Characteristic Analysis of Metabolic Profiles of Polygonatum odoratum (Mill.) Druce from Different Regions of Guizhou Province Based on Non-Targeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chromatographic Conditions
2.3. Data Analysis
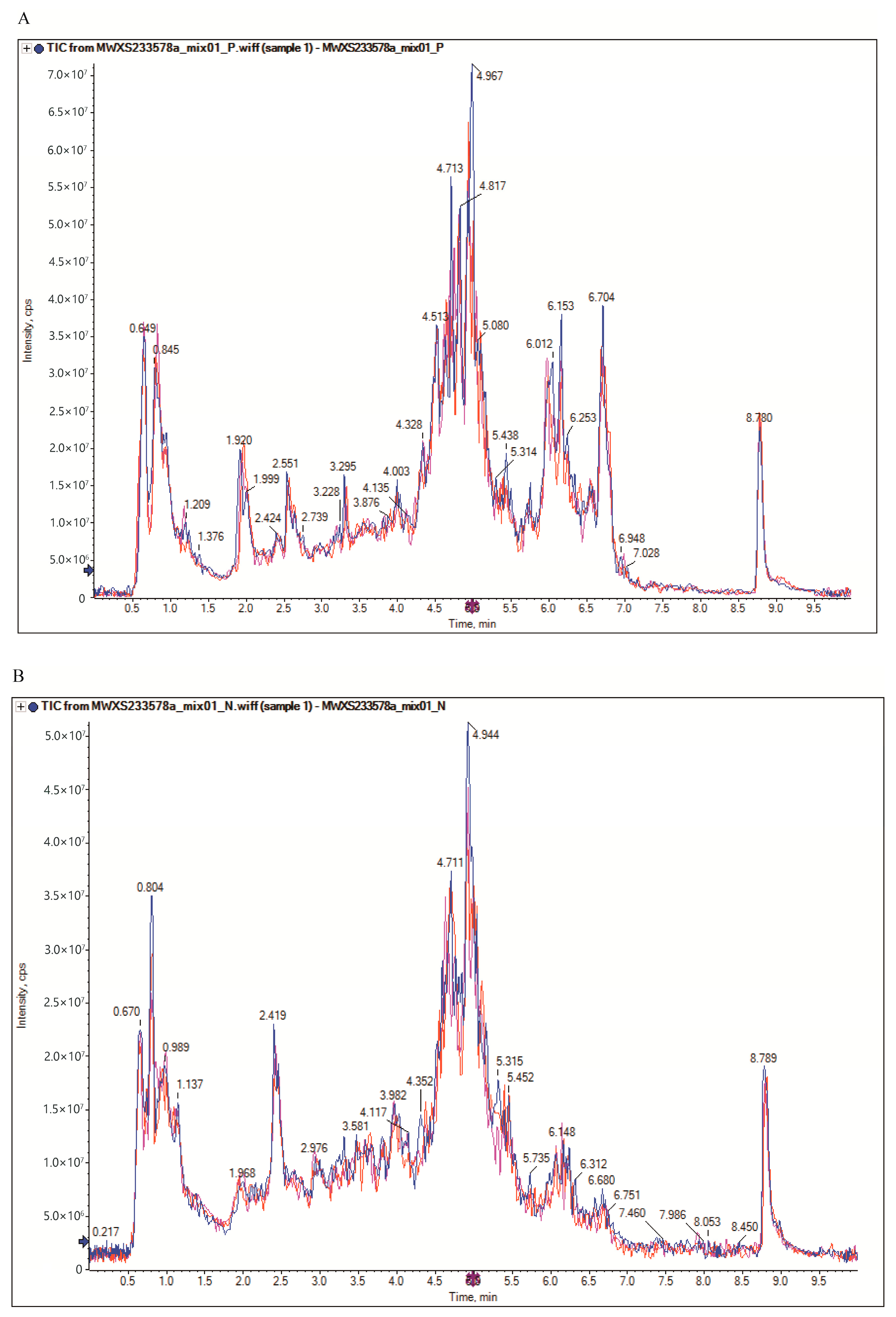
2.4. Quality Control Analysis
3. Results
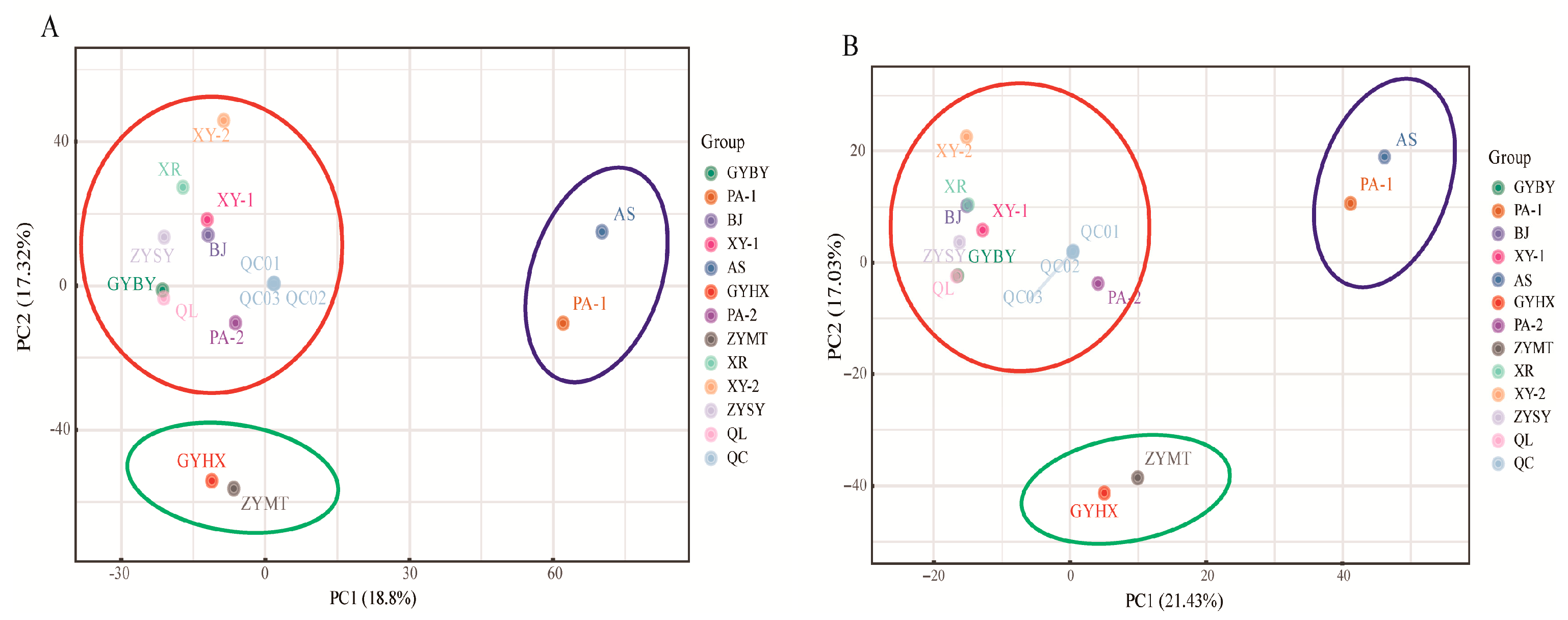
3.1. PCA Results of P. odoratum Samples from 12 Regions in Guizhou Province
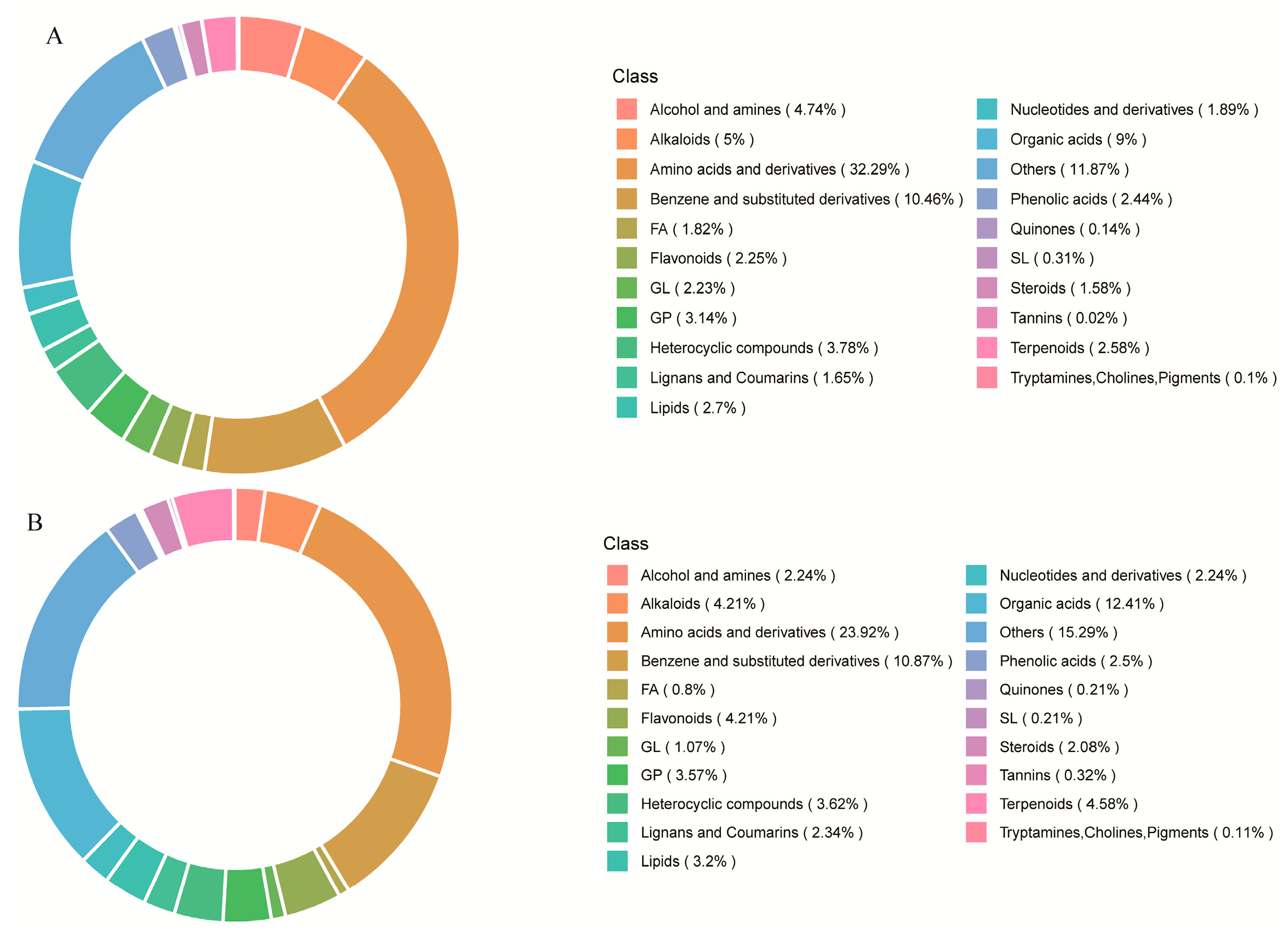
3.2. Identification of Metabolite Components in P. odoratum
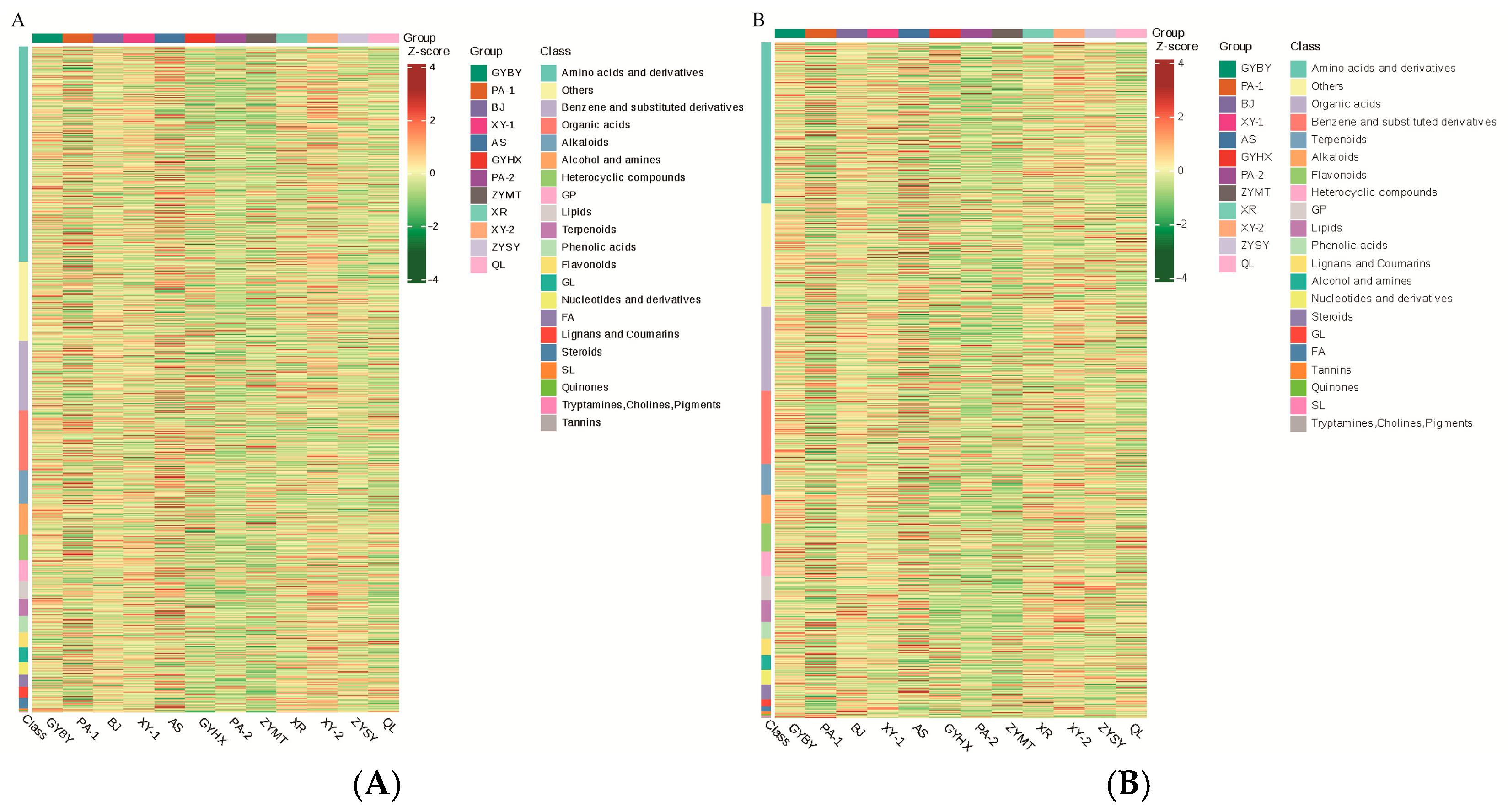
3.3. Comparison of Metabolite Components in P. odoratum from Different Regions
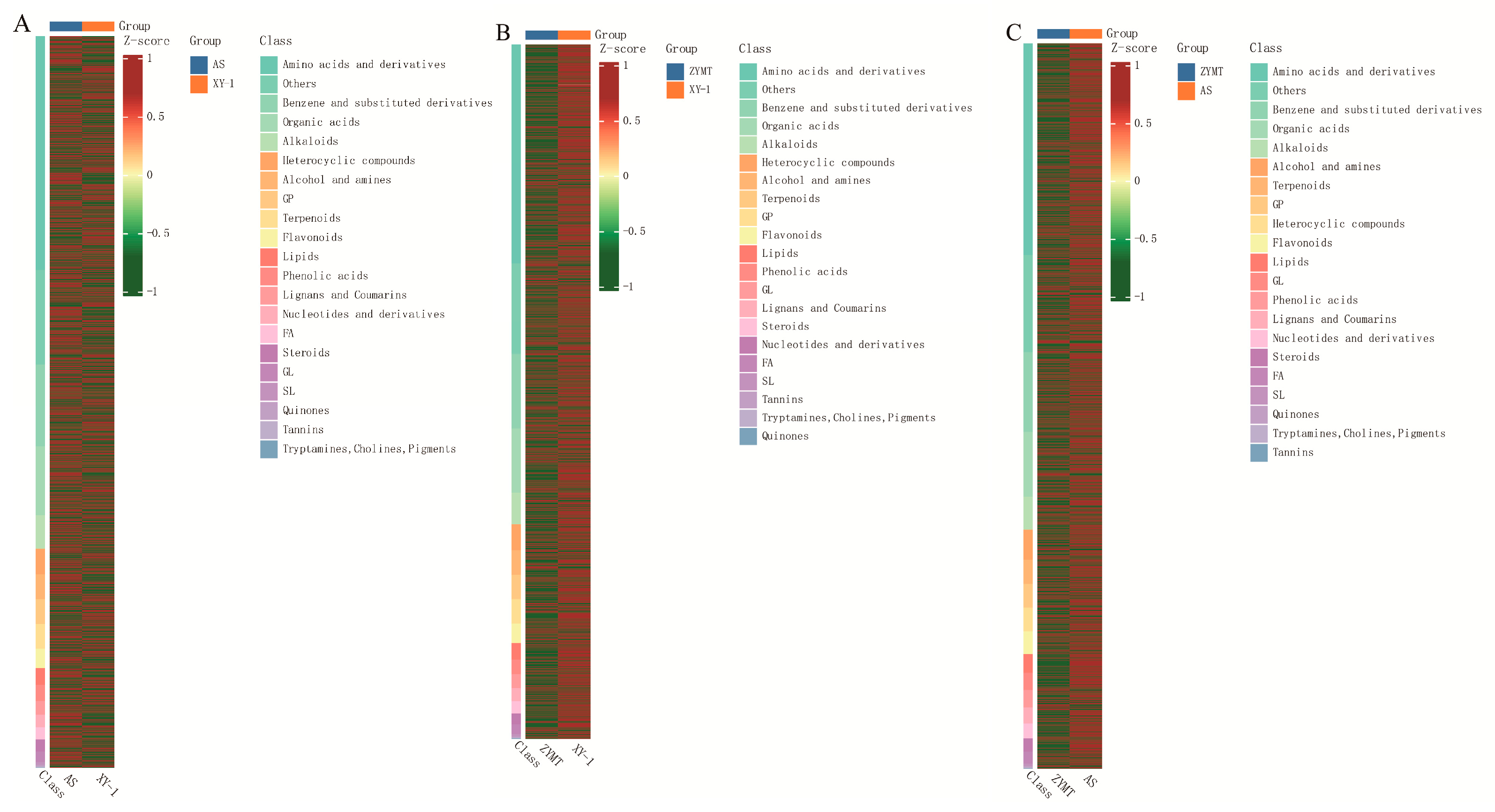
3.4. Screening of Differential Metabolites in P. odoratum from Different Regions of Guizhou Province
3.4.1. Analysis of Differential Metabolites in P. odoratum from Three Representative Origins
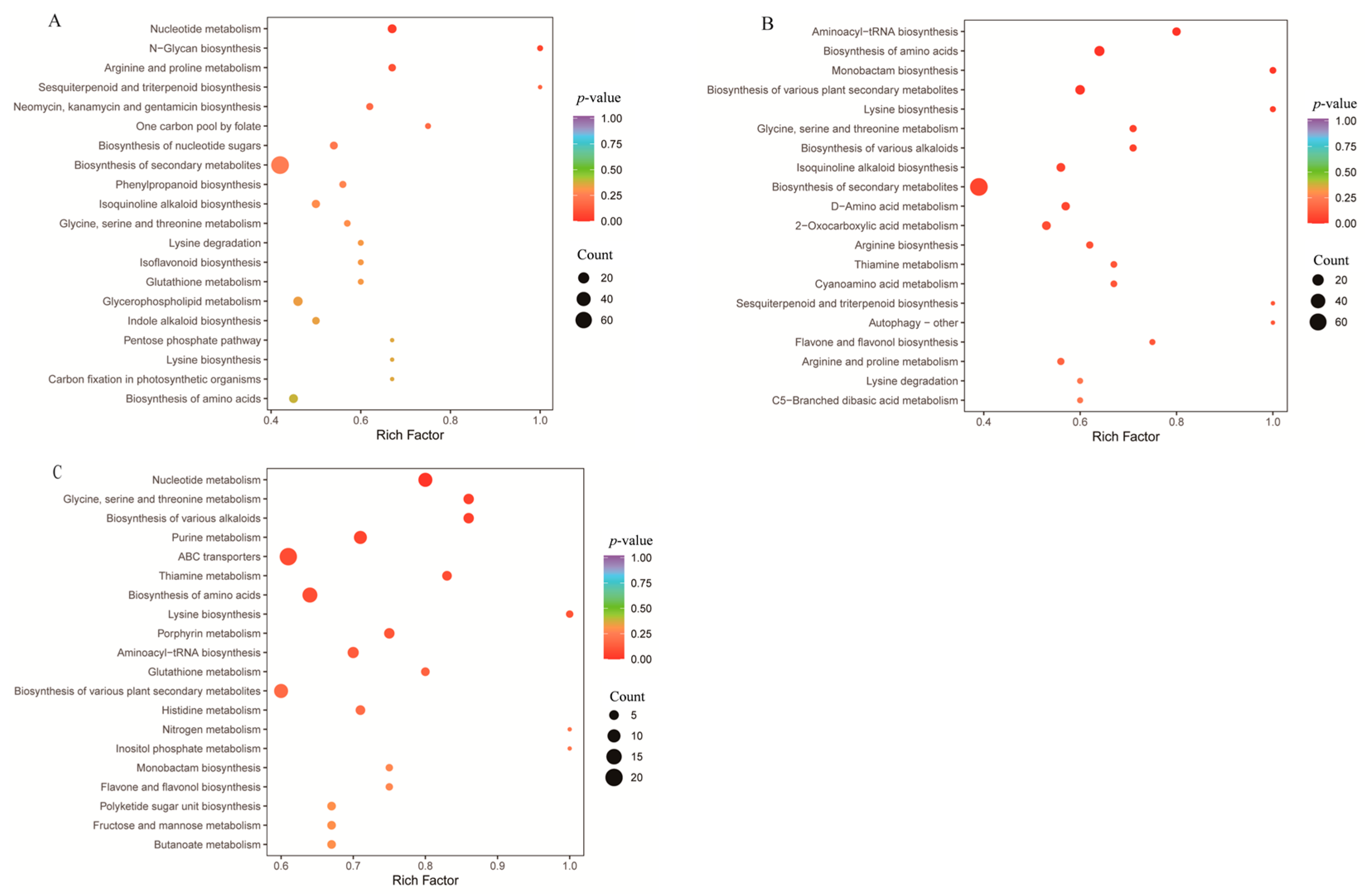
3.4.2. KEGG Enrichment Analysis of Differential Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Pingba, Anshun City |
| BJ | Qianxi, Bijie City |
| CV | Coefficient of Variation |
| ESI | Electrospray Ionization |
| FC | fold change |
| FDR | False Discovery Rate |
| GYBY | Baiyun, Guiyang City |
| GYHX | Huaxi, Guiyang City |
| HCA | hierarchical clustering analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KNN | k-Nearest Neighbors |
| PA−1 | Xidiantunjiaohe, Pu‘an County |
| PA−2 | Xidianlianglukou, Pu‘an County |
| PCA | principal component analysis |
| QC | Quality Control |
| QL | Dijiulaozhaihe, Qinglong County |
| SVR | Support Vector Regression |
| TIC | Total Ion Chromatogram |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| XR | Jiangjiawan, Xingren City |
| XY−1 | Muzhijidi, Xingyi City |
| XY−2 | Niubangzi, Xingyi City |
| ZYMT | Meitan, Zunyi City |
| ZYSY | Suiyang, Zunyi City |
Appendix A
| Code | Production Area | Cultivation Band Type | Soil Type | Soil Fertility | Altitude | Regional Environment Description |
|---|---|---|---|---|---|---|
| AS | Pingba, Anshun City | Farmland | Paddy soil | Fertile | ~1100 m | Riverside; terraced fields |
| BJ | Qianxi, Bijie City | Dry land | Limestone soil | Moderately fertile | ~1300 m | Mountainous area |
| GYBY | Baiyun, Guiyang City | Dry land | Yellow soil | Moderately fertile | ~1200 m | Mountainous area |
| GYHX | Huaxi, Guiyang City | Farmland | Paddy soil | Fertile | ~1000 m | Flat terrain |
| PA−1 | Xidiantunjiaohe, Pu‘an County | Farmland | Paddy soil | Fertile | ~1450 m | Riverside; terraced fields |
| PA−2 | Xidianlianglukou, Pu‘an County | Dry land | Limestone soil | Moderately fertile | ~1500 m | Mountainous area; prone to drought |
| QL | Dijiulaozhaihe, Qinglong County | Dry land | Limestone soil | Moderately fertile | ~1450 m | Mountainous area; prone to drought |
| XR | Jiangjiawan, Xingren City | Dry land | Yellow soil | Moderately fertile | ~1300 m | Mountainous area; prone to drought |
| XY−1 | Muzhijidi, Xingyi City | Dry land | Yellow soil | Moderately fertile | ~1300 m | Mountainous area; prone to drought; area with high temperature |
| XY−2 | Niubangzi, Xingyi City | Dry land | Yellow soil | Moderately fertile | ~1300 m | Mountainous area; prone to drought; area with high temperature |
| ZYMT | Meitan, Zunyi City | Farmland | Paddy soil | Fertile | ~900 m | Flat terrain |
| ZYSY | Suiyang, Zunyi City | Dry land | Yellow soil | Moderately fertile | ~1000 m | Mountainous area; prone to drought |
References
- Zhang, Y.; Li, X.; Yu, D.; Yang, Z.; Shen, Z.; Meng, Y.; Ding, Y.; Li, Y. Botany, chemistry, bio-activity, and application of Polygonatum odoratum (Mill.) Druce: A comprehensive review. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 13545–13566. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, H.; Zhao, C.; Li, X.; Wang, Y.; Wang, Y.; Huang, L.; Gao, W. Purification, characterization and immunomodulatory activity of fructans from Polygonatum odoratum and P. cyrtonema. Carbohydr. Polym. 2019, 214, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, F.; Chen, S.; Gao, Y. New homoisoflavanones from Polygonatum odoratum (Mill.) Druce. Yao Xue Xue Bao 2009, 44, 764–767. (In Chinese) [Google Scholar]
- Xia, G.; Li, X.; Zhang, Z.; Jiang, Y. Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce. Open Life Sci. 2021, 16, 92–101. [Google Scholar] [CrossRef]
- Ye, X.; Pi, X.; Zheng, W.; Cen, Y.; Ni, J.; Xu, L.; Wu, K.; Liu, W.; Li, L. The Methanol Extract of Polygonatum odoratum Ameliorates Colitis by Improving Intestinal Short-Chain Fatty Acids and Gas Production to Regulate Microbiota Dysbiosis in Mice. Front. Nutr. 2022, 9, 899421. [Google Scholar] [CrossRef]
- Liu, J.R.; Chen, B.X.; Jiang, M.T.; Cui, T.Y.; Lv, B.; Fu, Z.F.; Li, X.; Du, Y.D.; Guo, J.H.; Zhong, X.Q.; et al. Polygonatum odoratum polysaccharide attenuates lipopolysaccharide-induced lung injury in mice by regulating gut microbiota. Food Sci. Nutr. 2023, 11, 6974–6986. [Google Scholar] [CrossRef]
- Deng, Y.; He, K.; Ye, X.; Chen, X.; Huang, J.; Li, X.; Yuan, L.; Jin, Y.; Jin, Q.; Li, P. Saponin rich fractions from Polygonatum odoratum (Mill.) Druce with more potential hypoglycemic effects. J. Ethnopharmacol. 2012, 141, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, T.; Shi, T.; Chen, J.; Jin, L. Quality suitability regionalization analysis of Angelica sinensis in Gansu, China. PLoS ONE 2020, 15, e0243750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, D.; Liang, Z.; Liu, J.; Yan, K.; Zhu, Y.; Yang, S. Climatic factors control the geospatial distribution of active ingredients in Salvia miltiorrhiza Bunge in China. Sci. Rep. 2019, 9, 904. [Google Scholar] [CrossRef]
- Jiang, M.; Peng, M.; Li, Y.; Li, G.; Li, X.; Zhuang, L. Quality evaluation of four Ferula plants and identification of their key volatiles based on non-targeted metabolomics. Front. Plant Sci. 2024, 14, 1297449. [Google Scholar] [CrossRef]
- Liu, W.; Song, Q.; Cao, Y.; Xie, N.; Li, Z.; Jiang, Y.; Zheng, J.; Tu, P.; Song, Y.; Li, J. From 1H NMR-based non-targeted to LC-MS-based targeted metabolomics strategy for in-depth chemome comparisons among four Cistanche species. J. Pharm. Biomed. Anal. 2019, 162, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Dong, Q.; Wu, F.; Liu, Y.; Li, P.; Liu, J. Non-Targeted Metabolomic Analysis of Methanolic Extracts of Wild-Simulated and Field-Grown American Ginseng. Molecules 2019, 24, 1053. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Jin, J.; Liu, H.; Zhong, C.; Xie, J.; Qin, Y.; Zhang, S. Integrative analysis of the transcriptome and metabolome provides insights into polysaccharide accumulation in Polygonatum odoratum (Mill.) Druce rhizome. PeerJ Comput. Sci. 2024, 12, e17699. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Zhou, Y.; Zhang, J.; Zhang, X. Discrepancies in Karst Soil Organic Carbon in Southwest China for Different Land Use Patterns: A Case Study of Guizhou Province. Int. J. Environ. Res. Public Health 2019, 16, 4199. [Google Scholar] [CrossRef]
- He, J.; Yan, Y.J.; Yi, X.S.; Wang, Y.; Dai, Q.H. Soil heterogeneity and its interaction with plants in karst areas. Ying Yong Sheng Tai Xue Bao 2021, 32, 2249–2258. (In Chinese) [Google Scholar]
- Naz, S.; Liu, P.; Farooq, U.; Ma, H. Insight into de-regulation of amino acid feedback inhibition: A focus on structure analysis method. Microb. Cell Factories 2023, 22, 161. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: Essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Liu, D.H.; Zhang, D.K.; Wang, Y.H.; Li, G.; Yan, G.L.; Cao, L.J.; Xiao, X.H.; Huang, L.Q.; Wang, J.B. Quality Assessment of Panax notoginseng from Different Regions through the Analysis of Marker Chemicals, Biological Potency and Ecological Factors. PLoS ONE 2016, 11, e0164384. [Google Scholar] [CrossRef]
- Espinoza, K.S.; Snider, A.J. Therapeutic Potential for Sphingolipids in Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 789. [Google Scholar] [CrossRef]
- Gomez-Larrauri, A.; Larrea-Sebal, A.; Martín, C.; Gomez-Muñoz, A. The critical roles of bioactive sphingolipids in inflammation. J. Biol. Chem. 2025, 301, 110475. [Google Scholar] [CrossRef]
- Birt, D.F.; Merrill, A.H.; Barnett, T.; Enkvetchakul, B.; Pour, P.M.; Liotta, D.C.; Geisler, V.; Menaldino, D.S.; Schwartzbauer, J. Inhibition of skin carcinomas but not papillomas by sphingosine, N-methylsphingosine, and N-acetylsphingosine. Nutr. Cancer-Int. 1998, 31, 119–126. [Google Scholar] [CrossRef]
- Huang, S.; Jia, A.; Song, W.; Hessler, G.; Meng, Y.G.; Sun, Y.; Xu, L.; Laessle, H.; Jirschitzka, J.; Ma, S.; et al. Identification and receptor mechanism of TIR-catalyzed small molecules in plant immunity. Science 2022, 377, eabq3297. [Google Scholar] [CrossRef] [PubMed]
- Slocum, R.D.; Mejia Peña, C.; Liu, Z. Transcriptional reprogramming of nucleotide metabolism in response to altered pyrimidine availability in Arabidopsis seedlings. Front. Plant Sci. 2023, 14, 1273235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liao, J.; Xia, X.; Xiong, F.; Li, Y.; Shen, J.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W. Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol. 2019, 19, 43. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Zhao, C.; Li, S.; Kong, L.; Wu, W.; Kong, W.; Liu, Y.; Wei, Y.; Zhu, J.; et al. The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, H.; Tian, S.; Chang, X.; Jing, L. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Ibba, M.; Söll, D. The renaissance of aminoacyl-tRNA synthesis. EMBO Rep. 2001, 2, 382–387. [Google Scholar] [CrossRef]
- Ling, J.; Reynolds, N.; Ibba, M. Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 2009, 63, 61–78. [Google Scholar] [CrossRef]
- Jones, D.E.; Perez, L.; Ryan, R.O. 3-Methylglutaric acid in energy metabolism. Clin. Chim. Acta 2020, 502, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Haist, G.; Sidjimova, B.; Yankova-Tsvetkova, E.; Nikolova, M.; Denev, R.; Semerdjieva, I.; Bastida, J.; Berkov, S. Metabolite profiling and histochemical localization of alkaloids in Hippeastrum papilio (Ravena) van Scheepen. J. Plant Physiol. 2024, 296, 154223. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
| Metabolite Category | Retention Time (Min) | Adduct | Mass Error (×10−6) | Q1 Mass (Da) | Molecular Weight (Da) | Score | Database ID | Formula | Compound | Region with Maximum Content |
|---|---|---|---|---|---|---|---|---|---|---|
| Amino acids and derivatives | 3.33 | [M+]+ | 20.40 | 658.36 | 658.38 | 0.67 | Metlin (264109) | C32H50N8O7 | His-Leu-Lys-Tyr-Val | XY−2 |
| 2.07 | [M+CH3COO]− | 2.00 | 521.21 | 462.17 | 0.90 | Metlin (228141) | C16H26N6O10 | Ser-Asn-Gln-Asp | AS | |
| 5.00 | [M+CH3COO]− | 9.44 | 519.26 | 460.25 | 0.74 | Metlin (178582) | C20H36N4O8 | Leu-Ile-Ser-Glu | ZYMT | |
| GP | 4.72 | [M+Na]+ | 15.24 | 1065.55 | 1042.58 | 0.73 | PubChem (53480149) (HMDB0009954) | C50H92O18P2 | PIP (18:0/20:3 (5Z,8Z,11Z)) | QL |
| 6.74 | [M+H-H2O]+ | 1.06 | 520.34 | 537.34 | 0.87 | PubChem (16759367) | C26H52NO8P | PC (16:0/2:0) | XR | |
| 6.40 | [M-H]− | 9.90 | 515.24 | 727.55 | 0.63 | PubChem (53479648) (HMDB0009050) | C41H78NO7P | PE (18:1 (11Z)/P-18:1 (11Z)) | ZYSY | |
| Steroids | 4.57 | [M-H]− | 0.15 | 1095.53 | 1096.53 | 1.00 | PubChem (85125467) (HMDB0033401) | C51H84O25 | Yayoisaponin C | PA−2 |
| 4.66 | [M+H]+ | 3.03 | 917.47 | 916.47 | 0.93 | PubChem (85137950) (HMDB0033353) | C45H72O19 | Agavasaponin C | ZYMT | |
| 4.95 | [M+H-H2O]+ | 0.74 | 903.49 | 920.50 | 0.65 | PubChem (102482481) | C45H76O19 | Timosaponin BII | ZYMT | |
| SL | 8.62 | [M+Na]+ | 22.23 | 696.54 | 673.54 | 0.67 | PubChem (5283584) (HMDB0010702) | C38H76NO6P | CerP (d18:1/20:0) | GYBY |
| 8.74 | [M+Na]+ | 3.06 | 736.53 | 713.54 | 0.93 | PubChem (10169092) Metlin (85015) | C40H75NO9 | Glucosylceramide | GYBY | |
| Lipids | 6.01 | [M+NH4]+ | 0.77 | 318.30 | 300.27 | 0.70 | PubChem (12520) KEGG (C03195) | C18H36O3 | (R)-10-hydroxystearic acid | QL |
| GL | 8.56 | [M+NH4]+ | 2.84 | 792.56 | 774.53 | 0.93 | PubChem (21582567) | C45H74O10 | [(2S)-2-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]oxy-3-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxypropyl] (9Z,12Z,15Z)-octadeca-9,12,15-trienoate | GYBY |
| Organic acids | 1.10 | [M-H]− | 0.89 | 133.02 | 134.02 | 0.99 | PubChem (92824) (HMDB0031518) KEGG (C00497) | C4H6O5 | D-Malic acid | XY−1 |
| Benzene and substituted derivatives | 2.43 | [M-H]− | 0.02 | 475.15 | 476.15 | 1.00 | PubChem (3038513) | C20H28O13 | Primeverin | AS |
| Terpenoids | 4.65 | [M-H]− | 13.93 | 1065.52 | 1066.54 | 0.75 | PubChem (131751714) (HMDB0035343) | C54H82O21 | TR-saponin C | PA−2 |
| Heterocyclic compounds | 4.96 | [M-H]− | 1.00 | 919.49 | 920.49 | 0.98 | PubChem (131751196) (HMDB0031838) | C45H76O19 | Asparagoside E | ZYMT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.; Yang, Q.; Zheng, C.; Peng, F.; Pang, J.; Bao, N.; Sun, D. Characteristic Analysis of Metabolic Profiles of Polygonatum odoratum (Mill.) Druce from Different Regions of Guizhou Province Based on Non-Targeted Metabolomics. Metabolites 2025, 15, 733. https://doi.org/10.3390/metabo15110733
Liao C, Yang Q, Zheng C, Peng F, Pang J, Bao N, Sun D. Characteristic Analysis of Metabolic Profiles of Polygonatum odoratum (Mill.) Druce from Different Regions of Guizhou Province Based on Non-Targeted Metabolomics. Metabolites. 2025; 15(11):733. https://doi.org/10.3390/metabo15110733
Chicago/Turabian StyleLiao, Chaoxuan, Qianqian Yang, Chuanqi Zheng, Fuhai Peng, Junxiao Pang, Na Bao, and Dali Sun. 2025. "Characteristic Analysis of Metabolic Profiles of Polygonatum odoratum (Mill.) Druce from Different Regions of Guizhou Province Based on Non-Targeted Metabolomics" Metabolites 15, no. 11: 733. https://doi.org/10.3390/metabo15110733
APA StyleLiao, C., Yang, Q., Zheng, C., Peng, F., Pang, J., Bao, N., & Sun, D. (2025). Characteristic Analysis of Metabolic Profiles of Polygonatum odoratum (Mill.) Druce from Different Regions of Guizhou Province Based on Non-Targeted Metabolomics. Metabolites, 15(11), 733. https://doi.org/10.3390/metabo15110733





