Sex Modifies Metabolic Pathways Associated with Lipids in Untargeted Metabolomics: The Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–2006
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Plasma Sample Preparation and Untargeted Metabolomics Analysis
2.3. Clinical Lipid Measures
2.4. Biological Sex and Covariates
2.5. Statistical Analyses
2.5.1. Orthogonal Partial Least Squares–Regression (OPLS-R)
2.5.2. Unstratified Linear Regression Models with Sex-Metabolite Peak Interactions
2.5.3. Unstratified Pathway Enrichment
2.5.4. Sensitivity Analyses
2.6. Ethical Considerations and Approvals
3. Results
3.1. Participant Characters
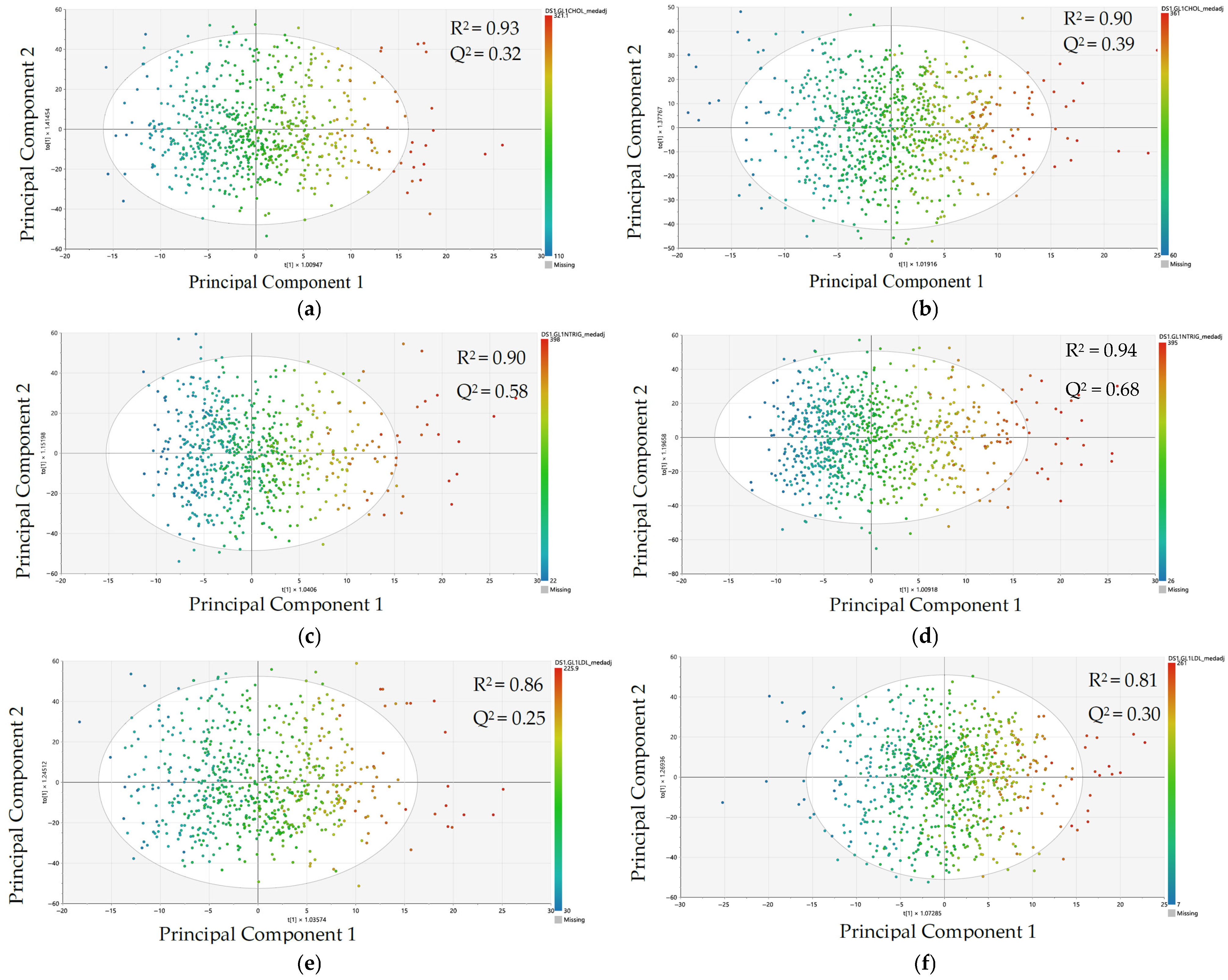
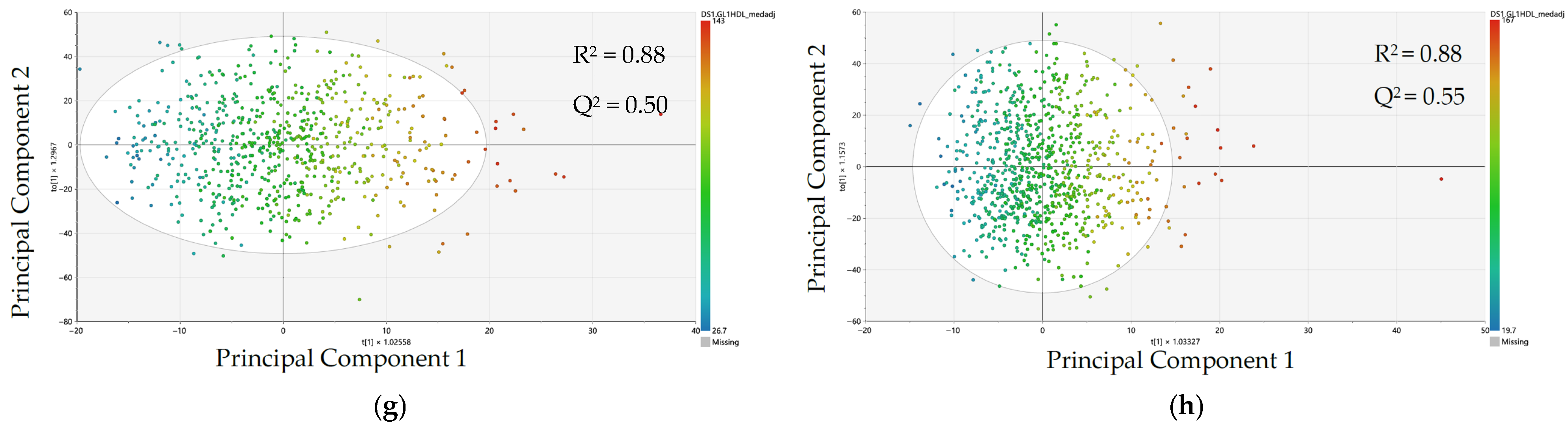
3.2. Comparison of Metabolomic Data Patterning and Model Performance by Sex Using Orthogonal Partial Least Squares–Regression (OPLS-R)
3.3. Sex Modification of Metabolite Peak-Lipid Associations in Unstratified Linear Regression Models (7255 Metabolite Peaks and 4 Clinical Lipid Measures)
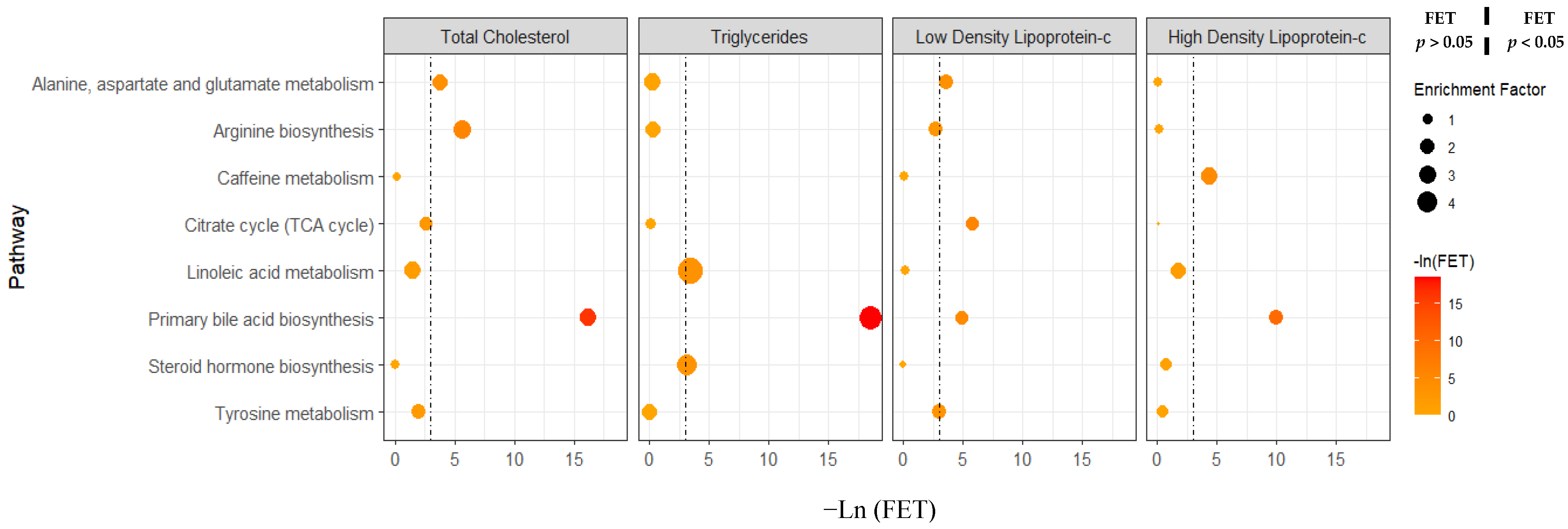
3.4. Sex Differences in Metabolic Pathway Activity Associated with Clinical Lipid Measures Using Pathway Enrichment Analyses
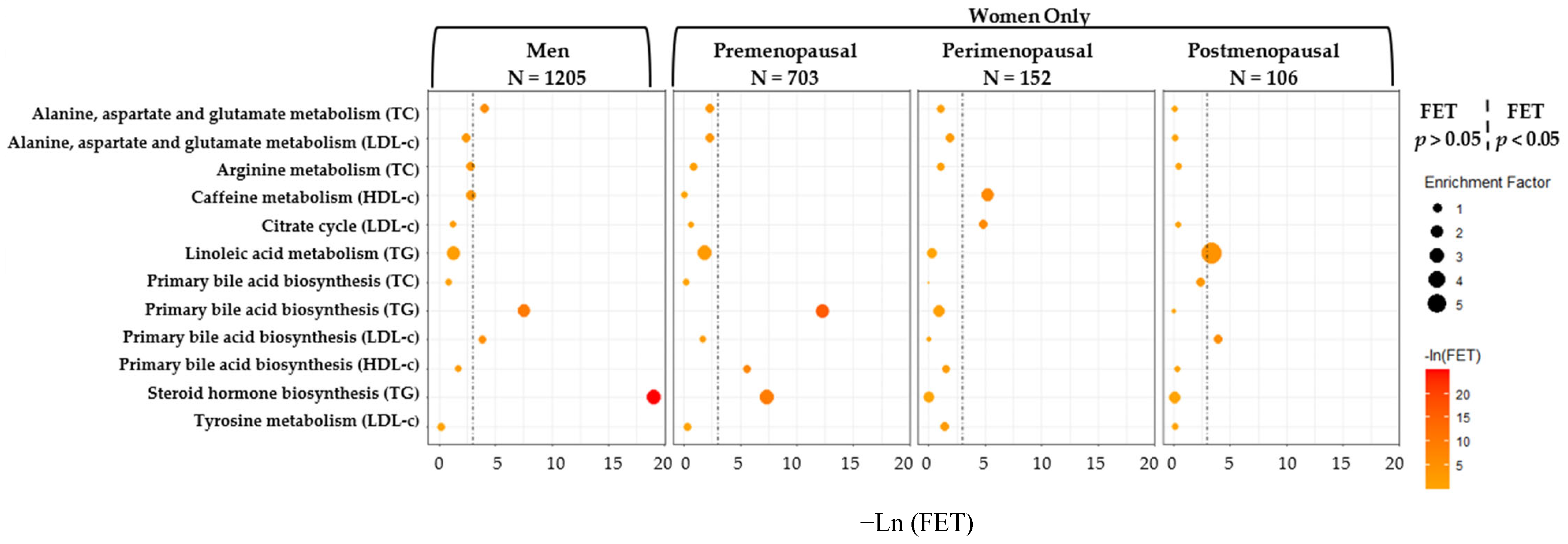
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAP | Automated Data Analysis Pipeline |
| BMI | Body mass index |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CVD | Cardiovascular diseases |
| FDR | False discovery rate |
| FET | Fisher’s exact test |
| HDL-c | High-density lipoprotein cholesterol |
| IPSL | In-house physical standard library |
| LDL-c | Low-density lipoprotein cholesterol |
| MS | Mass spectrometry |
| M/Z | Mass-to-charge ratio |
| nRMSE | Normalized root mean square error |
| nRMSEP | Normalized root mean square error of prediction |
| OPLS-R | Orthogonal partial least squares—regression |
| PUFA | Polyunsaturated fatty acid |
| RT | Retention time |
| SD | Standard deviation |
| TC | Total cholesterol |
| TG | Triglycerides |
| UHPLC-MS | Ultra-high performance liquid chromatography–high-resolution mass spectrometry |
| VIP | Variable Influence on Projection |
References
- Shah, N.S.; Lloyd-Jones, D.M.; O’Flaherty, M.; Capewell, S.; Kershaw, K.; Carnethon, M.; Khan, S.S. Trends in Cardiometabolic Mortality in the United States, 1999-2017. JAMA 2019, 322, 780. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145. [Google Scholar] [CrossRef]
- Bhargava, S.; de la Puente-Secades, S.; Schurgers, L.; Jankowski, J. Lipids and Lipoproteins in Cardiovascular Diseases: A Classification. Trends Endocrinol. Metab. 2022, 33, 409–423. [Google Scholar] [CrossRef]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and Lipids in Cardiovascular Disease: From Mechanistic Insights to Therapeutic Targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just about Sex Hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. [Google Scholar] [CrossRef]
- Chella Krishnan, K.; Mehrabian, M.; Lusis, A.J. Sex Differences in Metabolism and Cardiometabolic Disorders. Curr. Opin. Lipidol. 2018, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet Women and Cardiovascular Disease Commission: Reducing the Global Burden by 2030. The Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Knopp, R.H.; Paramsothy, P.; Retzlaff, B.M.; Fish, B.; Walden, C.; Dowdy, A.; Tsunehara, C.; Aikawa, K.; Cheung, M.C. Gender Differences in Lipoprotein Metabolism and Dietary Response: Basis in Hormonal Differences and Implications for Cardiovascular Disease. Curr. Atheroscler. Rep. 2005, 7, 472–479. [Google Scholar] [CrossRef]
- Wu, B.N.; O’Sullivan, A.J. Sex Differences in Energy Metabolism Need to Be Considered with Lifestyle Modifications in Humans. J. Nutr. Metab. 2011, 2011, 391809. [Google Scholar] [CrossRef]
- Bédard, A.; Lamarche, B.; Corneau, L.; Dodin, S.; Lemieux, S. Sex Differences in the Impact of the Mediterranean Diet on Systemic Inflammation. Nutr. J. 2015, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Artegoitia, V.M.; Krishnan, S.; Bonnel, E.L.; Stephensen, C.B.; Keim, N.L.; Newman, J.W. Healthy Eating Index Patterns in Adults by Sex and Age Predict Cardiometabolic Risk Factors in a Cross-Sectional Study. BMC Nutr. 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Audano, M.; Maldini, M.; De Fabiani, E.; Mitro, N.; Caruso, D. Gender-Related Metabolomics and Lipidomics: From Experimental Animal Models to Clinical Evidence. J. Proteom. 2018, 178, 82–91. [Google Scholar] [CrossRef]
- Patel, M.J.; Batch, B.C.; Svetkey, L.P.; Bain, J.R.; Turer, C.B.; Haynes, C.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Shah, S.H. Race and Sex Differences in Small-Molecule Metabolites and Metabolic Hormones in Overweight and Obese Adults. OMICS 2013, 17, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of Sex on Coronary Microvascular Dysfunction and Cardiac Outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet Quality and Major Chronic Disease Risk in Men and Women: Moving toward Improved Dietary Guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
- Pilote, L.; Dasgupta, K.; Guru, V.; Humphries, K.H.; McGrath, J.; Norris, C.; Rabi, D.; Tremblay, J.; Alamian, A.; Barnett, T.; et al. A Comprehensive View of Sex-Specific Issues Related to Cardiovascular Disease. Can. Med. Assoc. J. 2007, 176, S1–S44. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex Differences in Lipid and Lipoprotein Metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Robinson, G.A.; Pineda-Torra, I.; Ciurtin, C.; Jury, E.C. Sex Differences in Lipid Metabolism: Implications for Systemic Lupus Erythematosus and Cardiovascular Disease Risk. Front. Med. 2022, 9, 914016. [Google Scholar] [CrossRef]
- Freedman, D.S.; Otvos, J.D.; Jeyarajah, E.J.; Shalaurova, I.; Cupples, L.A.; Parise, H.; D’Agostino, R.B.; Wilson, P.W.F.; Schaefer, E.J. Sex and Age Differences in Lipoprotein Subclasses Measured by Nuclear Magnetic Resonance Spectroscopy: The Framingham Study. Clin. Chem. 2004, 50, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-Specific Pathway Differences in the Human Serum Metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef]
- Tabassum, R.; Ruotsalainen, S.; Ottensmann, L.; Gerl, M.J.; Klose, C.; Tukiainen, T.; Pirinen, M.; Simons, K.; Widén, E.; Ripatti, S. Lipidome- and Genome-Wide Study to Understand Sex Differences in Circulatory Lipids. J. Am. Heart Assoc. 2022, 11, e027103. [Google Scholar] [CrossRef]
- Friedman, G.D.; Cutter, G.R.; Donahue, R.P.; Hughes, G.H.; Hulley, S.B.; Jacobs, D.R.; Liu, K.; Savage, P.J. Cardia: Study Design, Recruitment, and Some Characteristics of the Examined Subjects. J. Clin. Epidemiol. 1988, 41, 1105–1116. [Google Scholar] [CrossRef]
- Funkhouser, E.; Wammack, J.; Roche, C.; Reis, J.; Sidney, S.; Schreiner, P. Where Are They Now? Retention Strategies over 25 Years in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Contemp. Clin. Trials Commun. 2018, 9, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Lewis, C.E.; Schreiner, P.J.; Shikany, J.M.; Sidney, S.; Reis, J.P. The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J. Am. Coll. Cardiol. 2021, 78, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, J.; Su, M.; Jia, E.; Chen, S.; Chen, T.; Ni, Y. Missing Value Imputation Approach for Mass Spectrometry-Based Metabolomics Data. Sci. Rep. 2018, 8, 663. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Bühlmann, P. MissForest—Non-Parametric Missing Value Imputation for Mixed-Type Data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef]
- Ghanbari, R.; Li, Y.; Pathmasiri, W.; McRitchie, S.; Etemadi, A.; Pollock, J.D.; Poustchi, H.; Rahimi-Movaghar, A.; Amin-Esmaeili, M.; Roshandel, G.; et al. Metabolomics Reveals Biomarkers of Opioid Use Disorder. Transl. Psychiatry 2021, 11, 103. [Google Scholar] [CrossRef]
- Kwak, M.; Kang, K.; Wang, Y. Methods of Metabolite Identification Using MS/MS Data. J. Comput. Inf. Syst. 2022, 62, 12–18. [Google Scholar] [CrossRef]
- Hullings, A.G. Sex Differences in Dietary Intake in Association with Cardiovascular Disease-Related Lipid Metabolites. Ph.D. Thesis, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2024. [Google Scholar] [CrossRef]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur Heart J 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Donahue, R.P.; Jacobs, D.R.; Sidney, S.; Wagenknecht, L.E.; Albers, J.J.; Hulley, S.B. Distribution of Lipoproteins and Apolipoproteins in Young Adults the CARDIA Study. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1989, 9, 656–664. [Google Scholar] [CrossRef]
- Sweeney, M.E.; Johnson, R.R. Ezetimibe: An Update on the Mechanism of Action, Pharmacokinetics and Recent Clinical Trials. Expert Opin. Drug Metab. Toxicol. 2007, 3, 441–450. [Google Scholar] [CrossRef]
- National Cholesterol Education Program NCEP Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Wu, J.; Province, M.A.; Coon, H.; Hunt, S.C.; Eckfeldt, J.H.; Arnett, D.K.; Heiss, G.; Lewis, C.E.; Ellison, R.C.; Rao, D.C.; et al. An Investigation of the Effects of Lipid-Lowering Medications: Genome-Wide Linkage Analysis of Lipids in the HyperGEN Study. BMC Genet. 2007, 8, 60. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Hahn, L.P.; Haskell, W.L.; Pirie, P.; Sidney, S. Validity and Reliability of Short Physical Activity History. J. Cardiopulm. Rehabil. 1989, 9, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Slattery, M.; Jacobs, D.; Cutter, G.; McDonald, A.; Van Horn, L.; Hilner, J.E.; Caan, B.; Bragg, C.; Dyer, A.; et al. A Study of the Reliability and Comparative Validity of the Cardia Dietary History. Ethn. Dis. 1994, 4, 15–27. [Google Scholar] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Sijtsma, F.P.; Meyer, K.A.; Steffen, L.M.; Shikany, J.M.; Van Horn, L.; Harnack, L.; Kromhout, D.; Jacobs, D.R. Longitudinal Trends in Diet and Effects of Sex, Race, and Education on Dietary Quality Score Change: The Coronary Artery Risk Development in Young Adults Study. Am. J. Clin. Nutr. 2012, 95, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, L.V.; Ballew, C.; Liu, K.; McDonald, A.; Hilner, J.E.; Burke, G.L.; Savage, P.J.; Bragg, C.; Caan, B.; Jacobs, D.; et al. Diet, Body Size, and Plasma Lipids-Lipoproteins in Young Adults: Differences by Race and Sex. Am. J. Epidemiol. 1991, 133, 9–23. [Google Scholar] [CrossRef]
- Kim, H.; Hu, E.A.; Wong, K.E.; Yu, B.; Steffen, L.M.; Seidelmann, S.B.; Boerwinkle, E.; Coresh, J.; Rebholz, C.M. Serum Metabolites Associated with Healthy Diets in African Americans and European Americans. J. Nutr. 2021, 151, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Vajargah, K.F.; Mehdizadeh, R.; Sadeghi-bazargani, H. Applications of Opls Statistical Method in Medicine. J. Math. Comput. Sci. 2014, 08, 411–422. [Google Scholar] [CrossRef]
- Ghosh, T.; Zhang, W.; Ghosh, D.; Kechris, K. Predictive Modeling for Metabolomics Data. In Computational Methods and Data Analysis for Metabolomics; Springer: New York, NY, USA, 2020; pp. 313–336. [Google Scholar]
- Wang, X.; Jones, D.R.; Shaw, T.I.; Cho, J.-H.; Wang, Y.; Tan, H.; Xie, B.; Zhou, S.; Li, Y.; Peng, J. Target-Decoy-Based False Discovery Rate Estimation for Large-Scale Metabolite Identification. J. Proteome Res. 2018, 17, 2328–2334. [Google Scholar] [CrossRef]
- Scheubert, K.; Hufsky, F.; Petras, D.; Wang, M.; Nothias, L.-F.; Dührkop, K.; Bandeira, N.; Dorrestein, P.C.; Böcker, S. Significance Estimation for Large Scale Metabolomics Annotations by Spectral Matching. Nat. Commun. 2017, 8, 1494. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2010. Diabetes Care 2010, 33, S11–S61. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef]
- McGranaghan, P.; Kirwan, J.A.; Garcia-Rivera, M.A.; Pieske, B.; Edelmann, F.; Blaschke, F.; Appunni, S.; Saxena, A.; Rubens, M.; Veledar, E.; et al. Lipid Metabolite Biomarkers in Cardiovascular Disease: Discovery and Biomechanism Translation from Human Studies. Metabolites 2021, 11, 621. [Google Scholar] [CrossRef]
- Lu, Y.; Pang, Z.; Xia, J. Comprehensive Investigation of Pathway Enrichment Methods for Functional Interpretation of LC–MS Global Metabolomics Data. Brief. Bioinform. 2023, 24, bbac553. [Google Scholar] [CrossRef]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and Women’s Cardiovascular Health: Is It Really an Obvious Relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma Acylcarnitines and Risk of Cardiovascular Disease: Effect of Mediterranean Diet Interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Fei, N.; Bernabé, B.P.; Lie, L.; Baghdan, D.; Bedu-Addo, K.; Plange-Rhule, J.; Forrester, T.E.; Lambert, E.V.; Bovet, P.; Gottel, N.; et al. The Human Microbiota Is Associated with Cardiometabolic Risk across the Epidemiologic Transition. PLoS ONE 2019, 14, e0215262. [Google Scholar] [CrossRef]
- Wen, Y.; Shang, Y.; Wang, Q. Exploration of the Mechanism of Linoleic Acid Metabolism Dysregulation in Metabolic Syndrome. Genet. Res. 2022, 2022, 6793346. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Benton, T.Z.; Bennett, B.J.; Jacobs, D.R.; Lloyd-Jones, D.M.; Gross, M.D.; Carr, J.J.; Gordon-Larsen, P.; Zeisel, S.H. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J. Am. Heart Assoc. 2016, 5, e003970. [Google Scholar] [CrossRef]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Vajdi, M. Gut Microbiota–Associated Trimethylamine N-Oxide and Increased Cardiometabolic Risk in Adults: A Systematic Review and Dose-Response Meta-Analysis. Nutr. Rev. 2021, 79, 1022–1042. [Google Scholar] [CrossRef]
- Ma, W.; Heianza, Y.; Huang, T.; Wang, T.; Sun, D.; Zheng, Y.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Dietary Glutamine, Glutamate and Mortality: Two Large Prospective Studies in US Men and Women. Int. J. Epidemiol. 2018, 47, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S. Alanine and Aspartate Aminotransferase and Glutamine-Cycling Pathway: Their Roles in Pathogenesis of Metabolic Syndrome. World J. Gastroenterol. 2012, 18, 3775. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L.; Salazar, C.; Ryff, C.D.; Coe, C.L.; Rigotti, A. Serum Sphingolipid Profiling as a Novel Biomarker for Metabolic Syndrome Characterization. Front. Cardiovasc. Med. 2022, 9, 1092331. [Google Scholar] [CrossRef] [PubMed]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine Learning Reveals Serum Sphingolipids as Cholesterol-Independent Biomarkers of Coronary Artery Disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef]
- Meikle, P.J.; Summers, S.A. Sphingolipids and Phospholipids in Insulin Resistance and Related Metabolic Disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef]
- Zhu, Q.; Scherer, P.E. Ceramides and Atherosclerotic Cardiovascular Disease: A Current Perspective. Circulation 2024, 149, 1624–1626. [Google Scholar] [CrossRef]
- Wittenbecher, C.; Cuadrat, R.; Johnston, L.; Eichelmann, F.; Jäger, S.; Kuxhaus, O.; Prada, M.; Del Greco, M.F.; Hicks, A.A.; Hoffman, P.; et al. Dihydroceramide- and Ceramide-Profiling Provides Insights into Human Cardiometabolic Disease Etiology. Nat. Commun. 2022, 13, 936. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Liu, H.-X.; Kalanetra, K.M.; Gonzalez, F.J.; French, S.W.; Krishnan, V.V.; Mills, D.A.; Wan, Y.-J.Y. Gender Differences in Bile Acids and Microbiota in Relationship with Gender Dissimilarity in Steatosis Induced by Diet and FXR Inactivation. Sci. Rep. 2017, 7, 1748. [Google Scholar] [CrossRef]
- Phelps, T.; Snyder, E.; Rodriguez, E.; Child, H.; Harvey, P. The Influence of Biological Sex and Sex Hormones on Bile Acid Synthesis and Cholesterol Homeostasis. Biol. Sex. Differ. 2019, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex Differences in Lipid Metabolism Are Affected by Presence of the Gut Microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, J.; Wu, W.; Zhu, Y.; Liu, X. The Role of Bile Acids in Cardiovascular Diseases: From Mechanisms to Clinical Implications. Aging Dis. 2023, 14, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Pantea Stoian, A.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Colantonio, L.D.; Zhao, H.; Bittner, V.; Dai, Y.; Farkouh, M.E.; Monda, K.L.; Safford, M.M.; Muntner, P.; Woodward, M. Sex Differences in High-Intensity Statin Use Following Myocardial Infarction in the United States. J. Am. Coll. Cardiol. 2018, 71, 1729–1737. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of Dietary, Circulating, and Supplement Fatty Acids with Coronary Risk. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Fat Intake and Risk of Cardiovascular Disease and All-Cause Mortality in a Population at High Risk of Cardiovascular Disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, R.; Shi, R.; Qin, H.; Chen, W.; Yu, Z.; Ding, Y.; Peng, C.; Shi, Y. Sex Differences in the Association between Plasma Polyunsaturated Fatty Acids Levels and Moderate-to-Severe Plaque Psoriasis Severity: A Cross-Sectional and Longitudinal Study. J. Transl. Med. 2023, 21, 834. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Gooren, L.J.; Toorians, A.W.; Katan, M.B.; Zock, P.L. Docosahexaenoic Acid Concentrations Are Higher in Women than in Men Because of Estrogenic Effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [CrossRef]
- Childs, C.E.; Romeu-Nadal, M.; Burdge, G.C.; Calder, P.C. Gender Differences in the n-3 Fatty Acid Content of Tissues. Proc. Nutr. Soc. 2008, 67, 19–27. [Google Scholar] [CrossRef]
- Kitson, A.P.; Marks, K.A.; Shaw, B.; Mutch, D.M.; Stark, K.D. Treatment of Ovariectomized Rats with 17β-Estradiol Increases Hepatic Delta-6 Desaturase Enzyme Expression and Docosahexaenoic Acid Levels in Hepatic and Plasma Phospholipids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Varì, R.; Scazzocchio, B.; D’Amore, A.; Giovannini, C.; Gessani, S.; Masella, R. Gender-Related Differences in Lifestyle May Affect Health Status. Ann. Ist. Super. Sanita 2016, 52, 158–166. [Google Scholar] [CrossRef]
- Wielsøe, M.; Berthelsen, D.; Mulvad, G.; Isidor, S.; Long, M.; Bonefeld-Jørgensen, E.C. Dietary Habits among Men and Women in West Greenland: Follow-up on the ACCEPT Birth Cohort. BMC Public Health 2021, 21, 1426. [Google Scholar] [CrossRef]
- Bédard, A.; Riverin, M.; Dodin, S.; Corneau, L.; Lemieux, S. Sex Differences in the Impact of the Mediterranean Diet on Cardiovascular Risk Profile. Br. J. Nutr. 2012, 108, 1428–1434. [Google Scholar] [CrossRef]
- Bédard, A.; Corneau, L.; Lamarche, B.; Dodin, S.; Lemieux, S. Sex Differences in the Impact of the Mediterranean Diet on LDL Particle Size Distribution and Oxidation. Nutrients 2015, 7, 3705–3723. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Sotgiu, G.; Ruoppolo, M.; Franconi, F.; Campesi, I. Sex Differences in the Human Metabolome. Biol. Sex. Differ. 2022, 13, 30. [Google Scholar] [CrossRef]
- Ruoppolo, M.; Campesi, I.; Scolamiero, E.; Pecce, R.; Caterino, M.; Cherchi, S.; Mercuro, G.; Tonolo, G.; Franconi, F. Serum Metabolomic Profiles Suggest Influence of Sex and Oral Contraceptive Use. Am. J. Transl. Res. 2014, 6, 614–624. [Google Scholar] [PubMed]
- Saito, K.; Maekawa, K.; Kinchen, J.M.; Tanaka, R.; Kumagai, Y.; Saito, Y. Gender- and Age-Associated Differences in Serum Metabolite Profiles among Japanese Populations. Biol. Pharm. Bull. 2016, 39, 1179–1186. [Google Scholar] [CrossRef]
- Andraos, S.; Lange, K.; Clifford, S.A.; Jones, B.; Thorstensen, E.B.; Wake, M.; Burgner, D.P.; Saffery, R.; O’Sullivan, J.M. Population Epidemiology and Concordance for Plasma Amino Acids and Precursors in 11–12-Year-Old Children and Their Parents. Sci. Rep. 2021, 11, 3619. [Google Scholar] [CrossRef]
- Jové, M.; Maté, I.; Naudí, A.; Mota-Martorell, N.; Portero-Otín, M.; De la Fuente, M.; Pamplona, R. Human Aging Is a Metabolome-Related Matter of Gender. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Tenori, L.; Luchinat, C.; Saccenti, E. Age and Sex Effects on Plasma Metabolite Association Networks in Healthy Subjects. J. Proteome Res. 2018, 17, 97–107. [Google Scholar] [CrossRef]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite Patterns Predicting Sex and Age in Participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) Study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef]
- Kochhar, S.; Jacobs, D.M.; Ramadan, Z.; Berruex, F.; Fuerholz, A.; Fay, L.B. Probing Gender-Specific Metabolism Differences in Humans by Nuclear Magnetic Resonance-Based Metabonomics. Anal. Biochem. 2006, 352, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Lin, W.; Broadhurst, D.; Begley, P.; Brown, M.; Zelena, E.; Vaughan, A.A.; Halsall, A.; Harding, N.; Knowles, J.D.; et al. Molecular Phenotyping of a UK Population: Defining the Human Serum Metabolome. Metabolomics 2015, 11, 9–26. [Google Scholar] [CrossRef]
- Mittelstrass, K.; Ried, J.S.; Yu, Z.; Krumsiek, J.; Gieger, C.; Prehn, C.; Roemisch-Margl, W.; Polonikov, A.; Peters, A.; Theis, F.J.; et al. Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers. PLoS Genet. 2011, 7, e1002215. [Google Scholar] [CrossRef]
- Gieger, C.; Geistlinger, L.; Altmaier, E.; Hrabé de Angelis, M.; Kronenberg, F.; Meitinger, T.; Mewes, H.-W.; Wichmann, H.-E.; Weinberger, K.M.; Adamski, J.; et al. Genetics Meets Metabolomics: A Genome-Wide Association Study of Metabolite Profiles in Human Serum. PLoS Genet. 2008, 4, e1000282. [Google Scholar] [CrossRef]
- Link, J.C.; Reue, K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Weersma, R.K.; Medema, M.H.; Netea, M.G.; et al. Influence of the Microbiome, Diet and Genetics on Inter-Individual Variation in the Human Plasma Metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Dai, C.L.; Wilmanski, T.; Baloni, P.; Smith, B.; Rappaport, N.; Hood, L.; Magis, A.T.; Gibbons, S.M. Genome-Microbiome Interplay Provides Insight into the Determinants of the Human Blood Metabolome. Nat. Metab. 2022, 4, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Hou, Y.; Zhang, H.; Yang, K.; Wang, J.; Guo, B.; Zhang, F.; Li, H.; Zhou, X.; Li, Y.; et al. Plasma Metabolic Profiles in Women Are Menopause Dependent. PLoS ONE 2015, 10, e0141743. [Google Scholar] [CrossRef]
| Variable | Women (n = 964) | Men (n = 1205) | ||
|---|---|---|---|---|
| Clinical lipid measures 1 | ||||
| Total cholesterol (mg/dL), mean (sd) * | 188.1 | (34.1) | 191.7 | (37.4) |
| Triglycerides (mg/dL), mean (sd) * | 90.7 | (50.2) | 121.3 | (67.0) |
| Low-density lipoprotein cholesterol (mg/dL), mean (sd) * | 109.8 | (31.2) | 119.8 | (34.4) |
| High-density lipoprotein cholesterol (mg/dL), mean (sd) * | 60.1 | (16.7) | 47.5 | (14.2) |
| Basic demographics 2 | ||||
| Study field center, n (col %) * | ||||
| Birmingham, AL | 222 | (23.0) | 334 | (27.7) |
| Chicago, IL | 241 | (25.0) | 290 | (24.1) |
| Minneapolis, MN | 213 | (22.1) | 306 | (25.4) |
| Oakland, CA | 288 | (29.9) | 275 | (22.8) |
| Self-reported race, n (col %) | ||||
| White | 544 | (56.4) | 714 | (59.3) |
| Black | 420 | (43.6) | 491 | (40.7) |
| Education, n (col %) * | ||||
| High school or less | 108 | (11.2) | 208 | (17.3) |
| College or more | 856 | (88.8) | 997 | (82.7) |
| Age (years), mean (sd) * | 44.7 | (3.8) | 45.3 | (3.5) |
| Total energy intake (kcals), median (25th, 75th) * | 1842.5 | (1447.0, 2401.1) | 2522.6 | (1964.0, 3259.6) |
| Lifestyle factors 3 | ||||
| Smoking status, n (col %) * | ||||
| Never | 593 | (61.5) | 758 | (62.9) |
| Former | 220 | (22.8) | 209 | (17.3) |
| Current | 151 | (15.7) | 238 | (19.8) |
| Taking birth control medication | ||||
| No | 834 | (86.5) | - | - |
| Yes | 130 | (13.5) | - | - |
| Physical activity score 4, median (25th, 75th) * | 234 | (106.5, 436.5) | 360 | (200.0, 588.0) |
| Alcohol consumption (mL/day), mean (sd) * | 8.2 | (14.5) | 14.5 | (25.9) |
| Body mass index and clinical factors 5 | ||||
| Diabetes status 6, n (col %) * | ||||
| No | 896 | (92.9) | 1087 | (90.2) |
| Yes | 68 | (7.1) | 118 | (9.8) |
| Hypertension status 7, n (col %) * | ||||
| No | 745 | (77.3) | 864 | (71.7) |
| Yes | 219 | (22.7) | 341 | (28.3) |
| Estimated glomerular filtration rate (mL/min/1.73 m2), mean (sd) | 95.6 | (14.3) | 94.8 | (15.2) |
| Body mass index (kg/m2), mean (sd) | 28.9 | (7.5) | 28.9 | (5.7) |
| Taking lipid-lowering medications, n (col %) * | ||||
| No | 912 | (94.6) | 1049 | (87.1) |
| Yes | 52 | (5.4) | 156 | (12.9) |
| Sensitivity analysis | ||||
| Self-reported menopausal status, n (column %) 8 | ||||
| Pre-menopausal | 703 | (73.2) | - | - |
| Peri-menopausal | 152 | (15.8) | - | - |
| Post-menopausal | 106 | (11.0) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hullings, A.G.; Howard, A.G.; Meyer, K.A.; Avery, C.L.; North, K.E.; Mhatre, S.; Sha, W.; Li, Y.; Rushing, B.R.; Sumner, S.; et al. Sex Modifies Metabolic Pathways Associated with Lipids in Untargeted Metabolomics: The Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–2006. Metabolites 2025, 15, 730. https://doi.org/10.3390/metabo15110730
Hullings AG, Howard AG, Meyer KA, Avery CL, North KE, Mhatre S, Sha W, Li Y, Rushing BR, Sumner S, et al. Sex Modifies Metabolic Pathways Associated with Lipids in Untargeted Metabolomics: The Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–2006. Metabolites. 2025; 15(11):730. https://doi.org/10.3390/metabo15110730
Chicago/Turabian StyleHullings, Autumn G., Annie Green Howard, Katie A. Meyer, Christy L. Avery, Kari E. North, Sachin Mhatre, Wei Sha, Yuanyuan Li, Blake R. Rushing, Susan Sumner, and et al. 2025. "Sex Modifies Metabolic Pathways Associated with Lipids in Untargeted Metabolomics: The Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–2006" Metabolites 15, no. 11: 730. https://doi.org/10.3390/metabo15110730
APA StyleHullings, A. G., Howard, A. G., Meyer, K. A., Avery, C. L., North, K. E., Mhatre, S., Sha, W., Li, Y., Rushing, B. R., Sumner, S., Du, X., Lewis, C. E., & Gordon-Larsen, P. (2025). Sex Modifies Metabolic Pathways Associated with Lipids in Untargeted Metabolomics: The Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–2006. Metabolites, 15(11), 730. https://doi.org/10.3390/metabo15110730








