xu, Z.; Ke, Y.; Sun, R.; Yang, H.; Zhang, X.; Zhen, X.; Zheng, L.T. Early glycolytic reprogramming controls microglial inflammatory activation. J. Neuroinflamm. 2021, 18, 129. [Google Scholar] [CrossRef] [PubMed]
- Devanney, N.A.; Stewart, A.N.; Gensel, J.C. Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp. Neurol. 2020, 329, 113310. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xu, C.B.; Chen, C.J.; Shi, G.N.; Guo, Q.L.; Zhou, Y.; Wei, Y.Z.; Wu, L.; Shi, J.G.; Zhang, T.T. Divanillyl sulfone suppresses NLRP3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice. J. Neuroinflamm. 2021, 18, 142. [Google Scholar] [CrossRef]
- Liu, P.; Chen, T.; Tan, F.; Tian, J.; Zheng, L.; Deng, Y.; Chen, J.; Chi, X. Dexmedetomidine alleviated neuropathic pain in dorsal root ganglion neurons by inhibition of anaerobic glycolysis activity and enhancement of ROS tolerance. Biosci. Rep. 2020, 40, BSR20191994. [Google Scholar] [CrossRef]
- Inquimbert, P.; Bartels, K.; Babaniyi, O.B.; Barrett, L.B.; Tegeder, I.; Scholz, J. Peripheral nerve injury produces a sustained shift in the balance between glutamate release and uptake in the dorsal horn of the spinal cord. Pain 2012, 153, 2422–2431. [Google Scholar] [CrossRef]
- Macaluso, A.; Bernabucci, M.; Trabucco, A.; Ciolli, L.; Troisi, F.; Baldini, R.; Gradini, R.; Battaglia, G.; Nicoletti, F.; Collini, S. Analgesic effect of a single preoperative dose of the antibiotic ceftriaxone in humans. J. Pain. 2013, 14, 604–612. [Google Scholar] [CrossRef]
- Albert, H.B.; Sorensen, J.S.; Christensen, B.S.; Manniche, C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): A double-blind randomized clinical controlled trial of efficacy. Eur. Spine J. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Shin, D.A.; Kim, T.U.; Chang, M.C. Minocycline for Controlling Neuropathic Pain: A Systematic Narrative Review of Studies in Humans. J. Pain. Res. 2021, 14, 139–145. [Google Scholar] [CrossRef]
- Falcucci, R.M.; Wertz, R.; Green, J.L.; Meucci, O.; Salvino, J.; Fontana, A.C.K. Novel Positive Allosteric Modulators of Glutamate Transport Have Neuroprotective Properties in an in Vitro Excitotoxic Model. ACS Chem. Neurosci. 2019, 10, 3437–3453. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.; Zhang, L.; Liu, W.; Yu, X.; Wang, Z.; Zheng, M. Electroacupuncture suppresses glucose metabolism and GLUT-3 expression in medial prefrontal cortical in rats with neuropathic pain. Biol. Res. 2021, 54, 24. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.X.; Wang, X.; Liu, Y.; Xu, T.C.; Yu, Z.; Xu, B. Electroacupuncture alleviates diabetic peripheral neuropathy through modulating mitochondrial biogenesis and suppressing oxidative stress. World J. Diabetes 2025, 16, 93130. [Google Scholar] [CrossRef] [PubMed]

| Neuropathic Pain Model | Research Object | Type of Metabolic Substrate | Change | Reference |
|---|---|---|---|---|
| BPA | Human | Glucose | Decreased glucose metabolism in the ipsilateral thalamus and S1; increased in the ipsilateral orbitofrontal cortex, contralateral insular cortex, and dorsolateral prefrontal cortex | [53] |
| Pain after hemorrhagic pontine stroke | Human | Glucose | Decreased glucose metabolism in the contralateral angular gyrus and ipsilateral supplementary motor cortex | [54] |
| BPA | Mouse | Glucose | The experimental group exhibited elevated standard glucose metabolic activity in both the right and left thalamus compared to control mice | [11] |
| SNI | Rat | Glucose | Glucose metabolism increased in the contralateral S1 posterior limb area, bilateral anterior insular cortex, thalamus, and cerebellar vermis; glucose metabolism decreased in the contralateral amygdala, bilateral splenoparietal cortex, prefrontal cortex, and hippocampus | [12,55,56] |
| TST | Rat | Glucose | 1–2 weeks: increase in metabolic activity was noted in the contralateral primary motor and sensory cortices 3–8 weeks: increase in activity in the central nucleus of the inferior colliculus and most of the cerebellum, accompanied by a decrease in activity in the periventricular gray matter and the primary and secondary motor cortices. | [57] |
| CCI | Rat | Lactate | Increased lactate levels | [14,15,62] |
| CCI | Mouse | BHB | Decreased BHB content | [67,68] |
| CCI | Rat | Glutamate | GLAST expression decreased, Glutamate levels increased | [79] |
| SNI | Rat | Glutamate | Glutamate levels increased | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, B.; Liu, J.; Hou, Z.; Xie, G.; Xiong, X.; Yu, S. The Crosstalk Between Brain Energy Metabolism and Neuropathic Pain: Mechanisms and Therapeutic Implications. Metabolites 2025, 15, 755. https://doi.org/10.3390/metabo15120755
Wang J, Liu B, Liu J, Hou Z, Xie G, Xiong X, Yu S. The Crosstalk Between Brain Energy Metabolism and Neuropathic Pain: Mechanisms and Therapeutic Implications. Metabolites. 2025; 15(12):755. https://doi.org/10.3390/metabo15120755
Chicago/Turabian StyleWang, Jiangtao, Baitong Liu, Jinyan Liu, Zhuoxi Hou, Guangxin Xie, Xiaoyi Xiong, and Shuguang Yu. 2025. "The Crosstalk Between Brain Energy Metabolism and Neuropathic Pain: Mechanisms and Therapeutic Implications" Metabolites 15, no. 12: 755. https://doi.org/10.3390/metabo15120755
APA StyleWang, J., Liu, B., Liu, J., Hou, Z., Xie, G., Xiong, X., & Yu, S. (2025). The Crosstalk Between Brain Energy Metabolism and Neuropathic Pain: Mechanisms and Therapeutic Implications. Metabolites, 15(12), 755. https://doi.org/10.3390/metabo15120755





