Dynamics of Urine Metabolomics and Tubular Inflammatory Cytokines in Type 1 Diabetes Across Disease Durations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Subjects
2.2. Quantitative ELISA Analysis of Urinary MCP-1, KIM-1, and NGAL
2.3. 1H-Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
2.4. Metabolite Differential Analysis and Pathway Evaluation
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Profiles of T1D Groups
3.2. Urinary MCP-1/Cr, KIM-1/Cr, and NGAL/Cr Levels in T1D Patients
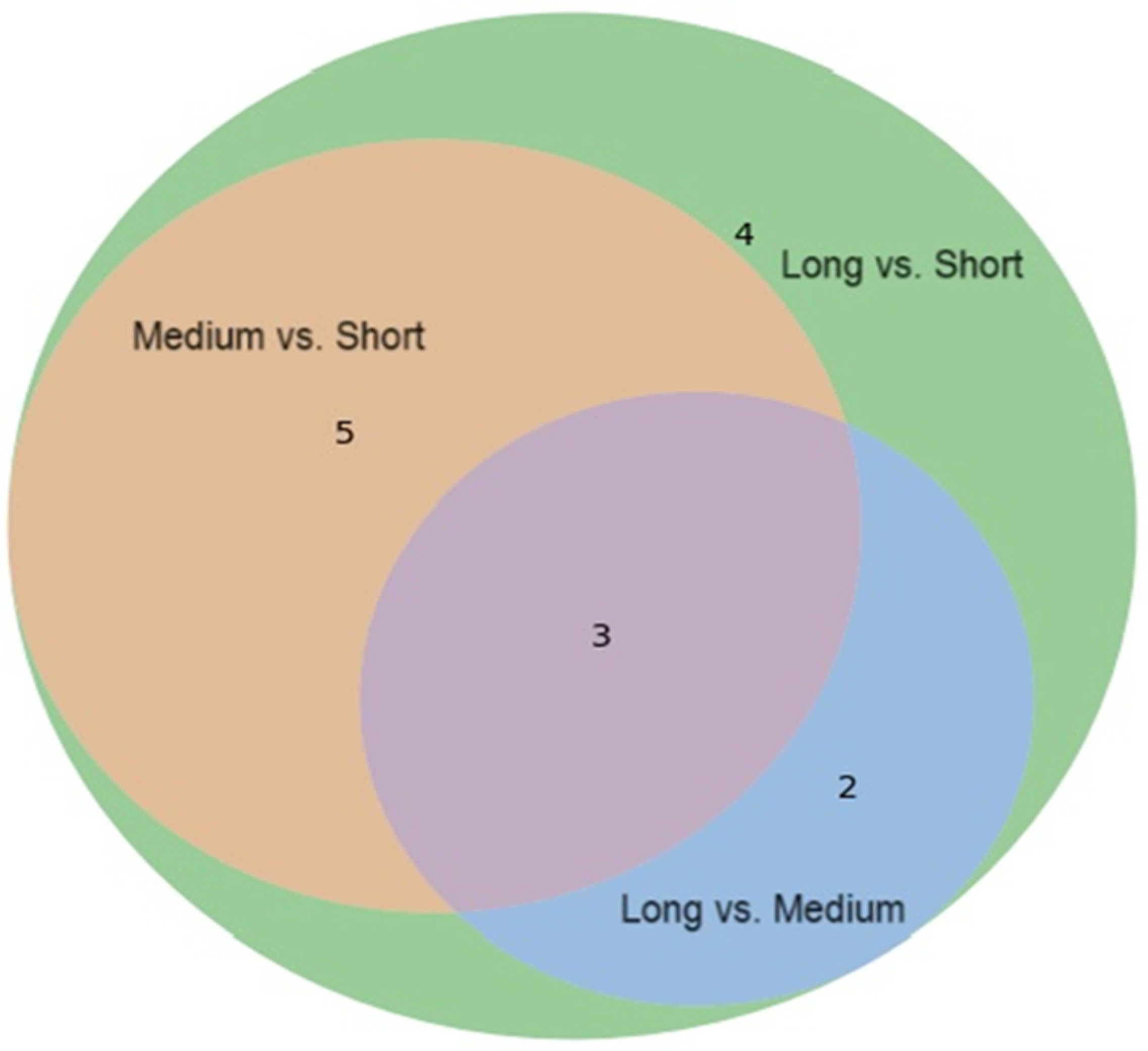
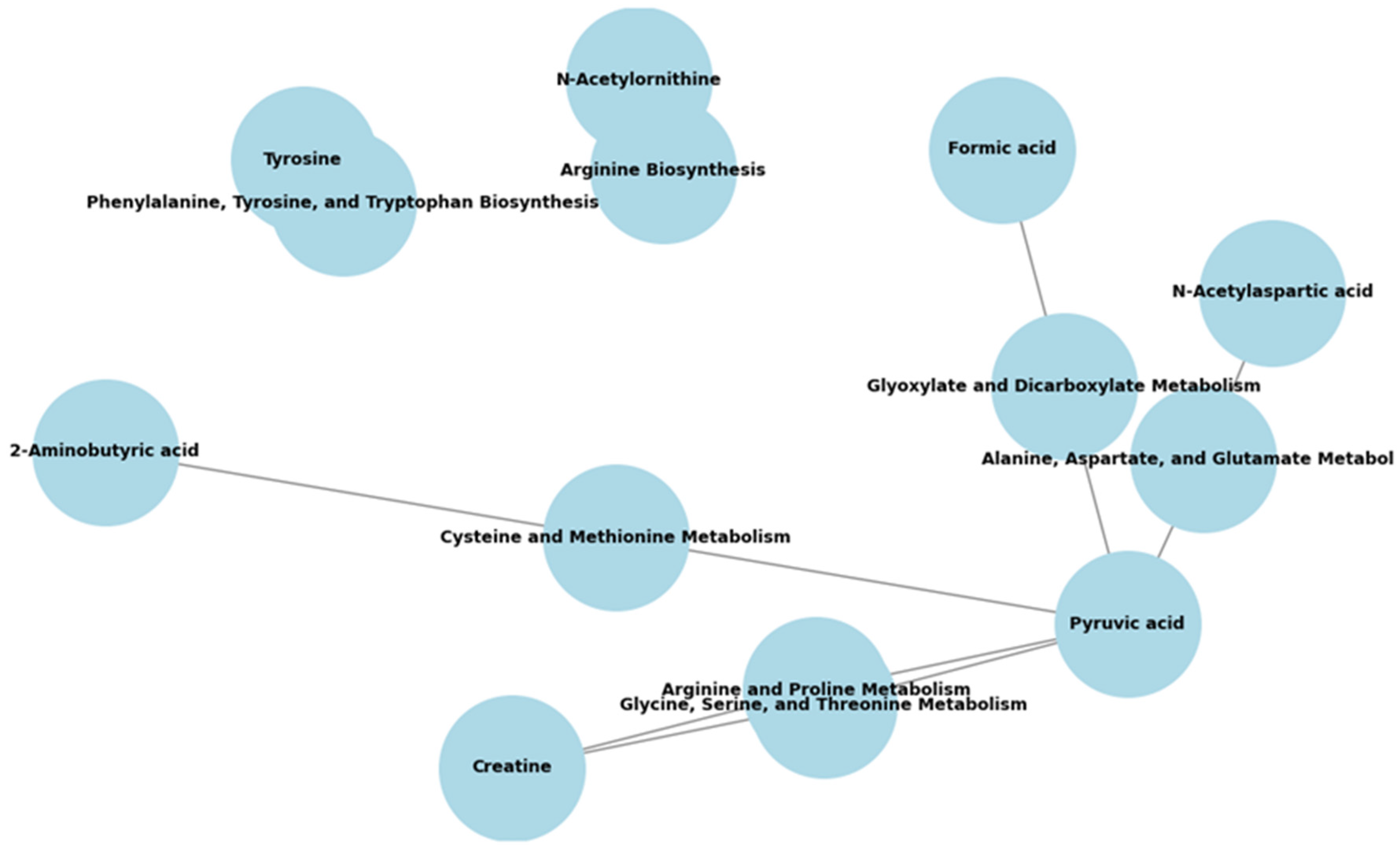
3.3. Distinct Urinary Metabolic Phenotypes and Functional Pathways of T1D-S, T1D-M, and T1D-L Patients
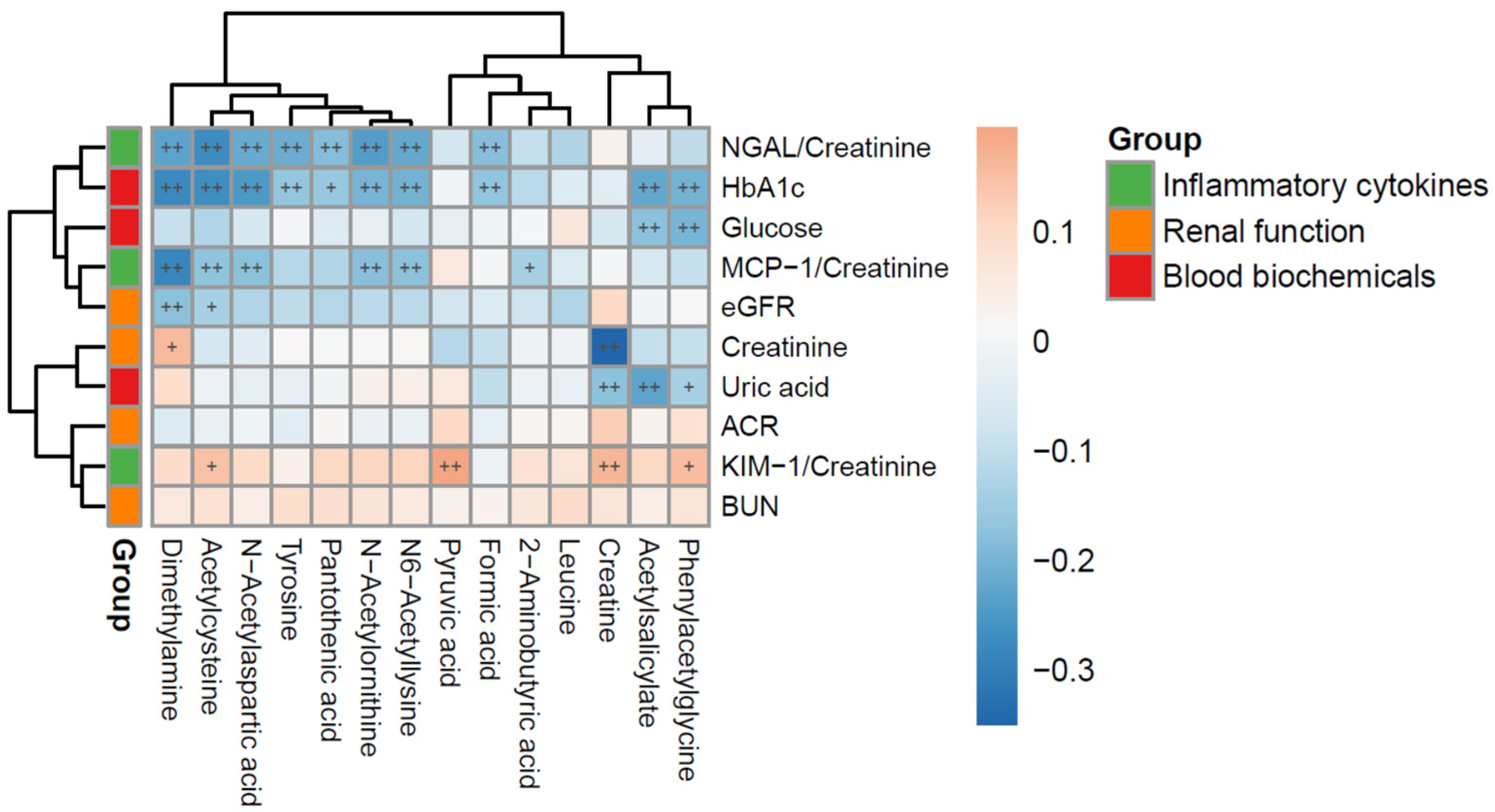
3.4. Relationships Between Urinary Cytokines (uKIM-1/Cr, uMCP-1/Cr, uNGAL/Cr) and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T1D | Type 1 diabetes |
| DKD | Diabetic kidney disease |
| CKD | Chronic kidney disease |
| ESKF | End-stage kidney failure |
| T1D-S | Type 1 diabetes-short duration |
| T1D-M | Type 1 diabetes-medium duration |
| T1D-L | Type 1 diabetes-long duration |
| KIM-1 | Kidney injury molecule-1 |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NAC | N-acetylcysteine |
| NAA | N-acetylaspartic acid |
| NAO | N-acetylornithine |
| BMI | Body mass index |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| FPG | Fasting plasma glucose |
| HbA1c | Glycohemoglobin |
| TC | Total cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| TG | Triglyceride |
| hs-CRP | High-sensitivity C-reactive protein |
| BUN | Blood urea nitrogen |
| Cr | Creatinine |
| eGFR | Estimated glomerular filtration rate |
| WBC | White blood cells |
| Hb | Hemoglobulin |
| UACR | Urine-albumin-to-creatinine ratio |
| ELISA | Enzyme-linked immunosorbent assay |
| NMR | Nuclear magnetic resonance |
References
- Kawasaki, E. Type 1 diabetes and autoimmunity. Clin. Pediatr. Endocrinol. 2014, 23, 99–105. [Google Scholar] [CrossRef]
- Smith, M.J.; Simmons, K.M.; Cambier, J.C. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat. Rev. Nephrol. 2017, 13, 712–720. [Google Scholar] [CrossRef]
- Ogle, G.D.; James, S.; Dabelea, D.; Pihoker, C.; Svennson, J.; Maniam, J.; Klatman, E.L.; Patterson, C.C. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res. Clin. Pract. 2022, 183, 109083. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef]
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef] [PubMed]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Agarwal, R.; Alpers, C.E.; Bakris, G.L.; Brosius, F.C.; Kolkhof, P.; Uribarri, J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022, 102, 248–260. [Google Scholar] [CrossRef]
- Jha, R.; Lopez-Trevino, S.; Kankanamalage, H.R.; Jha, J.C. Diabetes and Renal Complications: An Overview on Pathophysiology, Biomarkers and Therapeutic Interventions. Biomedicines 2024, 12, 1098. [Google Scholar] [CrossRef]
- Pleniceanu, O.; Twig, G.; Tzur, D.; Gruber, N.; Stern-Zimmer, M.; Afek, A.; Erlich, T.; Keinan-Boker, L.; Skorecki, K.; Calderon-Margalit, R.; et al. Kidney failure risk in type 1 vs. type 2 childhood-onset diabetes mellitus. Pediatr. Nephrol. 2021, 36, 333–340. [Google Scholar] [CrossRef]
- Tommerdahl, K.L.; Shapiro, A.L.B.; Nehus, E.J.; Bjornstad, P. Early microvascular complications in type 1 and type 2 diabetes: Recent developments and updates. Pediatr. Nephrol. 2022, 37, 79–93. [Google Scholar] [CrossRef]
- Mottl, A.K.; Tryggestad, J.B.; Isom, S.; Gubitosi-Klug, R.A.; Henkin, L.; White, N.H.; D’Agostino, R., Jr.; Hughan, K.S.; Dolan, L.M.; Drews, K.L. Major adverse events in youth-onset type 1 and type 2 diabetes: The SEARCH and TODAY studies. Diabetes Res. Clin. Pract. 2024, 210, 111606. [Google Scholar] [CrossRef]
- Lopez, L.N.; Wang, W.; Loomba, L.; Afkarian, M.; Butani, L. Diabetic kidney disease in children and adolescents: An update. Pediatr. Nephrol. 2022, 37, 2583–2597. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Steffes, M.; Sun, W.; Rutledge, B.; Cleary, P.; de Boer, I.H.; Zinman, B.; Lachin, J. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010, 33, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Gao, X.; Bebu, I.; de Boer, I.H.; Lachin, J.; Paterson, A.; Perkins, B.; Saenger, A.K.; Steffes, M.; Zinman, B. Early Glomerular Hyperfiltration and Long-Term Kidney Outcomes in Type 1 Diabetes: The DCCT/EDIC Experience. Clin. J. Am. Soc. Nephrol. 2019, 14, 854–861. [Google Scholar] [CrossRef]
- An, N.; Wu, B.T.; Yang, Y.W.; Huang, Z.H.; Feng, J.F. Re-understanding and focusing on normoalbuminuric diabetic kidney disease. Front. Endocrinol. 2022, 13, 1077929. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 2467. [Google Scholar] [CrossRef]
- Mutter, S.; Valo, E.; Aittomäki, V.; Nybo, K.; Raivonen, L.; Thorn, L.M.; Forsblom, C.; Sandholm, N.; Würtz, P.; Groop, P.H. Urinary metabolite profiling and risk of progression of diabetic nephropathy in 2670 individuals with type 1 diabetes. Diabetologia 2022, 65, 140–149. [Google Scholar] [CrossRef]
- Fufaa, G.D.; Weil, E.J.; Nelson, R.G.; Hanson, R.L.; Knowler, W.C.; Rovin, B.H.; Wu, H.; Klein, J.B.; Mifflin, T.E.; Feldman, H.I.; et al. Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol. Dial. Transpl. 2015, 30, 599–606. [Google Scholar] [CrossRef]
- Waijer, S.W.; Sen, T.; Arnott, C.; Neal, B.; Kosterink, J.G.W.; Mahaffey, K.W.; Parikh, C.R.; de Zeeuw, D.; Perkovic, V.; Neuen, B.L.; et al. Association between TNF Receptors and KIM-1 with Kidney Outcomes in Early-Stage Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2022, 17, 251–259. [Google Scholar] [CrossRef]
- Ugarte, F.; Santapau, D.; Gallardo, V.; Garfias, C.; Yizmeyián, A.; Villanueva, S.; Sepúlveda, C.; Rocco, J.; Pasten, C.; Urquidi, C.; et al. Urinary Extracellular Vesicles as a Source of NGAL for Diabetic Kidney Disease Evaluation in Children and Adolescents with Type 1 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 654269. [Google Scholar] [CrossRef]
- Yu, M.C.; Wang, T.M.; Chiou, Y.H.; Yu, M.K.; Lin, C.F.; Chiu, C.Y. Urine metabolic phenotyping in children with nocturnal enuresis and comorbid neurobehavioral disorders. Sci. Rep. 2021, 11, 16592. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Sinnaeve, D.; Van Gasse, B.; Tsiporkova, E.; Rietzschel, E.R.; De Buyzere, M.L.; Gillebert, T.C.; Bekaert, S.; Martins, J.C.; Van Criekinge, W. NMR-based characterization of metabolic alterations in hypertension using an adaptive, intelligent binning algorithm. Anal. Chem. 2008, 80, 3783–3790. [Google Scholar] [CrossRef]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Bloomgarden, Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef]
- Wang, C.; Feng, R.; Sun, D.; Li, Y.; Bi, X.; Sun, C. Metabolic profiling of urine in young obese men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC/Q-TOF MS). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bai, M.; Xie, X.; Wang, J.; Weng, C.; Dai, H.; Chen, J.; Han, F.; Lin, W. Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3345. [Google Scholar] [CrossRef] [PubMed]
- Yako, H.; Niimi, N.; Kato, A.; Takaku, S.; Tatsumi, Y.; Nishito, Y.; Kato, K.; Sango, K. Role of pyruvate in maintaining cell viability and energy production under high-glucose conditions. Sci. Rep. 2021, 11, 18910. [Google Scholar] [CrossRef]
- Zhang, X.M.; Deng, H.; Tong, J.D.; Wang, Y.Z.; Ning, X.C.; Yang, X.H.; Zhou, F.Q.; Jin, H.M. Pyruvate-Enriched Oral Rehydration Solution Improves Glucometabolic Disorders in the Kidneys of Diabetic db/db Mice. J. Diabetes Res. 2020, 2020, 2817972. [Google Scholar] [CrossRef]
- Nogueira, G.B.; Punaro, G.R.; Oliveira, C.S.; Maciel, F.R.; Fernandes, T.O.; Lima, D.Y.; Rodrigues, A.M.; Mouro, M.G.; Araujo, S.R.R.; Higa, E.M.S. N-acetylcysteine protects against diabetic nephropathy through control of oxidative and nitrosative stress by recovery of nitric oxide in rats. Nitric Oxide 2018, 78, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Nongkhlaw, B.; Limesh, M.; Pasanna, R.M.; Thomas, T.; Kuriyan, R.; Kurpad, A.V.; Mukhopadhyay, A. Acyl etha-nolamides in Diabetes and Diabetic Nephropathy: Novel targets from untargeted plasma metabolomic profiles of South Asian Indian men. Sci. Rep. 2019, 9, 18117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Hansen, T.M.; Frøkjær, J.B.; Selvarajah, D.; Muthulingam, J.A.; Tesfaye, S.; Juhl, A.; Drewes, A.M.; Jakobsen, P.E.; Karmisholt, J.; Brock, B.; et al. Reduced thalamic volume and metabolites in type 1 diabetes with polyneuropathy. Exp. Clin. Endocrinol. Diabetes 2022, 130, 327–334. [Google Scholar] [CrossRef]
- Cao, B.N.; Zhang, C.Y.; Wang, Z.; Wang, Y.X. Causal relationship between 412 gut microbiota, 1,400 blood metabolites, and diabetic nephropathy: A randomized Mendelian study. Front. Endocrinol. 2024, 15, 1450428. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Q.; He, J.; Li, Y. Immune responses in diabetic nephropathy: Pathogenic mechanisms and therapeutic target. Front. Immunol. 2022, 13, 958790. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef]
- Forbes, J.M.; McCarthy, D.A.; Kassianos, A.J.; Baskerville, T.; Fotheringham, A.K.; Giuliani, K.T.K.; Grivei, A.; Murphy, A.J.; Flynn, M.C.; Sullivan, M.A.; et al. T-cell expression and release of kidney injury molecule-1 in response to glucose variations initiates kidney injury in early diabetes. Diabetes 2021, 70, 1754–1766. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 2020, 180, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Massa, G.; Guedens, W.; Louis, E.; Noben, J.P.; Adriaensens, P. Metabolic profiling of type 1 diabetes mellitus in children and adolescents: A case-control study. Diabetol. Metab. Syndr. 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, G.; Lokhnygina, Y.; Ramaker, M.; Ilkayeva, O.; Muehlbauer, M.; Evans, W.; Rasbach, L.; Benjamin, R.; Freemark, M.; Gumus Balikcioglu, P. Catabolism of fats and branched-chain amino acids in children with Type 1 diabetes: Association with glycaemic control and total daily insulin dose. Endocrinol. Diabetes Metab. 2023, 6, e448. [Google Scholar] [CrossRef] [PubMed]
| T1D Duration (Years) | Significance | ||||
|---|---|---|---|---|---|
| All | T1D-S (≤5) | T1D-M (6–10) | T1D-L (≥10) | ||
| Variables | n = 247 | n = 62 | n = 67 | n = 118 | |
| Demographic parameters | |||||
| Diabetes duration (years) | 9.7 (5.0, 14.0) | 2.8 (1.5, 3.7) | 7.6 (6.6, 8.9) | 14.1 (12.6, 17.3) | 0.001 |
| Age (years) | 18.6 (13.6, 22.3) | 12.5 (9.3, 16.2) | 16.9 (13.2, 20.6) | 22.0 (19.0, 25.9) | 0.001 |
| Age at diagnosis (years) | 8.2 (4.7, 11.7) | 9.8 (6.3, 13.4) | 9.8 (5.9, 12.4) | 6.7 (4.0, 10.2) * | 0.001 |
| Male/female (number, %) | 121 (49.0%)/126 (51.0%) | 31 (44.9%)/38 (55.1%) | 35 (53.0%)/31 (47.0%) | 55 (49.1%)/57 (50.9%) | |
| BMI (kg/m2) | 21.0 (18.6, 23.0) | 18.7 (16.0, 20.4) | 20.9 (17.9, 23.2) | 22.1 (20.5, 24.4) | 0.001 |
| SBP (mmHg) | 118 (106, 128) | 108 (102, 118) | 118 (104, 128) | 124 (113, 135) | 0.001 |
| DBP (mmHg) | 71 (64, 79) | 66 (60, 72) | 70 (64, 77) | 76 (67, 82) | 0.001 |
| Glycemic control | |||||
| FBS (mg/dL) | 166 (109, 243) | 150 (100, 214) | 184 (99, 246) | 167 (120, 243) | 0.61 |
| HbA1c (%) | 8.2 (7.3, 9.4) | 8.2 (7.1, 9.1) | 8.0 (7.3, 9.4) | 8.4 (7.4, 9.5) | 0.70 |
| Plasma parameters | |||||
| TC (mg/dL) | 176 (158, 202) | 172 (153, 198) | 173 (152, 210) | 178 (164, 203) | 0.16 |
| LDL-C (mg/dL) | 99 (81, 120) | 90 (78, 118) | 94 (81, 123) | 104 (85, 119) * | 0.05 |
| HDL-C (mg/dL) | 61 (53, 72) | 62 (51, 73) | 62 (54, 73) | 61 (54, 70) | 0.78 |
| Triglyceride (mg/dL) | 59 (43, 84) | 59 (40, 79) | 55 (42, 90) | 63 (48, 85) | 0.70 |
| Uric acid (mg/dL) | 4.5 (3.7, 5.3) | 4.1 (3.2, 5.0) | 4.5 (3.6, 5.4) | 4.6 (4.0, 5.3) * | 0.02 |
| hs-CRP (mg/dL) | 0.6 (0.2, 1.6) | 0.3 (0.2, 0.87) | 0.7 (0.2, 1.51) | 0.8 (0.22, 2.06) | 0.41 |
| Homocysteine (μmol/L) | 7.9 (6.6, 9.6) | 6.9 (6.1, 8.9) | 7.7 (6.4, 9.3) | 8.2 (7.2, 10.4) * | 0.01 |
| Hematological parameters | |||||
| WBC (1000/uL) | 6.2 (5.2, 7.5) | 6.0 (5.0, 7.0) | 6.2 (5.3, 7.6) | 6.2 (5.2, 7.6) | 0.58 |
| Hb (g/dL) | 14.0 (13.2, 15) | 13.7 (13.2, 14.2) * | 14.0 (13.1, 14.8) | 14.5 (13.3, 15.3) | 0.01 |
| Platelet (1000/uL) | 283 (240, 327) | 279.0 (232.0, 317.0) | 275.0 (231.0, 329.0) | 289.5 (246.0, 328.0) | 0.34 |
| Nephrology | |||||
| Urine ACR (mg/g) | 5.6 (3.6, 10.6) | 6.3 (4.0, 10.6) | 5.4 (3.6, 9.5) | 5.4 (3.6, 12.4) | 0.73 |
| eGFR (ml/min/1.73 m2) * | 133.4 (114.2, 155.8) | 132.2 (116.9, 144.3) | 131.3 (110.9, 160.8) | 137.2 (115.1, 159.4) | 0.34 |
| T1D Patients | |||||
|---|---|---|---|---|---|
| Non-Diabetes (n = 60) | All T1D (n = 247) | T1D-S (n = 62) | T1D-M (n = 67) | T1D-L (n = 118) | |
| Age (years) | 9.2 (8.4, 10.8) | 18.6 (13.6, 22.3) * | 12.5 (9.3, 16.4) | 16.9 (13.2, 20.6) * | 22.0 (18.9, 25.6) * |
| Male (%) | 24 (40.0%) | 121 (49.0%) | 31 (44.9%) | 35 (53.0%) | 55 (49.1%) |
| BMI (kg/m2) | 17.2 (15.4, 19.5) | 21.0 (18.6, 23.0) * | 18.7 (16.0, 20.4) | 20.9 (17.9, 23.2) * | 22.1 (20.5, 24.4) * |
| Urine cytokines | |||||
| uMCP-1/Cr (µg/g) | 0 (0, 0.05) | 0.11 (0.07, 0.20) * | 0.14 (0.08, 0.25) * | 0.14 (0.07, 0.22) * | 0.10 (0.06, 0.17) * |
| uKIM-1/Cr (µg/g) | 0.39 (0.20, 0.61) | 0.68 (0.37, 1.21) * | 0.85 (0.41, 1.51) * | 0.85 (0.46, 1.49) * | 0.56 (0.30, 1.03) * |
| uNGAL/Cr (µg/g) | 2.53 (1.78, 4.22) | 7.56 (3.44, 25.93) * | 7.45 (4.68, 23.57) * | 7.38 (3.47, 18.21) * | 7.96 (2.93, 31.24) * |
| Urine Cytokines (µg/g) | UACR < 30 mg/g (n = 31) ф | UACR ≧ 30 mg/g (n = 216) ф | p-Value * | Adjusted Odds Ratio Ψ (95% CI) | p-Value * |
|---|---|---|---|---|---|
| uMCP-1/Cr | 0.108 (0.068, 0.189) | 0.177 * (0.071, 0.261) | 0.031 | 0.85 (0.09–7.80) | 0.887 |
| uKIM-1/Cr | 0.664 (0.364, 1.188) | 0.962 * (0.504, 2.488) | 0.020 | 0.74 (0.46–1.18) | 0.209 |
| uNGAL/Cr | 6.802 (3.028, 21.492) | 17.123 * (7.790, 55.674) | 0.0007 | 0.99 (0.98–1.00) | 0.078 |
| Medium vs. Short | Long vs. Short | Long vs. Medium | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Chemical Shift | VIP Score * | Fold Change † | p ‡ | VIP Score | Fold Change | p | VIP Score | Fold Change | p |
| Acetylcysteine | 2.081–2.092 (s) | 1.58 | 0.73 | 0.001 | 2.53 | 0.46 | <0.001 | 2.10 | 0.63 | <0.001 |
| N-Acetylaspartic acid | 2.026–2.030 (s) | 1.40 | 0.77 | 0.001 | 1.67 | 0.65 | <0.001 | 1.04 | 0.85 | 0.009 |
| N-Acetylornithine | 2.045–2.053 (s) | 0.91 | 0.83 | 0.026 | 1.20 | 0.73 | <0.001 | 0.83 | 0.88 | 0.031 |
| Acetylsalicylate | 2.340–2.355 (s) | 1.87 | 0.65 | 0.002 | 2.08 | 0.52 | <0.001 | 1.06 | 0.80 | 0.067 |
| Phenylacetylglycine | 7.423–7.446 (m) | 1.40 | 0.75 | 0.006 | 1.58 | 0.63 | <0.001 | 0.79 | 0.83 | 0.101 |
| Dimethylamine | 2.718–2.734 (s) | 0.93 | 0.86 | 0.006 | 0.64 | 1.03 | 0.016 | 0.57 | 1.20 | 0.854 |
| Creatine | 3.933–3.943 (s) | 2.96 | 0.35 | 0.025 | 1.83 | 0.33 | 0.042 | 1.14 | 0.94 | 0.564 |
| 2-Aminobutyric acid | 0.982–0.987 (t) | 1.57 | 0.65 | 0.033 | 0.95 | 0.71 | 0.005 | 1.07 | 1.09 | 0.221 |
| Pyruvic acid | 2.379–2.388 (s) | 0.81 | 0.90 | 0.123 | 1.54 | 0.75 | <0.001 | 1.60 | 0.83 | 0.008 |
| N6-Acetyllysine | 1.987–1.994 (s) | 0.74 | 0.85 | 0.063 | 1.05 | 0.76 | <0.001 | 0.76 | 0.89 | 0.026 |
| Formic acid | 8.460–8.470 (s) | 0.92 | 0.80 | 0.078 | 1.19 | 0.66 | 0.001 | 0.88 | 0.83 | 0.123 |
| Tyrosine | 6.891–6.916 (m) | 0.74 | 0.83 | 0.123 | 0.80 | 0.75 | 0.008 | 0.40 | 0.89 | 0.449 |
| Pantothenic acid | 0.934–0.940 (s) | 0.81 | 0.82 | 0.064 | 0.77 | 0.80 | 0.009 | 0.66 | 0.97 | 0.508 |
| Leucine | 0.951–0.968 (t) | 0.87 | 0.74 | 0.083 | 0.81 | 0.68 | 0.014 | 0.37 | 0.93 | 0.624 |
| Metabolites | Pathway Name | Total | Hits | Raw p | FDR | Function |
|---|---|---|---|---|---|---|
| T1D-M vs. T1D-S | ||||||
| None | ||||||
| T1D-L vs. T1D-S | ||||||
| N-Acetylaspartic acid, pyruvic acid | Alanine, aspartate, and glutamate metabolism | 28 | 2 | 0.025 | 0.299 | Amino acid metabolism |
| Pyruvic acid, formic acid | Glyoxylate and dicarboxylate metabolism | 32 | 2 | 0.032 | 0.599 | Carbohydrate metabolism |
| Creatine, pyruvic acid | Glycine, serine, and threonine metabolism | 33 | 2 | 0.034 | 0.299 | Amino acid metabolism |
| 2-Aminobutyric acid, pyruvic acid | Cysteine and methionine metabolism | 33 | 2 | 0.034 | 0.299 | Amino metabolism |
| Tyrosine | Phenylalanine, tyrosine, and tryptophan biosynthesis | 4 | 1 | 0.036 | 0.599 | Amino acid metabolism |
| Creatine, pyruvic acid | Arginine and proline metabolism | 38 | 2 | 0.044 | 0.619 | Amino acid metabolism |
| T1D-L vs. T1D-M | ||||||
| N-Acetylaspartic acid, pyruvic acid | Alanine, aspartate, and glutamate metabolism | 28 | 2 | 0.003 | 0.256 | Amino acid metabolism |
| N-Acetylornithine | Arginine biosynthesis | 14 | 1 | 0.044 | 1.000 | Amino acid metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.-S.; Chiu, C.-Y.; Lo, F.-S.; Lin, W.-C.; Wu, L.-J.; Yen, C.-Y.; Yu, M.-C. Dynamics of Urine Metabolomics and Tubular Inflammatory Cytokines in Type 1 Diabetes Across Disease Durations. Metabolites 2025, 15, 734. https://doi.org/10.3390/metabo15110734
Yu M-S, Chiu C-Y, Lo F-S, Lin W-C, Wu L-J, Yen C-Y, Yu M-C. Dynamics of Urine Metabolomics and Tubular Inflammatory Cytokines in Type 1 Diabetes Across Disease Durations. Metabolites. 2025; 15(11):734. https://doi.org/10.3390/metabo15110734
Chicago/Turabian StyleYu, Mei-Shiuan, Chih-Yung Chiu, Fu-Sung Lo, Wei-Cheng Lin, Li-Jia Wu, Cih-Yi Yen, and Mei-Ching Yu. 2025. "Dynamics of Urine Metabolomics and Tubular Inflammatory Cytokines in Type 1 Diabetes Across Disease Durations" Metabolites 15, no. 11: 734. https://doi.org/10.3390/metabo15110734
APA StyleYu, M.-S., Chiu, C.-Y., Lo, F.-S., Lin, W.-C., Wu, L.-J., Yen, C.-Y., & Yu, M.-C. (2025). Dynamics of Urine Metabolomics and Tubular Inflammatory Cytokines in Type 1 Diabetes Across Disease Durations. Metabolites, 15(11), 734. https://doi.org/10.3390/metabo15110734






