Abstract
Background/Objectives: Skeletal impairment has been reported as a common finding in Hyperphenylalaninemia (HPA)/Phenylketonuria (PKU) patients regardless of age and method of diagnosis, both in children and adults. Quantitative Ultrasound (QUS) is a radiation-free and low-cost method for assessing bone quality, used in various chronic conditions. Methods: Bone quality was evaluated using a calcaneal QUS device. Auxological parameters, nutritional intakes, and plasma levels of key bone biomarkers were also registered. The population was divided into four groups: PKU patients under diet therapy and HPA patients on a free diet, both divided into receiving or not receiving single vitamin D supplementation. Results: All HPA/PKU patients had median bone quality index (BQI) Z- and T-score values lower than −1, with slightly better values in HPA children and PKU-supplemented adults. Dietary vitamin D intake in PKU patients was significantly higher than in HPA subjects (p < 0.001), due to protein substitute supplementation. However, plasma 25(OH) vitamin D levels, although increased compared to baseline, were still overlapping among groups (p = 0.845) after supplementation. Approximately a quarter of both pediatric and adult non-supplemented PKU patients had Z-score and T-score levels below −2, and this percentage decreased with vitamin D supplementation in all groups. In PKU-supplemented patients, the Broadband Ultrasound Attenuation (BUA) was significantly higher than in the other groups (p = 0.040). Conclusions: The improvement in BUA may represent preliminary evidence of the effect of vitamin D on bone architecture, which could encourage this supplementation to prevent the worsening of bone structure and reduce the risk of fractures.
1. Introduction
Phenylketonuria (PKU, ORPHA79254, MIM 261600) is an inherited metabolic disorder caused by mutations in the Phenylalanine Hydroxylase (PAH) gene, coding the enzyme responsible for the metabolization of Phenylalanine (Phe) into Tyrosine (Tyr). As a consequence, Phe accumulates in the blood tissues and is transported across the blood–brain barrier. Its high concentration can lead to intellectual disability, behavioral abnormalities, and systemic issues in the life-long PKU follow-up [1,2].
Both PKU and mild HPA are caused by pathogenic variants in the PAH gene, leading to reduced activity of phenylalanine hydroxylase. The residual enzymatic activity determines the biochemical phenotype: PKU patients show markedly elevated blood phenylalanine concentrations and require a lifelong hypoproteic diet supplemented with amino acid mixtures, while HPA patients exhibit only mild elevation of phenylalanine levels and can maintain a normal diet.
In this context, low bone mineral density (BMD) has been described in both early-diagnosed and late-diagnosed Phenylketonuria (PKU) patients [3]. Adults with PKU showed significantly reduced BMD Z-Scores compared to the general population, although without a higher fracture risk [4]. Also, in pediatric patients with PKU, evidence regarding bone density status demonstrates lower than normal reference ranges [5,6], although without a clear correlation with the common determinants of osteopenia/osteoporosis in childhood [7].
Although traditionally it has been hypothesized that reduced intake of calcium and phosphorus from natural foods represents the main cause of decreased bone mass in patients with PKU, recent studies indicate that blood calcium status in treated patients is often normal or even higher than in the general population [8,9]. In our recent analysis, we also observed a tendency toward calcium over supplementation through protein substitutes, particularly in formulations designed for children over three years of age [10]. At the same time, it has been proposed that the composition of amino-acid-based medical foods—used in the dietary management of PKU—imposes an increased systemic acid load with consequent elevated urinary excretion of calcium and magnesium, a factor potentially relevant in the genesis of osteopenia/osteoporosis in these subjects [11]. On the contrary, it has been documented that patients with PKU are more often vitamin D sufficient than the general population [12] and lower than normal BMD values have also been found in Hyperphenylalaninemia (HPA) patients, who should be protected by a free diet regimen [13].
In the PKU diet, micronutrient supplementation happens mainly through amino acids (AA) mixtures, which are enriched with vitamins and oligoelements to supply the poor intake by diet [14,15]. Adequate intake, however, is not always reached, because physiological vitamin status is more dependent on natural protein intake than on their content as supplementation of AA formulas, and therefore single micronutrient supplementation is often necessary to reach physiological status [16]. However, studies on the effect of single vitamin D supplementation in this group of patients are rare and often focused more on the assessment of change in vitamin D plasma levels than on the effect on bone health status [17,18,19].
Quantitative ultrasound (QUS) has been reported in previous studies as a method to assess bone quality in children. In QUS, the attenuation and speed of propagation of the ultrasound wave provide information on the physical properties of bone [20]. Advantages of QUS are as follows: no ionizing radiation, lower cost, high portability, and lower time spent [21]. For these reasons, its clinical applications have recently been addressed by the International Society of Clinical Densitometry (ISCD), 2007 Pediatric Position Development Conference [22]. In particular, calcaneal QUS is considered the standard parameter for QUS study of bone health status and has been used in children and adolescents to assess osteopenia and fracture rate in subjects with bone and mineral disorders, in several chronic disorders [23,24].
In HPA/PKU patients, QUS has been rarely used as a method to follow up skeletal involvement. Evidence on bone status report lower values than the general population [25,26], but the effect of a hypoproteic diet has not been clearly elucidated, as osteopenia was also detected from an early age in PKU children, without a clear relationship to diet therapy [27]. Furthermore, the role of vitamin D supplementation in the context of multivitamin supplementation through AA mixtures remains a topic of discussion.
In this study, we aimed to assess QUS parameters in both free-diet HPA patients and PKU patients on a hypoproteic and amino-acid-supplemented diet, and to evaluate the effect of single vitamin D supplementation on those parameters.
2. Materials and Methods
We conducted a retrospective cross-sectional study on both PKU patients (Phe levels ≥ 360 umol/L) and HPA patients (120 umol/L > Phe < 360 umol/L). Data were collected between April and June 2025 at the Giovanni XXIII Pediatric Hospital. This study was purely observational and non-interventional. No experimental assignment or intervention was applied by the authors.
2.1. Patients
Inclusion criteria were as follows: patients with a confirmed diagnosis of either HPA or PKU who had undergone a QUS for bone health assessment over the follow-up were included in the study. Healthy control data were not available because of the retrospective design. Instead, QUS results were interpreted using validated age- and sex-specific reference ranges from the manufacturer and published pediatric/adult normative data, which serve as an internal reference comparable to a normal control population. The total study population was categorized based on clinical management and supplementation into four groups: PKU patients under diet therapy and AA mixtures, those also receiving single vitamin D supplementation, and patients with HPA on a free diet, with and without vitamin D supplementation. A supplementation period of three months was considered the minimum length to be included in the study, with a maximum duration of six months. Supplementation commenced in all cases of vitamin D levels < 20 ug/L, and the dose of supplementation was 400 UI/day [28].
Exclusion criteria were as follows: the absence of QUS measurement/results and incomplete records of laboratory tests at the time of ultrasound examination. Also, concomitant chronic disorders that could interfere with the patients’ bone status were considered as exclusion criteria.
2.2. Measurements
For each participant, anthropometric data, including weight and height to calculate body mass index (BMI), were collected at the time of the QUS. Protocol of assessment was the same as described in Tummolo et al. [29]. Anthropometric measurements were conducted using the InBody 230 analyzer (Biospace Corp., Seoul, Republic of Korea). Bone quality was evaluated using the SONOST 3000 (Osteosys, Seoul, Korea), a calcaneal QUS device which enabled the calculation of both the bone quality index (BQI) and its corresponding standard deviation score (SDS). According to the ISCD Pediatric Official Position (Z-score of −2.0 or lower was defined as below the expected range for age, and a Z-score above −2.0 was defined as within the expected range for age) [7]. The BQI was determined using two acoustic parameters: broadband ultrasound attenuation (BUA), expressed in dB/MHz, and speed of sound (SOS), expressed in m/s. BUA reflects bone microarchitecture and is sensitive to temperature variations, while SOS is suggestive of bone mineral density and inversely influenced by temperature [30]. The BQI was obtained by applying correlation coefficients (α, β) to an arithmetic formula, as described in a previous study by our group [13]. All QUS assessments were performed using the same equipment and standardized protocols across the entire patient cohort, ensuring consistency and reliability of the data. According to ISCD Pediatric Official Position, validated heel QUS devices predict fragility fracture in postmenopausal women and men over the age of 65 independently of central DXA BMD [7].
2.3. Laboratory Data
Laboratory data, including serum calcium and 25(OH) vitamin D, were obtained from routine blood tests performed at the time of the QUS. To assess dietary compliance, additional parameters, such as plasma Phe and Tyr concentrations, the Phe/Tyr ratio, and total serum proteins, were analyzed. All biochemical analyses were carried out at the same clinical laboratory to ensure reproducibility. High-performance liquid chromatography (HPLC) was used to measure plasma Phe and Tyr levels. The reference range for laboratory tests was determined according to the valid laboratory kit.
2.4. Nutritional Assessment
A nutritional analysis was conducted in patients with PKU undergoing dietary treatment with the addition of protein substitute supplementation and in HPA patients on a free-diet regimen. The intake of key bone-related nutrients, such as total proteins, vitamin D, calcium, and phosphorus, was examined, considering those provided by natural foods and those derived from protein substitutes. For this reason, patients were asked to complete a 3-day food diary. The Winfood Pro software (version 3.0.0, 2011, Medimatica Srl, Teramo, Italy) was used for intake calculations.
2.5. Statistical Methods
Descriptive statistics for continuous variables are reported as median and interquartile range (IQR), given the non-normal distribution of most variables, as assessed using the Shapiro–Wilk test. For inferential analysis, non-parametric tests were employed. The Mann–Whitney U test was used to compare two independent groups, while the Kruskal–Wallis test was applied for comparisons involving more than two groups. When significant differences were found with the Kruskal–Wallis test, post hoc pairwise comparisons were conducted using the correction to control for multiple testing. Associations between ordinal or non-normally distributed continuous variables were analyzed using Spearman’s rank correlation coefficient (ρ). All statistical tests were two-tailed, and p-values < 0.05 were considered statistically significant. Statistical analyses were performed using Jamovi (version 2.6.45).
3. Results
A total of 110 patients were included in the analysis: 16 with mild hyperphenylalaninemia (HPA), 6 with HPA receiving supplementation (HPA + SUP), 74 with PKU, and 14 with PKU receiving supplementation (PKU + SUP). The overall median age was 16 years (IQR: 9–24), with no significant differences among groups (p = 0.116). Of the total patients, 62% were younger than 18 years, and females were slightly more represented (61.8%), with no statistically significant differences in sex distribution (p = 0.326). Median body mass index (BMI) values were also comparable across groups (p = 0.086) (Table 1).

Table 1.
Demographic and clinical characteristics of patients.
Sixty-four PKU patients (86%) were administered with AA mixtures and four (5%) took glycomacropeptides (GMP). The nutritional intake by protein substitutes for the PKU group is reported in Table 2. No statistically significant differences were found for protein equivalent, calcium, phosphorus, and vitamin D intakes between the two groups of PKU patients. Considering intakes from natural foods, natural protein, calcium, phosphorus, and vitamin D intake, were instead significantly lower in PKU groups than in HPA groups (Table 2).

Table 2.
Nutritional intakes from both amino acid substitutes and natural foods.
When considering the total intake of nutrients from both protein substitutes and natural foods, calcium and vitamin D intakes become significantly higher in PKU patients than in HPA patients (p = 0.05; p = 0.001, respectively), due to the contribution from protein substitutes. In contrast, total protein intake becomes overlapping between the two groups, as well as phosphorus intake. (Figure 1).
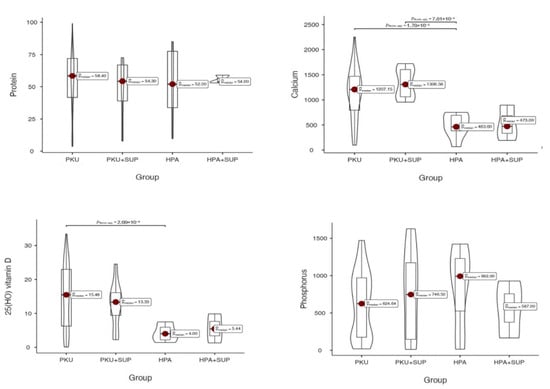
Figure 1.
Total nutrient intakes in the study groups (PKU, PKU with supplementation [PKU + SUP], HPA, and HPA with supplementation [HPA + SUP]). Violin plots display the distribution of intakes for protein (top left, 0 = 0.831), calcium (top right, p = 0.052), 25(OH) vitamin D (bottom left, p = 0.001), and phosphorus (bottom right, p = 0.713). Red dots indicate the median values, with boxes showing the interquartile range. p-values refer to pairwise group comparisons.
Biochemical parameters among the four patient groups, and normal values are reported in Table 3. Calcium levels fell in all cases within the normal range and were highest in the HPA + SUP group, although this difference did not reach statistical significance. The median 25(OH)Vitamin D levels were in all cases higher than 20 ng/mL, with the upper limit of the range reaching the sufficiency levels (>30 ng/mL) in all groups but in the HPA + SUP group (p = 0.8). Phe levels and Phe/Tyr ratio were significantly different, and tyrosine level was comparable among groups (p = 0.576).

Table 3.
Median (interquartile range) of biochemical parameters.
Bone quality assessment results were as follows: bone mineral density, expressed as a T-score in adults and a Z-score in children, did not differ significantly across the four groups. In adults, the median T-score in the overall population was –1.40 (IQR –2.18 to –0.93). Patients with HPA had the highest values (median –0.82, IQR –1.20 to –0.58), whereas patients with PKU showed lower scores (median –1.47, IQR –2.22 to –1.04), with PKU + SUP patients presenting intermediate values (median –1.35, IQR –1.55 to –1.10). Despite this apparent gradient, differences did not reach statistical significance (Kruskal–Wallis p = 0.889). In children, the overall median Z-score was –1.54 (IQR –2.11 to –1.04). Values were similar across groups, ranging from –1.71 (IQR –2.41 to –1.14) in HPA + SUP patients to –1.13 (IQR –2.03 to –0.86) in PKU + SUP patients, with no significant differences (p = 0.577). Thus, although bone density indices tended to be slightly better in HPA patients and lower in PKU patients, variability within groups prevented these trends from achieving statistical significance (Figure 2).
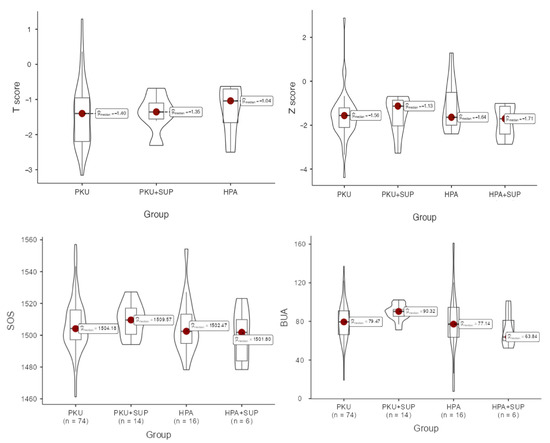
Figure 2.
Comparison of data distributions across groups using box and violin plots. For each group, the box plot displays the median (horizontal line) and the interquartile range (represented by the box limits). The accompanying violin plots display the estimated probability density of the data, allowing for a visual comparison of distributional patterns between groups. T-scores were used for adults (top left, p = 0.889), while Z-scores were used for children (top right, p = 0.577), to account for age-related differences in normative data. SOS (bottom left, p = 0.580) and BUA (bottom right, p = 0.040) were shown.
Distributions of all the available QUS output values are reported in Table 4. BQI was higher in the PKU supplemented group, although without significant difference compared to other groups. BUA was significantly higher in the above group (p = 0.040), whereas it showed the lowest levels in the HPA-supplemented group. Z-score reached the highest levels in HPA children, and the T-score in PKU-supplemented adults.

Table 4.
QUS outputs among different groups.
In adult patients, when applying the <−2 threshold, the prevalence of PKU patients falling below this cut-off level was 35% (n = 11). In contrast, only 17% of PKU + SUP and 20% of HPA adult patients presented T-score levels below −2. No HPA patients supplemented with vitamin D had a T-score level under −2.
In pediatric patients, the Z score < −2 threshold was observed in 37% of the PKU sample (n = 16), while smaller proportions were found in PKU + SUP (25%), HPA (27%), and HPA + SUP (33%) (Figure 3). Spearman’s rank correlation coefficient did not reveal any significant correlation with total protein, vitamin D, phosphorus, calcium intake, gender, age, or BMI. Notably there was no correlation of QUS parameters with Phe levels and patients falling within the target Phe levels according to European guidelines did not result in better bone quality (73.9 vs. 70.6; p = 0.724).
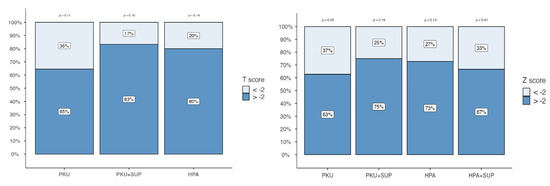
Figure 3.
Percentage distribution of T-scores (left panel) and Z-scores (right panel) across patient groups: PKU, PKU with supplementation (PKU + SUP), HPA, and HPA with supplementation (HPA + SUP). Bars represent the proportion of subjects with values below −2 (light blue) and above −2 (dark blue). Percentages are shown within each bar segment. p-values reported above the columns indicate statistical comparisons between groups.
4. Discussion
There is a long-standing concern that bone health in HPA/PKU patients is poorer than that of the general population [3,5], due to the diet regimen, potentially leading to growth failure and fractures, and to the hyperphenylalaninemia itself [31]. However, studies conducted to date have produced conflicting findings in terms of bone mineral density in this group of patients, possibly due to differences in the age of the patients studied, type of HPA considered, methods of assessment, and the criteria applied.
It is increasingly acknowledged that the development of the osteoporotic state involves the interaction of multiple mechanisms. Understanding the pathogenesis of osteoporosis is closely linked to understanding the pathophysiology of hyperphenylalaninemia in the human body.
The dietary protein content has been largely involved in maintaining a satisfactory bone health status. Studies on children fed with a vegetarian diet, similar to the PKU diet pattern, suggest that the quality of protein in vegan diets is controversial for bone health, due to the pattern of amino acids. Of note, the so-called protein digestibility corrected amino acid score [32] of most plant proteins is lower than that of animal proteins. Studies confirm lower intakes of vitamin D in such a diet pattern and conclude that an adequate supply of this vitamin is significant in a plant-based diet [33].
In this context, the hypoproteic diet in PKU may be involved in the pathogenesis of osteopenia, due to reduced bone-forming substrates. However, osteopenia also occurs in patients who have never received dietary therapy, such as HPA patients. Humans and animal studies find no correlation between bone formation and resorption markers and plasma hyperphenylalaninemia [18,34]. Evidence on mouse models showed a mesenchymal stem cell developmental defect in the osteoblast pathway, secondary to energy dysregulation and oxidative stress, a well-known pathological mechanism linked to hyperphenylalaninemia [35].
In our study, all HPA/PKU patients had median BQI Z- and T-score values lower than −1, with slightly better values in HPA children and PKU-supplemented adults. Dietary vitamin D intake in PKU patients was significantly higher than in HPA subjects, likely due to supplementation by amino acid mixtures [36]. Despite that, plasma 25(OH) vitamin D levels were comparable among groups.
The necessity of vitamin D supplementation in the PKU diet has not been clarified yet, and supplementation is generally prescribed based on the vitamin status, regardless of the vitamin D intake via amino acid mixtures. The vitamin status assessment in studies on PKU patients demonstrates that in many cases, vitamin levels were higher than those of the general population, with vitamin D deficiency ranging from 35% of patients [31] to 53.57% of the population considered [16]. In a systematic review with meta-analysis, vitamin D levels in PKU patients did not significantly differ from those of healthy controls [37].
Although no differences were found in our sample across groups regarding vitamin D levels, when assessing bone quality, approximately a quarter of both pediatric and adult non-supplemented PKU patients had Z-score and T-score levels below −2. This percentage decreased in the case of vitamin D supplementation. This difference was not noticeable for the HPA group. Furthermore, in PKU-supplemented patients, the Broadband ultrasound attenuation, one of the QUS parameters measuring the differential attenuation of sound waves transmitted through the bone, was significantly higher than in the other groups.
In recent years, this technique of quantitative assessment of the skeleton has been developed as an alternative method to bone mass density assessment for the study of bone quality and as a predictor of fracture risk, focusing on the assessment of bone morphology and microarchitecture [38].
BUA has been considered an index of hip fractures in the osteoporosis-risky population, such as postmenopausal women. In a recent study on 6189 postmenopausal women older than 65 years, QUS was performed and compared with the results of bone mineral density measurements of the calcaneus and hip. BUA was associated with a doubling of the risk for hip fractures in an overlapping way with calcaneus BMD [39].
Calcaneal bone mineral density and BUA were also compared in 236 patients, including 77 with osteoporotic fractures. BUA showed the most significant difference between the two populations and discriminated between fractures and nonfracture subjects [40]. Finally, BUA has also been implicated as a potential marker to stratify individuals at risk of deterioration in physical health [41].
Despite the increasing use of quantitative ultrasound techniques in bone assessment, the vast majority of bone health assessments in HPA/PKU patients are still based on BMD measurements, both in adults and children. In a recent study on 18 adults with PKU and matched controls, a reduced bone mineral density T-score was detected in patients [18], although the vast majority were vitamin D sufficient. Neither Phe blood concentration nor dietary habits or lifestyle were associated with BMD in regression analysis. A metanalysis by Rocha on 4097 PKU adults highlighted that mean BMD Z-scores were statistically significantly lower compared with an age-matched control or reference population, but still within the expected range for age (>−2.0). The estimated prevalence of BMD Z-scores ≤−2.0 was 8%. However, the small number of observations in the subgroup analyses limited interpretation.
Male sex and low BMI conferred a higher risk for low BMD in classic PKU, whereas Phe levels and dietary adherence do not, in a study on PKU adults [42]. Compliance to hypoproteic diet with more proteins from AA supplements assumption and low BMI were associated with low BMD in another study [43].
In the pediatric setting, a case series on 48 children who took either amino acid (L-AA) or casein glycomacropeptide substitutes (CGMP-AA) as their main protein source [19], reports that bone density was clinically normal, although the median Z-scores were below the population mean. Blood biochemistry was within the reference ranges, with no advantage to bone density observed from taking CGMP-AA compared with L-AAs. In our sample, only a few patients were administered with GMP (n = 4); therefore, a comparison between the two types of protein substitutes was not possible.
This study has some strengths and some limitations. It provides evidence on QUS parameters on a relatively large sample of patients and associates, and, for the first time, single vitamin D supplementation with parameters related to bone structure and architecture. However, due to its retrospective design, group sizes were not balanced. This imbalance, reflecting the real-world distribution of cases and not influenced by predefined allocation criteria, may have affected the statistical power of some comparisons and could increase the risk of Type II error. In this context, the use of non-parametric tests, which are robust to unequal group sizes and non-normal distributions, partially mitigates this issue. Finally, as with all retrospective studies, causality cannot be firmly established, as temporal relationships between exposures and outcomes are more difficult to verify in this type of study.
5. Conclusions
Although there is still no unanimous consensus on how to assess bone health in HPA/PKU patients [44], a regular skeletal evaluation should be part of the follow-up of both children and adults affected by this metabolic disorder. The increasing evidence on the capability of QUS parameters, such as BUA, in evaluating the bone architecture and the fracture risk should facilitate the introduction of this low-cost and radiation-free technique in the instrumental evaluation of bone. Our data are in line with the results of bone health measurements and bone mineral density from other studies. Although we did not observe an amelioration of vitamin D levels after this micronutrient supplementation, the improvement of BUA may represent preliminary evidence of the effect of vitamin D on bone architecture that could encourage this supplementation to prevent worsening of bone structure and reduce the risk of fractures.
Author Contributions
Conceptualization, A.T.; methodology, G.D.R., D.D.G., and R.C.; formal analysis, M.D.N.; data curation, M.D.N., G.D.R., L.M., and V.D.T.; writing—original draft preparation, A.T.; writing—review and editing, A.T., D.D.G., and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and did not require approval by the Local Ethics Committee. According to the rules from the Italian Medicines Agency, retrospective studies without direct contact with patients do not need a written consent to process personal data when they are used for research aims. However, the study has been notified to the institutional review board of the Children Hospital Giovanni XXIII of Bari and data security was ensured by the pseudo-anonymization of identification codes. No patients were personally identifiable at any time during the entire study period. Privacy-by-design (or data protection-by-design) principles were adopted in this study.
Informed Consent Statement
Patient consent was waived due to retrospective studies without direct contact with patients do not need a written consent to process personal data when they are used for research aims.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. (The datasets generated and/or analyzed during the current study are not publicly available due to privacy protection rules).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPA | Hyperphenylalaninemia |
| PKU | Phenylketonuria |
| QUS | Quantitative Ultrasound |
| BQI | Bone Quality Index |
| BUA | Broadband Ultrasound Attenuation |
| Phe | Phenylalanine |
| Tyr | Tyrosine |
| PAH | Phenylalanine Hydroxylase |
| BMD | Bone Mineral Density |
| AA | Amino Acids |
| ISCD | International Society of Clinical Densitometry |
| BMI | Body Mass Index |
| SDS | Standard Deviation Score |
| SOS | Speed of Sound |
| HPLC | High-performance Liquid Chromatography |
| IQR | Interquartile Range |
| GMP | Glycomacropeptide |
| CGMP | Casein Glycomacropeptide |
References
- Kylies, J.; Brunne, B.; Rune, G.M. A culture model for the assessment of phenylalanine neurotoxicity in phenylketonuria. In Vitro Models 2022, 1, 103–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Castro, M.J.; de Lamas, C.; Sánchez-Pintos, P.; González-Lamuño, D.; Couce, M.L. Bone Status in Patients with Phenylketonuria: A Systematic Review. Nutrients 2020, 12, 2154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocha, J.C.; Hermida, Á.; Jones, C.J.; Wu, Y.; Clague, G.E.; Rose, S.; Whitehall, K.B.; Ahring, K.K.; Pessoa, A.L.S.; Harding, C.O.; et al. Meta-analysis of bone mineral density in adults with phenylketonuria. Orphanet J. Rare Dis. 2024, 19, 338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demirdas, S.; Coakley, K.E.; Bisschop, P.H.; Hollak, C.E.; Bosch, A.M.; Singh, R.H. Bone health in phenylketonuria: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2015, 10, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, J.R.; Humphries, I.R.; Waters, D.L.; Roberts, D.C.; Lipson, A.H.; Howman-Giles, R.G.; Gaskin, K.J. Decreased bone mineral density in children with phenylketonuria. Am. J. Clin. Nutr. 1994, 59, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Baim, S.; Leonard, M.B.; Bianchi, M.L.; Hans, D.B.; Kalkwarf, H.J.; Langman, C.B.; Rauch, F. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Pediatric Position Development Conference. J. Clin. Densitom. 2008, 11, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Venegas, E.; Langeveld, S.; Ahring, K.; Benitez, R.; Desloovere, A.; Dios, E.; Gomez, E.; Hermida, A.; Marsaux, C.; Verloo, P.; et al. Nutrient Status and Intakes of Adults with Phenylketonuria. Nutrients 2024, 16, 2724. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Beblo, S.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Coşkun, T.; et al. European guidelines on diagnosis and treatment of phenylketonuria: First revision. Mol. Genet. Metab. 2025, 145, 109125. [Google Scholar] [CrossRef] [PubMed]
- Tummolo, A.; Carella, R.; De Giovanni, D.; Di Tullio, V.; Lorusso, L.; Bartolomeo, N. A Cross-Sectional Study on Protein Substitutes for Paediatric Phenylketonuria Diet: Time to Pay Attention. Nutrients 2025, 17, 1767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stroup, B.M.; Sawin, E.A.; Murali, S.G.; Binkley, N.; Hansen, K.E.; Ney, D.M. Amino Acid Medical Foods Provide a High Dietary Acid Load and Increase Urinary Excretion of Renal Net Acid, Calcium, and Magnesium Compared with Glycomacropeptide Medical Foods in Phenylketonuria. J. Nutr. Metab. 2017, 2017, 1909101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanusch, B.; Schlegtendal, A.; Lücke, T.; Sinningen, K. Ex Vivo Osteoclastogenesis from Peripheral Blood Mononuclear Cells Is Unchanged in Adults with Phenylketonuria, Regardless of Dietary Compliance. Int. J. Mol. Sci. 2025, 26, 5776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tummolo, A.; Brunetti, G.; Giordano, M.; Carbone, V.; Faienza, M.F.; Aricò, M.; Pesce, S. The use of quantitative ultrasound in a tertiary-level children hospital: Role in the follow-up of chronically ill patients. J. Ultrasound 2022, 25, 563–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salera, S.; Coacci, S.; Cipriani, A.; Gentilucci, M.; Pierattini, V. Special low protein foods in the management of Inborn Errors of Metabolism: Overview on availability, composition and comparison with regular foods in Italy. JIM 2024, 1, e532. [Google Scholar]
- Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Bélanger-Quintana, A.; MacDonald, A.; Dokoupil, K.; Gokmen Ozel, H.; Lammardo, A.M.; Goyens, P.; et al. Micronutrient status in phenylketonuria. Mol. Genet. Metab. 2013, 110, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Kose, E.; Arslan, N. Vitamin/mineral and micronutrient status in patients with classical phenylketonuria. Clin. Nutr. 2019, 38, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Hanusch, B.; Falkenstein, M.; Volkenstein, S.; Dazert, S.; Lücke, T.; Sinningen, K. No Impairment in Bone Turnover or Executive Functions in Well-Treated Preschoolers with Phenylketonuria-A Pilot Study. Nutrients 2024, 16, 2072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanusch, B.; Schlegtendal, A.; Grasemann, C.; Lücke, T.; Sinningen, K. Adults with Phenylketonuria have suboptimal bone mineral density apart from vitamin D and calcium sufficiency. Front. Endocrinol. 2025, 16, 1488215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daly, A.; Högler, W.; Crabtree, N.; Shaw, N.; Evans, S.; Pinto, A.; Jackson, R.; Ashmore, C.; Rocha, J.C.; Strauss, B.J.; et al. A Three-Year Longitudinal Study Comparing Bone Mass, Density, and Geometry Measured by DXA, pQCT, and Bone Turnover Markers in Children with PKU Taking L-Amino Acid or Glycomacropeptide Protein Substitutes. Nutrients 2021, 13, 2075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiba, K.; Suetoshi, R.; Cretin, D.; Arai, T.; Kawajiri, T.; Okayama, A.; Tsuji, S.; Okazaki, N.; Osaki, M.; Yoh, K. Development of a QUS Device to Evaluate Deterioration of Cortical Bone: Verification by HR-pQCT and Measurements in Healthy Individuals and Dialysis Patients. J. Clin. Densitom. 2021, 24, 94–105. [Google Scholar] [CrossRef]
- Hans, D.; Baim, S. Quantitative Ultrasound (QUS) in the Management of Osteoporosis and Assessment of Fracture Risk. J. Clin. Densitom. 2017, 20, 322–333. [Google Scholar] [CrossRef]
- Krieg, M.A.; Barkmann, R.; Gonnelli, S.; Stewart, A.; Bauer, D.C.; Del Rio Barquero, L.; Kaufman, J.J.; Lorenc, R.; Miller, P.D.; Olszynski, W.P.; et al. Quantitative ultrasound in the management of osteoporosis: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Shamir, R.; Eshach-Adiv, O.; Iosilevsky, G.; Brik, R. Assessment of osteoporosis by quantitative ultrasound versus dual energy X-ray absorptiometry in children with chronic rheumatic diseases. J. Rheumatol. 2004, 31, 981–985. [Google Scholar] [PubMed]
- Schalamon, J.; Singer, G.; Schwantzer, G.; Nietosvaara, Y. Quantitative ultrasound assessment in children with fractures. J. Bone Miner. Res. 2004, 19, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.Y.Y.; Turcato, M.F.; Nicoletti, C.F.; Nonino, C.B.; Martins, L.D.; Iannetta, O.; Guerreiro, C.T.; Santos, G.G.; Marchini, J.S. Effects of Short-Term Calcium Supplementation in Children and Adolescents with Phenylketonuria. J. Clin. Densitom. 2018, 21, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.; Mussa, A.; Zanin, A.; Greggio, N.A.; Burlina, A.; Spada, M. Impact of metabolic control on bone quality in phenylketonuria and mild hyperphenylalaninemia. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 345–350. [Google Scholar] [CrossRef] [PubMed]
- de Groot, M.J.; Hoeksma, M.; van Rijn, M.; Slart, R.H.; van Spronsen, F.J. Relationships between lumbar bone mineral density and biochemical parameters in phenylketonuria patients. Mol. Genet. Metab. 2012, 105, 566–570. [Google Scholar] [CrossRef]
- Adami, S.; Romagnoli, E.; Carnevale, V.; Scillitani, A.; Giusti, A.; Rossini, M.; Gatti, D.; Nuti, R.; Minisola, S. Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Linee guida su prevenzione e trattamento dell’ipovitaminosi D con colecalciferolo. SIOMMMS [Guidelines on prevention and treatment of vitamin D deficiency. Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS)]. Reumatismo 2011, 63, 129–147. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- Tummolo, A.; Carella, R.; Paterno, G.; Bartolomeo, N.; Giotta, M.; Dicintio, A.; De Giovanni, D.; Fischetto, R. Body Composition in Adolescent PKU Patients: Beyond Fat Mass. Children 2022, 9, 1353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manuals and User Guides for OsteoSys SONOST 3000, version 3.03.06; OsteoSys Co., Ltd.: Seoul, Republic of Korea. Available onlne: https://www.manualslib.com/manual/1285276/Osteosys-Sonost-3000.html (accessed on 10 November 2025).
- Ahmadzadeh, M.; Sohrab, G.; Alaei, M.; Eini-Zinab, H.; Mohammadpour-Ahranjani, B.; Rastgoo, S.; Namkhah, Z. Growth and Nutritional Status of Phenylketonuric Children and Adolescents. BMC Pediatr. 2022, 22, 664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthews, J.J.; Arentson-Lantz, E.J.; Moughan, P.J.; Wolfe, R.R.; Ferrando, A.A.; Church, D.D. Understanding Dietary Protein Quality: Digestible indispensable Amino Acid Scores and Beyond. J. Nutr. 2025, 155, 3152–3167. [Google Scholar] [CrossRef]
- Reis, D.; Schwermer, M.; Nowak, L.; Naami, N.; Zuzak, T.J.; Längler, A. Vegetarian Diet and Dietary Intake, Health, and Nutritional Status in Infants, Children, and Adolescents: A Systematic Review. Nutrients 2025, 17, 2183. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, S.F.; Tourkova, I.L.; Sudano, C.R.; Larrouture, Q.C.; Blair, H.C. A New View of Bone Loss in Phenylketonuria. Organogenesis 2021, 17, 50–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobrowolski, S.F.; Sudano, C.; Phua, Y.L.; Tourkova, I.L.; Spridik, K.; Goetzman, E.S.; Vockley, J.; Blair, H.C. Mesenchymal stem cell energy deficit and oxidative stress contribute to osteopenia in the Pahenu2 classical PKU mouse. Mol. Genet. Metab. 2021, 132, 173–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bokayeva, K.; Jamka, M.; Walkowiak, D.; Duś-Żuchowska, M.; Herzig, K.H.; Walkowiak, J. Vitamin Status in Patients with Phenylketonuria: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 5065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, F.; Zheng, L.; Theopold, J.; Schleifenbaum, S.; Heyde, C.E.; Osterhoff, G. Methods for bone quality assessment in human bone tissue: A systematic review. J. Orthop. Surg. Res. 2022, 17, 174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bauer, D.C.; Glüer, C.C.; Cauley, J.A.; Vogt, T.M.; Ensrud, K.E.; Genant, H.K.; Black, D.M. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Arch. Intern. Med. 1997, 157, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Fournier, B.; Laugier, P.; Chappard, C.; Kolta, S.; Dougados, M.; Berger, G. Broadband ultrasound attenuation imaging: A new imaging method in osteoporosis. J. Bone Miner. Res. 1996, 11, 1112–1118. [Google Scholar] [CrossRef]
- Perrott, S.L.; Martin, K.; Keevil, V.L.; Wareham, N.J.; Khaw, K.T.; Myint, P.K. Calcaneal broadband ultrasound attenuation predicts physical capability: EPIC-Norfolk prospective population-based study. Maturitas 2023, 173, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Çıkı, K.; Kahraman, A.B.; Akar, H.T.; Yıldız, Y.; Dursun, A.; Tokatlı, A.; Coşkun, T.; Sivri, S. High prevalence of low bone mineral density in young adults with phenylketonuria. Postgrad. Med. 2025, 137, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Dybal, E.; Maillot, F.; Feillet, F.; Fouilhoux, A.; Astudillo, L.; Lavigne, C.; Arnoux, J.B.; Odent, S.; Gay, C.; Schiff, M.; et al. Bone mineral density in French adults with early-treated phenylketonuria. Mol. Genet. Metab. 2025, 144, 109044. [Google Scholar] [CrossRef] [PubMed]
- Paterno, G.; Di Tullio, V.; Carella, R.; De Ruvo, G.; Furioso, F.; Skublewska-D’Elia, A.; De Giovanni, D.; Tummolo, A. Growth Parameters and Prevalence of Obesity in PKU Patients and Peers: Is This the Right Comparison? Pediatr. Rep. 2024, 16, 892–901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lowe, T.B.; DeLuca, J.; Arnold, G.L. Similarities and differences in key diagnosis, treatment, and management approaches for PAH deficiency in the United States and Europe. Orphanet J. Rare Dis. 2020, 15, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).