Metabolomic Profiling of Commercial Tomato Puree by One-Shot Mass Spectrometry-Based Analysis: A Qualitative Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Extraction Protocol
2.3. LC-MS/MS Analysis
2.4. Data Processing and Annotation
2.5. Multivariate Statistical Analysis
3. Results
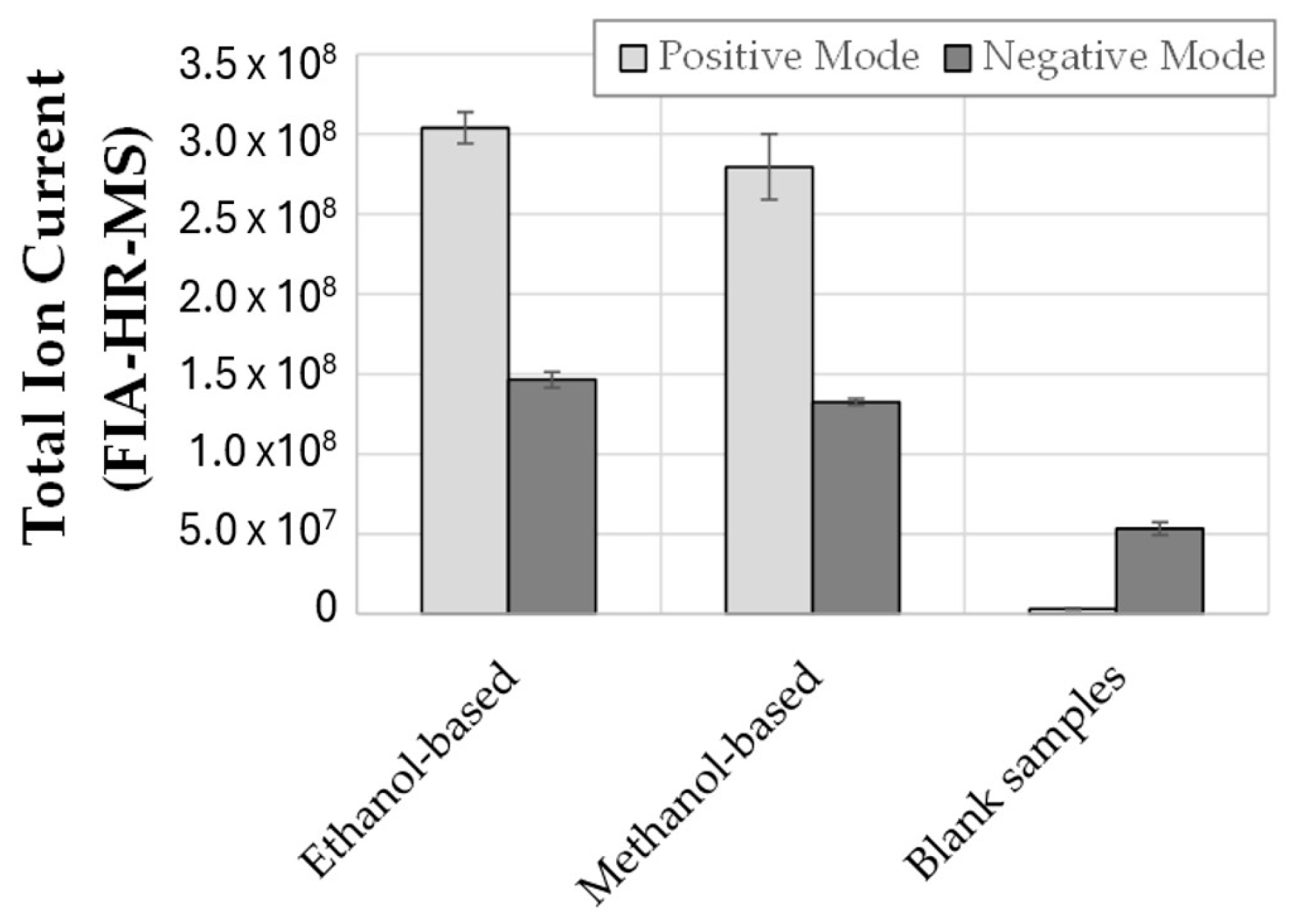
3.1. Metabolomic Data Acquisition, Processing, and Annotation
3.2. Metabolite Profiling: Molecular Function and Beneficial Effects
3.2.1. Organic Acids and Derivatives
3.2.2. Organoheterocyclic, Organic Oxygen Compounds and Alkaloids
| Chemical Taxonomy | Name | Formula | Δmass [ppm] | m/z | Reference Ion | Main Fragments | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Class | Sub Class | |||||||
| Carboxylic acids and derivatives | Amino acids, peptides, and analogues | DL-Arginine | C6H14N4O2 | −0.21 | 1751.189 | [M + H] + 1 | 600.655; 700.659; 1160.709; 1580.926; 1751.191 | [43] * |
| DL-Glutamic acid | C5H9NO4 | −0.37 | 1480.604 | [M + H] + 1 | 560.504; 840.450; 1020.555; 1300.501; 1480.611 | [19,43] * | ||
| DL-Histidine | C6H9N3O2 | −0.50 | 1560.767 | [M + H] + 1 | 830.609; 930.452; 950.608; 1100.716; 1560.767 | [43] * | ||
| DL-Phenylalanine | C9H11NO2 | −0.07 | 1660.863 | [M + H] + 1 | 1030.546; 1070.496; 1200.810; 1310.494; 1660.865 | [19,43] * | ||
| L-(−)-Asparagine | C4H8N2O3 | 0.11 | 1330.608 | [M + H] + 1 | 700.295; 740.244; 870.557; 880.400; 1160.351 | [43] * | ||
| DL-Tyrosine | C9H11NO3 | 0.11 | 1820.812 | [M + H] + 1 | [18,43] * | |||
| L-Aspartic acid | C4H7NO4 | −10.02 | 1320.288 | [M−H]−1 | 880.389; 1140.182; 1150.023; 1320.289 | [43] * | ||
| Methionine S-oxide | C5H11NO3S | −0.58 | 1480.426 | [M + H-H2O] + 1 | ||||
| L-Proline | C5H9NO2 | 2.02 | 1160.708 | [M + H] + 1 | 700.669; 710.692; 1160.709 | [43] * | ||
| L-Norleucine | C6H13NO2 | 0.56 | 1321.020 | [M + H] + 1 | 690.706; 860.970; 871.003 | |||
| Stachydrine | C7H13NO2 | −0.53 | 1441.018 | [M + H] + 1 | 580.660; 840.814; 1441.018 | |||
| γ-Aminobutyric acid | C4H9NO2 | 4.13 | 1040.710 | [M + H] + 1 | [19,43] * | |||
| N-(Phenylacetyl)aspartic acid | C12H13NO5 | −1.13 | 2500.718 | [M−H]−1 | ||||
| Indoleacetylaspartate | C14H14N2O5 | −0.30 | 2910.975 | [M + H] + 1 | ||||
| Amino acids, peptides, and analogues | NILP | C21H37N5O6 | −0.30 | 4562.812 | [M + H] + 1 | 860.700; 1160.708; 2001.393; 2281.341; 3412.180 | ||
| LVL | C17H33N3O4 | −0.86 | 3442.540 | [M + H] + 1 | 720.815; 860.970; 1321.020; 1851.650; 2311.702 | |||
| VF | C14H20N2O3 | −0.13 | 2651.546 | [M + H] + 1 | 720.815; 2651.556 | |||
| IPI | C17H31N3O4 | −1.05 | 3422.384 | [M + H] + 1 | ||||
| LLL | C18H35N3O4 | −0.64 | 3582.696 | [M + H] + 1 | ||||
| LL | C12H24N2O3 | −0.80 | 2451.858 | [M + H] + 1 | [19] * | |||
| LF | C15H22N2O3 | −0.95 | 2791.700 | [M + H] + 1 | ||||
| Tricarboxylic acids and derivatives | Citric acid | C6H8O7 | −5.14 | 1910.187 | [M−H]−1 | 850.297; 870.072; 1110.074; 1290.180; 1910.186 | [19] *, [20] **, [43] * | |
| Alkaloids | Not available | Trigonelline | C7H7NO2 | 0.05 | 1380.550 | [M + H] + 1 | 920.500; 940.656; 1100.603; 1380.550; 1390.584 | [43] * |
| Harmala alkaloids | Not available | 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid | C13H14N2O2 | −1.04 | 2311.126 | [M + H] + 1 | ||
| Harmala alkaloids | Not available | Flazin | C17H12N2O4 | −0.29 | 3090.869 | [M + H] + 1 | ||
| Harmala alkaloids | Not available | Perlolyrine | C16H12N2O2 | −0.48 | 2650.970 | [M + H] + 1 | [44] ** | |
| Phenols | Benzenediols | Kukoamine A | C28H42N4O6 | −0.37 | 2661.624 | [M + 2H] + 2 | [45] * | |
| Phenols | Methoxyphenols | Sinapyl alcohol | C11H14O4 | −3.57 | 2090.812 | [M−H]−1 | ||
| Benzene and substituted derivatives | Benzoic acids and derivatives | Salicylic acid | C7H6O3 | −10.15 | 1370.230 | [M−H]−1 | 930.330; 1370.231 | [43] * |
| Fatty acyls | Fatty acyl glycosides | (−)-11-hydroxy-9,10-dihydrojasmonic acid 11-β-D-glucoside | C18H30O9 | −0.33 | 3891.820 | [M−H]−1 | ||
| Fatty acyls | Fatty acyl glycosides | 12-hydroxyjasmonic acid 12-O-β-D-glucoside | C19H30O8 | −0.79 | 3872.010 | [M + H] + 1 | ||
| Fatty acyls | Linoleic acids and derivatives | (−)-trans-Methyl dihydrojasmonate | C13H22O3 | −0.81 | 2271.640 | [M + H] + 1 | ||
| Fatty acyls | Linoleic acids and derivatives | Methyl Jasmonate | C13H20O3 | −0.51 | 2251.484 | [M + H] + 1 | ||
| Prenol lipids | Sesquiterpenoids | (±)-(2E)-Abscisic acid | C15H20O4 | −0.68 | 2651.433 | [M + H] + 1 | ||
| Steroids and steroid derivatives | Steroidal glycosides | Tomatin | C50H83NO21 | 0.16 | 1034.516 | [M + H] + 1 | [19] *, [20] **, [46] ** | |
| Steroids and steroid derivatives | Steroidal alkaloids | Tomatidine | C27H45NO2 | −1.04 | 4163.519 | [M + H] + 1 | 1611.325; 2552.107; 2732.212; 3983.420; 4163.523 | [43] * |
| Organooxygen compounds | Alcohols and polyols | D-(-)-Quinic acid | C7H12O6 | −4.91 | 1910.552 | [M−H]−1 | 850.279; 930.330; 1910.553; 1920.587 | [18] * |
| Organooxygen compounds | Alcohols and polyols | Chlorogenic acid | C16H18O9 | −0.12 | 3530.879 | [M−H]−1 | [19] *, [20] **, [43] *, [47] **, [48] ** | |
| Organooxygen compounds | Alcohols and polyols | Cynarine | C25H24O12 | −0.32 | 5151.193 | [M−H]−1 | [48] ** | |
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | α-D-Mannose 1-phosphate | C6H13O9P | −0.10 | 2590.224 | [M−H]−1 | 789.575; 969.681; 1389.790; 1990.001; 2590.227 | |
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | Sucrose | C12H22O11 | −0.58 | 3411.088 | [M−H]−1 | 690.343; 850.290; 1270.391; 1450.498; 1630.605 | [19] * |
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | D-(+)-Glucose | C6H12O6 | −4.77 | 1790.550 | [M−H]−1 | [19,43] * | |
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | Glucosamine | C6H13NO5 | 0.21 | 1800.867 | [M + H] + 1 | ||
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | D-Gluconic acid | C6H12O7 | −4.84 | 1950.501 | [M−H]−1 | [19,43] * | |
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 1-O-(4-coumaroyl)-β-D-glucose | C15H18O8 | −0.27 | 3710.984 | [M−H]−1 | ||
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | Caffeic acid 3-glucoside | C15H18O9 | −0.09 | 3410.878 | [M−H]−1 | [20,48] ** | |
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | Zeatin 7-glucoside | C16H23N5O6 | −3.65 | 3801.562 | [M−H]−1 | ||
| Imidazopyrimidines | Purines and purine derivatives | Adenine | C5H5N5 | −0.07 | 1360.618 | [M + H] + 1 | 940.405; 1070.491; 1190.356; 1360.619; 1370.459 | [43] * |
| Imidazopyrimidines | Purines and purine derivatives | Guanine | C5H5N5O | −0.35 | 1520.566 | [M + H] + 1 | 1100.352; 1280.458; 1350.303; 1520.568; 1530.409 | |
| Diazines | Pyrimidines and pyrimidine derivatives | Cytosine | C4H5N3O | 2.47 | 1120.508 | [M + H] + 1 | 690.455; 940.404; 950.245; 1120.509 | |
| Diazines | Pyrimidines and pyrimidine derivatives | Uracil | C4H4N2O2 | 2.52 | 1130.348 | [M + H] + 1 | 700.295; 850.289; 950.244; 960.085; 1130.349 | |
| Pyridines and derivatives | Pyridinecarboxylic acids and derivatives | Nicotinic acid | C6H5NO2 | 0.77 | 1240.394 | [M + H] + 1 | 780.344; 800.501; 960.448; 1240.395; 1250.429 | |
| Indoles and derivatives | Indoles and derivatives | Indole-3-acetaldehyde | C10H9NO | −0.46 | 1600.756 | [M + H] + 1 | ||
| Indoles and derivatives | Pyridoindoles | Norharman | C11H8N2 | −0.14 | 1690.760 | [M + H] + 1 | 1680.670; 1690.761 | |
| Indoles and derivatives | Indolyl carboxylic acids and derivatives | DL-Tryptophan | C11H12N2O2 | 0.31 | 2050.972 | [M + H] + 1 | 1180.655; 1440.809; 1460.601; 1590.919; 1880.707 | [19,43] * |
| Dihydrofurans | Furanones | Ascorbic acid | C6H8O6 | −6.50 | 1750.237 | [M−H]−1 | 590.123; 710.122; 870.072; 1150.023; 1750.239 | [20] ** |
| Flavonoids | Flavans | (±)-Naringenin | C15H12O5 | −0.67 | 2730.754 | [M−H]−1 | 1070.124; 1190.489; 1510.026; 1770.184; 2710.616 | [20] **, [19] *, [43] *, [46] **, [47] **, [48] ** |
| Flavonoids | Flavans | Eriodictyol | C15H12O6 | 0.32 | 2870.562 | [M−H]−1 | 830.123; 1070.124; 1250.230; 1350.440; 1510.026 | [18] *, [43] *, [48] ** |
| Flavonoids | Flavones | Quercetin | C15H10O7 | −0.96 | 3030.496 | [M + H] + 1 | 1530.183; 1770.548; 2290.495; 2850.402; 3030.501 | [19] *, [43] *, [48] ** |
| Flavonoids | Flavonoids | Apiorutin | C32H38O20 | 0.30 | 7411.891 | [M−H]−1 | [20] ** | |
| Flavonoids | O-methylated flavonoids | (±)-Hesperetin | C16H14O6 | −0.36 | 3010.717 | [M−H]−1 | [48] ** | |
| Flavonoids | Flavonoid glycosides | Rutin | C27H30O16 | 1.02 | 6091.471 | [M−H]−1 | 710.499; 850.290; 1290.548; 1530.183; 3030.501 | [20] **, [43] *, [46] **, [47] **, [48] ** |
| Flavonoids | Flavonoid glycosides | Astilbin | C21 H22 O11 | 0.14 | 4491.091 | [M−H]−1 | ||
| Flavonoids | Flavonoid glycosides | isoquercetin | C21 H20 O12 | −0.14 | 4651.026 | [M + H] + 1 | ||
| Flavonoids | Flavonoid glycosides | Kaempferol 3-O-glucoside-7-O-rhamnoside | C27H30O15 | 0.01 | 5931.515 | [M−H]−1 | [48] ** | |
| Flavonoids | Flavonoid glycosides | Naringin | C27H32O14 | 2.64 | 5791.735 | [M−H]−1 | [49] * | |
| Flavonoids | Flavonoid glycosides | Prunin | C21H22O10 | −0.15 | 4331.140 | [M−H]−1 | [43] * | |
| Flavonoids | Flavonoid glycosides | Quercetin 3- (2G-xylosylrutinoside) | C32 H38 O20 | −2.05 | 7432.014 | [M + H] + 1 | [19] * | |
| Coumarins and derivatives | Coumarins and derivatives | 7-Hydroxycoumarine | C9H6O3 | −0.41 | 1630.389 | [M + H] + 1 | 790.549; 890.391; 1070.495; 1350.441; 1630.390 | [43] * |
| Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives | Feruloylagmatine | C15H22N4O3 | −0.46 | 3071.763 | [M + H] + 1 | ||
| Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives | (E)-p-coumaric acid | C9H8O3 | 0.12 | 1650.546 | [M + H] + 1 | [18] *, [47] **, [48] ** | |
| Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives | N-Caffeoyltyramine | C17H17NO4 | −0.78 | 2981.083 | [M−H]−1 | ||
| Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives | N-trans-Feruloyloctopamine | C18H19NO5 | −0.64 | 3281.190 | [M−H]−1 | ||
| Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives | Paprazine | C17H17NO3 | −0.51 | 2841.279 | [M + H] + 1 | ||
3.2.3. Phenylpropanoids and Polyketides
3.2.4. Lipids and Lipid-like Molecules
3.3. Multivariate Statistical Analysis of Metabolite Profiles
4. Discussion
Biological and Health Implications of the Main Identified Bioactive Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Mass Spectrometry |
| LC-QTOF-MS | Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry |
| LC | Liquid Chromatography |
| UHPLC | Ultra-High-Pressure Liquid Chromatography |
| AGC | Automatic Gain Control |
| HESI | Heated Electrospray Ionization |
| HR-MS | High-Resolution Mass Spectrometry |
| S/N | Signal/Noise |
| IAA | Indol-3-acetic acid |
| DPP-IV | Dipeptyl peptidase IV |
| ACE | Angiotensin converting enzyme |
| AAs | Amino acids |
| GABA | ɣ-aminobutyric acid |
| PAA | n-(phenylacetyl)aspartic acid |
| IA-Asp | Indoleacetylaspartate |
| NAD | Nicotinamide adenine dinucleotide |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| βCs | β-carbolines |
| THβCs | Tetrahydro-β-carbolines |
| ABA | Abscistic acid |
| JA | Jasmoic acid |
References
- FAOSTAT Food Balance Sheets. 2020. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 24 April 2020).
- European Commission, Fruit and Vegetables Production. Available online: https://agridata.ec.europa.eu/extensions/DashboardFruitAndVeg/FruitandVegetableProduction.html (accessed on 30 September 2025).
- Tomatoes Global Market Report 2025. Available online: https://www.researchandmarkets.com/reports/5807115/tomatoes-global-market-report?srsltid=AfmBOookOcZF1BRCwk7mqat444c9rVQDNGEua7crwJtpOw7YFfOcfB02 (accessed on 30 September 2025).
- Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Analytical determination of antioxidants in tomato: Typical components of the Mediterranean diet. J. Sep. Sci. 2007, 30, 452–461. [Google Scholar] [CrossRef]
- Beecher, G.R. Nutrient Content of Tomatoes and Tomato Products. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef]
- Weisburger, J.H. Lycopene and Tomato Products in Health Promotion. Exp. Biol. Med. 2002, 227, 924–927. [Google Scholar] [CrossRef]
- Wu, X.; Yu, L.; Pehrsson, P.R. Are Processed Tomato Products as Nutritious as Fresh Tomatoes? Scoping Review on the Effects of Industrial Processing on Nutrients and Bioactive Compounds in Tomatoes. Adv. Nutr. Int. Rev. J. 2021, 13, 138–151. [Google Scholar] [CrossRef]
- Ebadi, M.; Mohammadi, M.; Pezeshki, A.; Jafari, S.M. Health Benefits of Beta-Carotene. In Handbook of Food Bioactive Ingredients: Properties and Applications; Jafari, S.M., Rashidinejad, A., Gandara, J.S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–26. [Google Scholar] [CrossRef]
- Tufail, T.; Ain, H.B.U.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef] [PubMed]
- Far, B.F.; Lomer, N.B.; Gharedaghi, H.; Sahrai, H.; Mahmoudvand, G.; Rouzbahani, A.K. Is beta-carotene consumption associated with thyroid hormone levels? Front. Endocrinol. 2023, 14, 1089315. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidantcontent of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Ali, M.Y.; Ibn Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 142. [Google Scholar] [CrossRef]
- World Processing Tomato Council. Available online: https://www.wptc.to/production (accessed on 10 May 2025).
- Thakur, B.R.; Singh, R.K.; Nelson, P.E. Quality attributes of processed tomato products: A review. Food Rev. Int. 1996, 12, 375–401. [Google Scholar] [CrossRef]
- Code of Federal Regulations. Title 21—Food and Drugs; Part 155—Canned Vegetables; Section 155.191—Tomato Concentrates; U.S. Government Publishing Office: Washington, DC, USA, 2024.
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef]
- Otify, A.M.; Ibrahim, R.M.; Abib, B.; Laub, A.; Wessjohann, L.A.; Jiang, Y.; Farag, M.A. Unveiling metabolome heterogeneity and new chemicals in 7 tomato varieties via multiplex approach of UHPLC-MS/MS, GC–MS, and UV–Vis in relation to antioxidant effects as analyzed using molecular networking and chemometrics. Food Chem. 2023, 417, 135866. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; de Vos, R. Changes in Antioxidant and Metabolite Profiles during Production of Tomato Paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in Carotenoids and Antioxidant Vitamins in Tomato as a Function of Varietal and Technological Factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Dao, L.; Flessa, S.; Gillespie, D.M.; Jewell, W.T.; Huebner, B.; Bertow, D.; Ebeler, S.E. Processing Effects on Lycopene Content and Antioxidant Activity of Tomatoes. J. Agric. Food Chem. 2001, 49, 3713–3717. [Google Scholar] [CrossRef]
- Chanforan, C.; Loonis, M.; Mora, N.; Caris-Veyrat, C.; Dufour, C. The impact of industrial processing on health-beneficial tomato microconstituents. Food Chem. 2012, 134, 1786–1795. [Google Scholar] [CrossRef]
- Jacob, K.; García-Alonso, F.; Ros, G.; Periago, M. Stability of carotenoids, phenolic compounds, ascorbic acid and antioxidant capacity of tomatoes during thermal processing. Arch. Latinoam. Nutr. 2025, 60, 192–198. [Google Scholar] [CrossRef]
- Georgé, S.; Tourniaire, F.; Gautier, H.; Goupy, P.; Rock, E.; Caris-Veyrat, C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011, 124, 1603–1611. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Sagdic, O.; Boyacioglu, D.; Capanoglu, E. Industrial processing versus home processing of tomato sauce: Effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chem. 2017, 220, 51–58. [Google Scholar] [CrossRef]
- da Silva, W.B.; Hispagnol, G.F.; Nunes, E.V.d.S.; Castro-Gamboa, I.; Pilon, A.C. Plant Sample Preparation for Metabolomics, Lipidomics, Ionomics, Fluxomics, and Peptidomics. Separations 2025, 12, 21. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green Solvents for the Extraction of High Added-Value Compounds from Agri-food Waste. Food Eng. Rev. 2019, 12, 83–100. [Google Scholar] [CrossRef]
- The Human Metabolome Database. Available online: https://www.hmdb.ca/metabolites/ (accessed on 2 July 2025).
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Takayama, M.; Ezura, H. How and why does tomato accumulate a large amount of GABA in the fruit? Front. Plant Sci. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, P.; Liu, P.; Xu, P. Exploring stachydrine: From natural occurrence to biological activities and metabolic pathways. Front. Plant Sci. 2024, 15, 1442879. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Motyka, V.; Pokorná, E.; Filepová, R.; Dobrev, P.I.; Handa, A.K.; Mattoo, A.K. A comprehensive endogenous phytohormone metabolite landscape identifies new metabolites associated with tomato fruit. Plant Growth Regul. 2024, 104, 343–357. [Google Scholar] [CrossRef]
- Riov, J.; Bangerth, F. Metabolism of Auxin in Tomato Fruit Tissue. Plant Physiol. 1992, 100, 1396–1402. [Google Scholar] [CrossRef]
- BIOPEP-UWM Database. Available online: http://www.uwm.edu.pl/biochemia/index.php/pl/biopep (accessed on 20 July 2025).
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Yousr, M.; Howell, N. Antioxidant and ACE Inhibitory Bioactive Peptides Purified from Egg Yolk Proteins. Int. J. Mol. Sci. 2015, 16, 29161–29178. [Google Scholar] [CrossRef]
- Pratley, R.E. Overview of glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Medscape J. Med. 2008, 10, 171. [Google Scholar]
- Barz, W. Metabolism and degradation of nicotinic acid in plant cell cultures. In Primary and Secondary Metabolism of Plant Cell Cultures; Springer: Berlin/Heidelberg, Germany, 1985; pp. 186–195. [Google Scholar] [CrossRef]
- Tareq, F.S.; Singh, J.; Ferreira, J.F.S.; Sandhu, D.; Suarez, D.L.; Luthria, D.L. A Targeted and an Untargeted Metabolomics Approach to Study the Phytochemicals of Tomato Cultivars Grown Under Different Salinity Conditions. J. Agric. Food Chem. 2024, 72, 7694–7706. [Google Scholar] [CrossRef]
- Herraiz, T.; Peña, A.; Salgado, A. Identification, Formation, and Occurrence of Perlolyrine: A β-Carboline Alkaloid with a Furan Moiety in Foods. J. Agric. Food Chem. 2023, 71, 13451–13461. [Google Scholar] [CrossRef]
- Saller, J.; List, C.; Hübner, H.; Gmeiner, P.; Clark, T.; Pischetsrieder, M. Identification and quantification of kukoamine A and kukoamine B as novel μ-opioid receptor agonists in potato and other solanaceous plants. Food Chem. 2023, 427, 136637. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Sforza, S.; Dossena, A.; Marchelli, R. A multiresidual method for the simultaneous determination of the main glycoalkaloids and flavonoids in fresh and processed tomato (Solanum lycopersicum L.) by LC-DAD-MS/MS. J. Sep. Sci. 2009, 32, 3664–3671. [Google Scholar] [CrossRef]
- Di Lecce, G.; Martínez-Huélamo, M.; Tulipani, S.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Setup of a UHPLC–QqQ-MS Method for the Analysis of Phenolic Compounds in Cherry Tomatoes, Tomato Sauce, and Tomato Juice. J. Agric. Food Chem. 2013, 61, 8373–8380. [Google Scholar] [CrossRef]
- González-Coria, J.; Mesirca-Prevedello, C.; Lozano-Castellón, J.; Casadei, E.; Valli, E.; López-Yerena, A.; Jaime-Rodríguez, C.; Pinto, D.; Illan, M.; Torrado, X.; et al. Chemometric study on the effect of cooking on bioactive compounds in tomato pomace enriched sauces. npj Sci. Food 2024, 8, 58. [Google Scholar] [CrossRef]
- Choe, U.; Sun, J.; Bailoni, E.; Chen, P.; Li, Y.; Gao, B.; Wang, T.T.Y.; Rao, J.; Yu, L. Chemical Composition of Tomato Seed Flours, and Their Radical Scavenging, Anti-Inflammatory and Gut Microbiota Modulating Properties. Molecules 2021, 26, 1478. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Peña, A.; Mateo, H.; Herraiz, M.; Salgado, A. Formation, Characterization, and Occurrence of β-Carboline Alkaloids Derived from α-Dicarbonyl Compounds and l-Tryptophan. J. Agric. Food Chem. 2022, 70, 9143–9153. [Google Scholar] [CrossRef]
- Herraiz, T.; Salgado, A. Formation, Identification, and Occurrence of the Furan-Containing β-Carboline Flazin Derived from l-Tryptophan and Carbohydrates. J. Agric. Food Chem. 2024, 72, 6575–6584. [Google Scholar] [CrossRef]
- Seong, S.H.; Jung, H.A.; Choi, J.S. Discovery of Flazin, an Alkaloid Isolated from Cherry Tomato Juice, As a Novel Non-Enzymatic Protein Glycation Inhibitor via in Vitro and in Silico Studies. J. Agric. Food Chem. 2021, 69, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, L.; Kohlen, W.; Oplaat, C.; Charnikhova, T.; Cristescu, S.; Michieli, P.; Wolters-Arts, M.; Bouwmeester, H.; Mariani, C.; Vriezen, W.; et al. ABA-deficiency results in reduced plant and fruit size in tomato. J. Plant Physiol. 2012, 169, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wu, Q.; Huang, S.; Zhu, B.; Chen, F.; Liu, B.; Cai, L.; Mao, L.; Luo, Z.; Li, L.; et al. Exogenous abscisic acid regulates primary metabolism in postharvest cherry tomato fruit during ripening. Sci. Hortic. 2022, 299, 111008. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Wu, Y.; Tang, Z.; Zhang, D.; Lyu, J.; Khan, K.S.; Xiao, X.; Yu, J. Evaluation of nutritional composition, biochemical, and quality attributes of different varieties of tomato (Solanum lycopersicum L.). J. Food Compos. Anal. 2024, 132, 106384. [Google Scholar] [CrossRef]
- Dong, M.; Xin, R.; Li, Z.-Y.; Li, Y.-L.; Huang, X.-H.; Dong, X.-P.; Zhu, B.-W.; Qin, L. Simultaneously quantification of organic acids metabolites by HPLC mass spectrometry to reveal the postharvest quality change in cherry tomato. J. Food Compos. Anal. 2022, 117, 105105. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant Activity of Citrus Limonoids, Flavonoids, and Coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2022, 80, 241–262. [Google Scholar] [CrossRef]
- Khalilabad, S.N.; Mirzaei, A.; Askari, V.R.; Mirzaei, A.; Khademi, R.; Rahimi, V.B. How hesperidin and Hesperetin, as promising food Supplements, combat cardiovascular Diseases: A systematic review from bench to bed. J. Funct. Foods 2024, 120, 106358. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Minoggio, M.; Bramati, L.; Simonetti, P.; Gardana, C.; Iemoli, L.; Santangelo, E.; Mauri, P.; Spigno, P.; Soressi, G.; Pietta, P. Polyphenol Pattern and Antioxidant Activity of Different Tomato Lines and Cultivars. Ann. Nutr. Metab. 2003, 47, 64–69. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Chang, C. Chlorogenic acid intake guidance: Sources, health benefits, and safety. Asia Pac. J. Clin. Nutr. 2022, 31, 602–610. [Google Scholar] [CrossRef]
- Mashurabad, P.C.; Palika, R.; Jyrwa, Y.W.; Bhaskarachary, K.; Pullakhandam, R. Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits. J. Food Sci. Technol. 2017, 54, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chandran, D.; Tomar, M.; Bhuyan, D.J.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Radha; Dhumal, S.; Singh, S.; et al. Valorization Potential of Tomato (Solanum lycopersicum L.) Seed: Nutraceutical Quality, Food Properties, Safety Aspects, and Application as a Health-Promoting Ingredient in Foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Ruan, M.; Wang, R.; Ye, Q.; Wan, H.; Zhou, G.; Cheng, Y.; Guo, S.; Liu, C.; et al. The PLA Gene Family in Tomato: Identification, Phylogeny, and Functional Characterization. Genes 2025, 16, 130. [Google Scholar] [CrossRef]
- Zhao, L.; Maimaitiyiming, R.; Hong, J.; Wang, L.; Mu, Y.; Liu, B.; Zhang, H.; Chen, K.; Aihaiti, A. Optimization of tomato (Solanum lycopersicum L.) juice fermentation process and analysis of its metabolites during fermentation. Front. Nutr. 2024, 11, 1344117. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostag. Leukot. Ess. Fat. Acids 2015, 101, 41–51. [Google Scholar] [CrossRef]
- Ngo, T.H.; Park, J.; Jo, Y.D.; Jin, C.H.; Jung, C.-H.; Nam, B.; Han, A.-R.; Nam, J.-W. Content of Two Major Steroidal Glycoalkaloids in Tomato (Solanum lycopersicum cv. Micro-Tom) Mutant Lines at Different Ripening Stages. Plants 2022, 11, 2895. [Google Scholar] [CrossRef]
- Passera, A.; Casati, P.; Abbasi-Parizad, P.; Pagnoni, S.; Carullo, D.; Farris, S.; Scaglia, B. Evaluation of the biocide activity of tomatine-rich extracts from tomato cannery residues against fungi and bacteria. Environ. Technol. Innov. 2024, 36, 103807. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, R. p-Coumaric Acid: A Naturally Occurring Chemical with Potential Therapeutic Applications. Curr. Org. Chem. 2022, 26, 1333–1349. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Chen, B.; Xie, H.; Chen, D. Antioxidant and Cytoprotective Effects of Kukoamines A and B: Comparison and Positional Isomeric Effect. Molecules 2018, 23, 973. [Google Scholar] [CrossRef]
- Liao, L.; Tang, Y.; Li, B.; Tang, J.; Xu, H.; Zhao, K.; Zhang, X. Stachydrine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Biomed. Pharmacother. 2023, 161, 114489. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxidative Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Mandal, N.; Sen, G.; Pathak, A.; Gauliya, K.; Manjhi, M.K.; Upadhyay, D.C.; Khan, M.L.; Upadhyay, C.P. Comprehensive validation of bactericidal efficacy of umbelliferone against multidrug-resistant pathogens through multifaceted antimicrobial assays. Arch. Microbiol. 2025, 207, 180. [Google Scholar] [CrossRef] [PubMed]



| Variable | Type | Counts/Type | Total |
|---|---|---|---|
| Variety | Baby Plum (Datterino) | 20 | 43 |
| Oblong (Allungato) | 2 | ||
| Cherry (Ciliegino) | 3 | ||
| Rossoro | 1 | ||
| 30% Baby Plum (Datterino) | 2 | ||
| 30% Cherry (Ciliegino) | 2 | ||
| Not specified | 13 | ||
| Geographical origin | Emilia Romagna | 12 | 43 |
| Sicily | 10 | ||
| Apulia | 15 | ||
| Italy | 6 | ||
| Farming condition | Organic | 12 | 43 |
| Conventional | 31 |
| Super Class | N° Identified Features | |
|---|---|---|
| Level IIa | Level III | |
| Organic acids and derivatives | 34 | 100 |
| Organoheterocyclic compounds | 19 | 30 |
| Lipids and lipid-like molecules | 14 | 103 |
| Phenylpropanoids and polyketides | 11 | 23 |
| Organic oxygen compounds | 4 | 35 |
| Nucleosides, nucleotides, and analogues | 6 | 5 |
| Benzenoids | 5 | 17 |
| Alkaloids and derivatives | 1 | 7 |
| Organic nitrogen compounds | 2 | 2 |
| Homogeneous non-metal compounds | 0 | 2 |
| Other | 4 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamonaca, A.; De Angelis, E.; Pilolli, R. Metabolomic Profiling of Commercial Tomato Puree by One-Shot Mass Spectrometry-Based Analysis: A Qualitative Perspective. Metabolites 2025, 15, 732. https://doi.org/10.3390/metabo15110732
Lamonaca A, De Angelis E, Pilolli R. Metabolomic Profiling of Commercial Tomato Puree by One-Shot Mass Spectrometry-Based Analysis: A Qualitative Perspective. Metabolites. 2025; 15(11):732. https://doi.org/10.3390/metabo15110732
Chicago/Turabian StyleLamonaca, Antonella, Elisabetta De Angelis, and Rosa Pilolli. 2025. "Metabolomic Profiling of Commercial Tomato Puree by One-Shot Mass Spectrometry-Based Analysis: A Qualitative Perspective" Metabolites 15, no. 11: 732. https://doi.org/10.3390/metabo15110732
APA StyleLamonaca, A., De Angelis, E., & Pilolli, R. (2025). Metabolomic Profiling of Commercial Tomato Puree by One-Shot Mass Spectrometry-Based Analysis: A Qualitative Perspective. Metabolites, 15(11), 732. https://doi.org/10.3390/metabo15110732








