Personalized Delivery of Probiotics and Prebiotics via 3D Food Printing
Abstract
1. Introduction
2. Diet, Probiotics, and Prebiotics in Microbiota Modulation
2.1. Diet in Microbiota Modulation
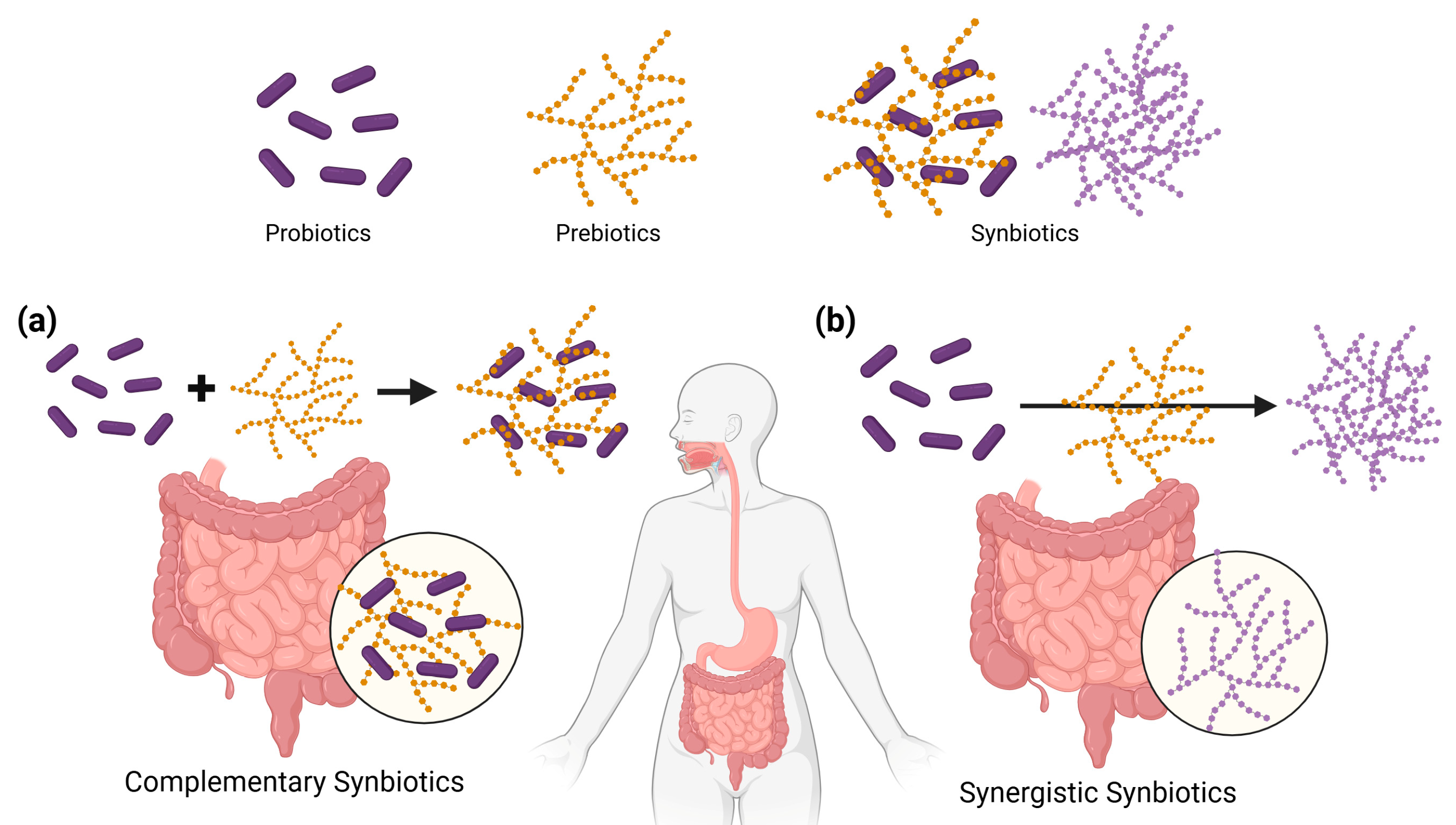
2.2. Probiotics, Prebiotics, and Synbiotics in Microbiota Modulation
2.3. Limitation
3. Three-Dimensional Food Printing for Personalized Nutrition
3.1. General Overview of 3D Food Printing
3.2. Three-Dimensional Food Printing for Personalization
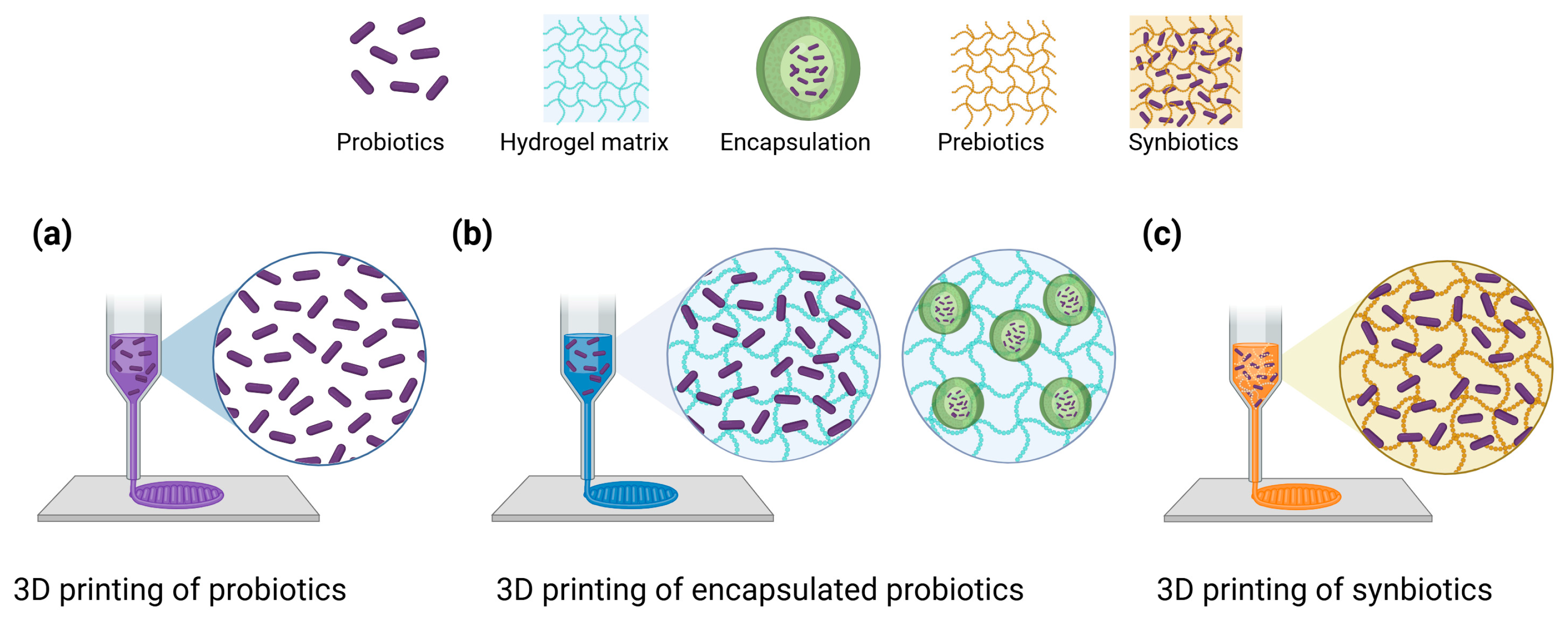
3.3. Three-Dimensional Food Printing for Probiotics
4. Advances in Personalized Delivery of Probiotics and Prebiotics via 3D Food Printing
4.1. Encapsulation and Targeted Release
4.2. Printing Conditions and Process Parameters
4.2.1. Temperature
4.2.2. Nozzle Sizes
4.2.3. Infill Patterns
4.2.4. Post-Processing
5. Synbiotic Applications in 3D Printing
6. Challenges and Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three dimensional |
| SCFA | Short-chain fatty acid |
| CAD | Computer-aided design |
| CFU | Colony Forming Unit |
| GI | Gastrointestinal |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| AI | Artificial intelligence |
References
- Yoon, Y.S.; Lee, H.I.; Oh, S.W.; Lee, H. A Life-Stage Approach to Precision Nutrition: A Narrative Review. Cureus 2024, 16, e66813. [Google Scholar] [CrossRef] [PubMed]
- Brügger, V.; Kowatsch, T.; Jovanova, M. Predicting postprandial glucose excursions to personalize dietary interventions for type-2 diabetes management. Sci. Rep. 2025, 15, 25920. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Linenberg, I.; Polidori, L.; Asnicar, F.; Arre, A.; Wolf, J.; Badri, F.; Bernard, H.; Capdevila, J.; Bulsiewicz, W.J.; et al. Effects of a personalized nutrition program on cardiometabolic health: A randomized controlled trial. Nat. Med. 2024, 30, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ehlert, B.; Metwally, A.A.; Perelman, D.; Park, H.; Brooks, A.W.; Abbasi, F.; Michael, B.; Celli, A.; Bejikian, C.; et al. Individual variations in glycemic responses to carbohydrates and underlying metabolic physiology. Nat. Med. 2025, 31, 2232–2243. [Google Scholar] [CrossRef]
- Bashiardes, S.; Godneva, A.; Elinav, E.; Segal, E. Towards utilization of the human genome and microbiome for personalized nutrition. Curr. Opin. Biotechnol. 2018, 51, 57–63. [Google Scholar] [CrossRef]
- Hugon, P.; Lagier, J.C.; Colson, P.; Bittar, F.; Raoult, D. Repertoire of human gut microbes. Microb. Pathog. 2017, 106, 103–112. [Google Scholar] [CrossRef]
- Abeles, S.R.; Pride, D.T. Molecular bases and role of viruses in the human microbiome. J. Mol. Biol. 2014, 426, 3892–3906. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Chassaing, B.; Langella, P. Exploring the interaction and impact of probiotic and commensal bacteria on vitamins, minerals and short chain fatty acids metabolism. Microb. Cell Factories 2024, 23, 172. [Google Scholar] [CrossRef]
- Şenel, S. An overview of physical, microbiological and immune barriers of oral mucosa. Int. J. Mol. Sci. 2021, 22, 7821. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, 4–17. [Google Scholar] [CrossRef]
- Li, J.; Ghosh, T.S.; Arendt, E.; Shanahan, F.; O’Toole, P.W. Cross-Cohort Gut Microbiome Signatures of Irritable Bowel Syndrome Presentation and Treatment. Adv. Sci. 2024, 11, 2308313. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes 2024, 16, 2304900. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikaram, M.A.; Uitterlinden, A.; Zhernakova, A.; Fu, J.; et al. Association of insulin resistance and type 2 diabetes with gut microbial diversity: A microbiome-wide analysis from population studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chi, L.; Zhu, Y.; Shi, X.; Tu, P.; Li, B.; Yin, J.; Gao, N.; Shen, W.; Schnabl, B. An introduction to next generation sequencing bioinformatic analysis in gut microbiome studies. Biomolecules 2021, 11, 530. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.b.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- di Vito, R.; Conte, C.; Traina, G. A multi-strain probiotic formulation improves intestinal barrier function by the modulation of tight and adherent junction proteins. Cells 2022, 11, 2617. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Afrakoti, L.G.M.P.; Kavyani, Z.; Nogourani, Z.S.; Musazadeh, V.; Jafarlou, M.; Dehghan, P. The role of probiotic supplementation in inflammatory biomarkers in adults: An umbrella meta-analysis of randomized controlled trials. Inflammopharmacology 2023, 31, 2253–2268. [Google Scholar] [CrossRef]
- Higuchi, T.; Furuichi, M.; Maeda, N.; Tsugawa, T.; Ito, K. Effects of probiotics in children with acute gastroenteritis: A systematic review and meta-analysis focusing on probiotics utilized in Japan. J. Infect. Chemother. 2024, 30, 337–342. [Google Scholar] [CrossRef]
- Cuthill, S.; Muroke, V.; Dubois, A.; Dubé, M.P.; Guertin, M.C.; Millette, M.; Tardif, J.C. Effect of probiotic supplementation on glycemic control in patients with type 2 diabetes: A randomized controlled trial. Clin. Nutr. ESPEN 2025, 68, 148–152. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Karen, S.; Catherine, S.; Kelly, S.S.; Patrice, D.C.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.; Walter, J.; Gibson, G.R.; Bedu-Ferrari, C.; Scott, K.; Tancredi, D.J.; Wijeyesekera, A.; Sanders, M.E. Classifying compounds as prebiotics—Scientific perspectives and recommendations. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Karen, P.S.; Hannah, D.H.; Meghan, B.A.; Nathalie, M.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Mkilima, T. Engineering artificial microbial consortia for personalized gut microbiome modulation and disease treatment. Ann. N. Y. Acad. Sci. 2025, 1548, 29–55. [Google Scholar] [CrossRef]
- Baral, K.C.; Bajracharya, R.; Lee, S.H.; Han, H.K. Advancements in the pharmaceutical applications of probiotics: Dosage forms and formulation technology. Int. J. Nanomed. 2021, 16, 7535–7556. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Guimarães, J.F.; Coutinho, N.M.; Pimentel, T.C.; Ranadheera, C.S.; Santillo, A.; Albenzio, M.; Cruz, A.G.; Sant’Ana, A.S. The future of functional food: Emerging technologies application on prebiotics, probiotics and postbiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2560–2586. [Google Scholar] [CrossRef]
- Krishna Kumar, R.; Foster, K.R. 3D printing of microbial communities: A new platform for understanding and engineering microbiomes. Microb. Biotechnol. 2023, 16, 489–493. [Google Scholar] [CrossRef]
- Millan, M.G.D. 3D food printing: Technological advances, personalization and future challenges in the food industry. Int. J. Gastron. Food Sci. 2024, 37, 10096. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Trujillo-de Santiago, G.; Álvarez, M.M.; Chuck-Hernández, C. Advances and prospective applications of 3D food printing for health improvement and personalized nutrition. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5722–5741. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Duarte, L.G.R.; Bonfim, D.O.; Salgaço, M.K.; Mattoso, L.H.C.; Egea, M.B. Shaping the future of functional foods: Using 3D printing for the encapsulation and development of new probiotic foods. Probiotics Antimicrob. Proteins 2025, 17, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2023, 62, 101828. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.D.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Sidhu, S.R.K.; Kok, C.W.; Kunasegaran, T.; Ramadas, A. Effect of plant-based diets on gut microbiota: A systematic review of interventional studies. Nutrients 2023, 15, 1510. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.l.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef]
- Gundogdu, A.; Nalbantoglu, O.U. The role of the Mediterranean diet in modulating the gut microbiome: A review of current evidence. Nutrition 2023, 114, 112118. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Nshanian, M.; Gruber, J.J.; Geller, B.S.; Chleilat, F.; Lancaster, S.M.; White, S.M.; Alexandrova, L.; Camarillo, J.M.; Kelleher, N.L.; Zhao, Y.; et al. Short-chain fatty acid metabolites propionate and butyrate are unique epigenetic regulatory elements linking diet, metabolism and gene expression. Nat. Metab. 2025, 7, 196–211. [Google Scholar] [CrossRef]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Cotter, P.; Ross, R.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Kołodziej, M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther. 2015, 42, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhu, Y.; Zhang, Y.; Hamza, T.; Yu, H.; Saint Fleur, A.; Galen, J.; Yang, Z.; Feng, H. A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Sci. Transl. Med. 2020, 12, eaax4905. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Ni, H.; Asche, C.V.; Kim, M.; Walayat, S.; Ren, J. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: A meta-analysis. Curr. Med. Res. Opin. 2017, 33, 1191–1197. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Gómez, M.X.; Martínez, I.; Bottacini, F.; O’Callaghan, A.; Ventura, M.; van Sinderen, D.; Hillmann, B.; Vangay, P.; Knights, D.; Hutkins, R.W.; et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 2016, 20, 515–526. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; Federic, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Kaewarsar, E.; Chaiyasut, C.; Lailerd, N.; Makhamrueang, N.; Peerajan, S.; Sirilun, S. Optimization of mixed inulin, fructooligosaccharides, and galactooligosaccharides as prebiotics for stimulation of probiotics growth and function. Foods 2023, 12, 1591. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef]
- Abrams, S.A.; Hawthorne, K.; Aliu, O.; Hicks, P.; Chen, Z.; Griffin, I. An inulin-type fructan enhances calcium absorption in young adults throughout the GI tract with the largest effect occurring in the colon. FASEB J. 2007, 21, A175. [Google Scholar] [CrossRef]
- Giacco, R.; Clemente, G.; Luongo, D.; Lasorella, G.; Fiume, I.; Brouns, F.; Bornet, F.; Patti, L.; Cipriano, P.; Rivellese, A.A.; et al. Effects of short-chain fructo-oligosaccharides on glucose and lipid metabolism in mild hypercholesterolaemic individuals. Clin. Nutr. 2004, 23, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Cheng, L.; Wang, J.; Raghavan, V. Gut microbiome modulation by probiotics, prebiotics, synbiotics and postbiotics: A novel strategy in food allergy prevention and treatment. Crit. Rev. Food Sci. Nutr. 2024, 64, 5984–6000. [Google Scholar] [CrossRef]
- Olivares, M.; Laparra, M.; Sanz, Y. Oral administration of Bifidobacterium longum CECT 7347 modulates jejunal proteome in an in vivo gliadin-induced enteropathy animal model. J. Proteom. 2012, 77, 310–320. [Google Scholar] [CrossRef]
- Musazadeh, V.; Assadian, K.; Rajabi, F.; Faghfouri, A.H.; Soleymani, Y.; Kavyani, Z.; Najafiyan, B. The effect of synbiotics on liver enzymes, obesity indices, blood pressure, lipid profile, and inflammation in patients with non-alcoholic fatty liver: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2024, 208, 107398. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Graziani, A.; Frühbeck, G.; Baffy, G.; Garruti, G.; Ciaula, A.D.; Bonfrate, L. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations? Eur. J. Intern. Med. 2024, 119, 13–30. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 61. [Google Scholar] [CrossRef]
- Kong, C.; Yang, M.; Yue, N.; Zhang, Y.; Tian, C.; Wei, D.; Shi, R.; Yao, J.; Wang, L.; Li, D. Restore intestinal barrier integrity: An approach for inflammatory bowel disease therapy. J. Inflamm. Res. 2024, 17, 5389–5413. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghinia, R.; Nemati, H.; Ebrahimi, A.; Shekouh, D.; Karami, S.; Eraghi, M.M.; Mohagheghzadeh, H.; Hunter, J.; Pasalar, M. The Impact of Probiotics, Prebiotics, and Synbiotics on Depression and Anxiety Symptoms of Patients with Depression: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2025, 188, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic GI transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Puvanasundram, P.; Chong, C.M.; Sabri, S.; Yusoff, M.S.; Karim, M. Multi-strain probiotics: Functions, effectiveness and formulations for aquaculture applications. Aquac. Rep. 2021, 21, 100905. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef]
- D’Amico, V.; Cavaliere, M.; Ivone, M.; Lacassia, C.; Celano, G.; Vacca, M.; Forgia, F.A.; Fontana, S.; Angelis, M.D.; Denora, N.; et al. Microencapsulation of probiotics for enhanced stability and health benefits in dairy functional foods: A focus on pasta filata cheese. Pharmaceutics 2025, 17, 185. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Z.; Zhou, W.; Fuh, J.Y.; Hong, G.S.; Chiu, A. A review on 3D printing for customized food fabrication. Procedia Manuf. 2015, 1, 308–319. [Google Scholar] [CrossRef]
- Demei, K.; Zhang, M.; Phuhongsung, P.; Mujumdar, A.S. 3D food printing: Controlling characteristics and improving technological effect during food processing. Food Res. Int. 2022, 156, 111120. [Google Scholar] [CrossRef]
- Zhu, S.; Ramos, P.V.; Heckert, O.R.; Stieger, M.; van der Goot, A.J.; Schutyser, M. Creating protein-rich snack foods using binder jet 3D printing. J. Food Eng. 2022, 332, 111124. [Google Scholar] [CrossRef]
- Jonkers, N.; Van Dommelen, J.A.W.; Geers, M.G.D. Selective Laser Sintered food: A unit cell approach to design mechanical properties. J. Food Eng. 2022, 335, 111183. [Google Scholar] [CrossRef]
- Tan, C.; Toh, W.Y.; Wong, G.; Li, L. Extrusion-based 3D food printing–Materials and machines. Int. J. Bioprinting 2018, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Bugday, Z.Y.; Venkatachalam, A.; Anderson, P.D.; van der Sman, R.G.M. Rheology of paste-like food inks for 3D printing: Effects of nutrient and water content. Curr. Res. Food Sci. 2024, 9, 100847. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, M.; Bhandari, B. Materials properties of printable edible inks and printing parameters optimization during 3D printing: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3074–3081. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Gruppi, A.; Vieira, M.V.; Matos, G.S.; Vicente, A.A.; Teixeira, J.A.; Fuciños, P.; Spigno, G.; Pastrana, L.M. How additive manufacturing can boost the bioactivity of baked functional foods. J. Food Eng. 2021, 294, 110394. [Google Scholar] [CrossRef]
- Yu, J.Y.; Park, H.J. Customized oral mucosal adhesive film applied with β-carotene-loaded delivery systems using an embedded 3D printing method. Food Control 2024, 158, 110229. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.J.; Yu, J.Y. Coaxial 3D printing for customized orodispersible film based on an embedding system with curcumin liposome and phycocyanin. J. Food Eng. 2024, 371, 111984. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Varvara, R.A.; Szabo, K.; Vodnar, D.C. 3D food printing: Principles of obtaining digitally-designed nourishment. Nutrients 2021, 13, 3617. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, A.; Ghorani, B.; Emadzadeh, B.; Sarabi-Jamab, M.; Emadzadeh, M.; Modiri, A.; Mendes, A.C. Double protection of probiotics in alginate hydrogel through emulsification incorporated with freeze drying and coaxial wet-electrospraying: Survivability and targeted delivery. LWT 2024, 204, 116459. [Google Scholar] [CrossRef]
- Hamayun, M.; Ahmed, E.; Wedamulla, N.; Kanth, B.; Kim, E.K.; Kim, H.Y.; Lee, B. Next-Gen Nutrition: Challenges, Innovations and Opportunities in 3D Food Printing with Probiotics. Future Foods 2025, 11, 100620. [Google Scholar] [CrossRef]
- Mora-Castaño, G.; Domínguez-Robles, J.; Himawan, A.; Millán-Jiménez, M.; Caraballo, I. Current trends in 3D printed gastroretentive floating drug delivery systems: A comprehensive review. Int. J. Pharm. 2024, 663, 124543. [Google Scholar] [CrossRef]
- Uboldi, M.; Chiappa, A.; Briatico-Vangosa, F.; Melocchi, A.; Zema, L. 3D printing of partially-coated floating systems for controlled release of drugs into the stomach. Int. J. Pharm. 2025, 675, 125513. [Google Scholar] [CrossRef]
- Derossi, A.; Spence, C.; Corradini, M.G.; Jekle, M.; Fahmy, A.R.; Caporizzi, R.; Devahastin, S.; Moses, J.A.; Le-Bail, A.; Zhou, W.; et al. Personalized, digitally designed 3D printed food towards the reshaping of food manufacturing and consumption. Sci. Food 2024, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Sohrabvandi, S.; Sadighara, P.; Hosseini, H.; Farhoodi, M.; Assadpour, E.; Sani, M.A.; Zhang, F.; Seyyedi-Mansour, S.; Jafari, S.M.; et al. Personalized nutrition with 3D-printed foods: A systematic review on the impact of different additives. Adv. Colloid Interface Sci. 2024, 328, 103181. [Google Scholar] [CrossRef]
- Truong-Le, Q.A.; Lee, S.O.; Ubeyitogullari, A. Encapsulation of Bifidobacterium bifidum into a pH-sensitive alginate-pectin gel system using 3D food printing: Enhanced viability and targeted release. Int. J. Biol. Macromol. 2025, 318, 145134. [Google Scholar] [CrossRef]
- Fan, D.; Diller, S.; Mansi, S.; Wang, C.; Mela, P.; Özkale, B.; Lieleg, O. A multi-functional 3D-printable gel-in-gel system for the delivery of probiotics to the intestine. Food Hydrocoll. 2024, 156, 110267. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Zhang, M. Incorporation of probiotics (Bifidobacterium animalis subsp. Lactis) into 3D printed mashed potatoes: Effects of variables on the viability. Food Res. Int. 2020, 128, 108795. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, Z.; An, Z.; Hu, L.; Li, H.; Mo, H.; Hati, S. Incorporation of probiotics into 3D printed Pickering emulsion gel stabilized by tea protein/xanthan gum. Food Chem. 2023, 409, 135289. [Google Scholar] [CrossRef]
- Zhang, L.; Lou, Y.; Schutyser, M.A. 3D printing of cereal-based food structures containing probiotics. Food Struct. 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Yoha, K.S.; Anukiruthika, T.; Anila, W.; Moses, J.A.; Anandharamakrishnan, C. 3D printing of encapsulated probiotics: Effect of different post-processing methods on the stability of Lactiplantibacillus plantarum (NCIM 2083) under static in vitro digestion conditions and during storage. LWT 2021, 146, 111461. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.; Tian, B.; Xu, J.; Wang, X.; Dai, H.; Wang, H.; Xu, F.; Wang, C. 3D printed spiral tube–like cellulose scaffold for oral delivery of probiotics. Sci. Adv. 2024, 10, eadp3654. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Führen, J. Synergistic vs. complementary synbiotics: The complexity of discriminating synbiotic concepts using a Lactiplantibacillus plantarum exemplary study. Microbiome Res. Rep. 2024, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Fu, L.; Pan, D.; Chu, Y.; Feng, M.; Lu, Y.; Yang, C.; Wang, Y.; Xia, J.; Sun, G.; et al. Role of probiotics/synbiotic supplementation in glycemic control: A critical umbrella review of meta-analyses of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 1467–1485. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karaca, A.C.; Ebrahimi, M.; Assadpour, E.; Jafari, S.M. The 3D printed probiotic products; an emerging category of the functional foods for the next-generations. Trends Food Sci. Technol. 2024, 148, 104526. [Google Scholar] [CrossRef]
- Jiménez-Villeda, B.E.; Falfán-Cortés, R.N.; Rangel-Vargas, E.; Santos-López, E.M.; Gómez-Aldapa, C.A.; Torres-Vitela, M.R.; Villarruel-López, A.; Castro-Rosas, J. Synbiotic encapsulation: A trend towards increasing viability and probiotic effect. J. Food Process. Preserv. 2023, 2023, 7057462. [Google Scholar] [CrossRef]
- Rivas-Llamas, O.D.; Lara-Ceniceros, T.E.; Gallegos-Infante, J.A.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M.; Bermúdez-Quiñones, G.; González-Herrera, S.M. Advances in 3D printing of functional foods: A focus on plant-based inks. Food Humanit. 2025, 5, 100852. [Google Scholar] [CrossRef]
- Zidarič, T.; Gradišnik, L.; Frangež, T.; Šoštarič, M.; Korunič, E.; Maver, T.; Maver, U. Novel 3D printed polysaccharide-based materials with prebiotic activity for potential treatment of diaper rash. Int. J. Biol. Macromol. 2024, 269, 131958. [Google Scholar] [CrossRef] [PubMed]
- Prithviraj, V.; Díaz, L.P.; Lemus-Mondaca, R.; Ullah, A.; Roopesh, M.S. Emerging advancements in 3D food printing. Front. Food Sci. Technol. 2025, 5, 1607449. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Nahid, M. Smart nutrition: AI and 3D printing for personalized diets. Food Nutr. 2025, 1, 100032. [Google Scholar] [CrossRef]
- Wang, N.; Li, R.; Wang, X.; Yang, X. 4D food printing: Key factors and optimization strategies. Trends Food Sci. Technol. 2024, 145, 104380. [Google Scholar] [CrossRef]
| Type | Strain | Design | Matrix/Scaffold | Key Process Parameters | Outcomes-GI | Outcomes–Storage/In Vivo | Note | Ref |
|---|---|---|---|---|---|---|---|---|
| Probiotics | B. bifidum | Coaxial core–shell | · Core: starch · Shell: alginate + pectin (pH-responsive) | · Post-printing survival > 96% | · SGF pH 1.2 (2 h) : 83.1% · SIF pH 6.8: >90% | - | Strong gastric acid protection Potential for targeted intestinal delivery | [90] |
| L. rhamnosus GG | Gel-in -Gel | · Alginate microgels (with CaCO3) in gelatin–alginate matrix | · Print ~30 °C · 4–5 layers | · SCF 2 h: ~86% (encapsulated) | - | CaCO3 buffering and microenvironmental protection | [91] | |
| B. animalis subsp. lactis BB-12 | Extrusion | · Mashed potato ink | · 24–45 °C: ns · 55 °C (45min) · Nozzle: 0.6/1.0.1.4 mm | - | · 55 °C (45 min) : 10.07→7.99 log CFU/g · 0.6 mm: −0.19 log | Prolonged high temperature and narrow nozzle (shear/oxygen) are detrimental | [92] | |
| B. animalis subsp. Lactis | Extrusion (pickering) | · Tea protein + Xanthan gum | · 45–55 °C: ns · 65 °C (10 min) | - | · 65 °C : 8.07→6.59 log CFU/g · Nozzle effect: ns | Thermal-stress threshold identified | [93] | |
| L. plantarum NCIM 2083 | Extrusion Infill + baking | · Cereal (wheat) dough | · Infill : Honeycomb vs. concentric · Baking 145–205 °C | - | · Honeycomb: 107 · Concentric: 105 · Baking: ~109→105 CFU/g | Internal geometry and baking conditions govern survival | [94] | |
| Synbiotics | L. plantarum NCIM 2083 | Encapsulation + extrusion | · Fructo-oligosaccharides, whey protein, maltodextrin | · Spray freeze + freeze dry | · 4 h GI : 79%, : 6.43 ± 0.17 log CFU/mL | · 35 d (4 °C & ambient) : 96–98% : 7.98 ± 0.48 log CFU/mL · Free: GI −3~−4 log · Storage: ~−2 log | Prebiotics serve both protective and nutritive roles | [95] |
| Roseburia intestinalis | Cellulose spital tube | · Cellulose hydrogel (barrier + fermentable substrate) | · Oral delivery | - | · Intestinal retention ~72 h vs. ~ 12h (free) : butyrate/SCFAs ↑ · In HFD/IBD mice : Weight/adiposity ↓ : lipids ↑ : inflammation ↓ | Scaffold provides simultaneous physical protection and metabolic support | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J. Personalized Delivery of Probiotics and Prebiotics via 3D Food Printing. Metabolites 2025, 15, 744. https://doi.org/10.3390/metabo15110744
Yu J. Personalized Delivery of Probiotics and Prebiotics via 3D Food Printing. Metabolites. 2025; 15(11):744. https://doi.org/10.3390/metabo15110744
Chicago/Turabian StyleYu, Jiyoung. 2025. "Personalized Delivery of Probiotics and Prebiotics via 3D Food Printing" Metabolites 15, no. 11: 744. https://doi.org/10.3390/metabo15110744
APA StyleYu, J. (2025). Personalized Delivery of Probiotics and Prebiotics via 3D Food Printing. Metabolites, 15(11), 744. https://doi.org/10.3390/metabo15110744






