Plant Metabolome Between Root and Aerial Parts of Cichorium intybus L. and Anti-Hyperuricemia Mechanisms Based on Cell Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Metabolome Between Chicory Roots and Leaves Extracts
2.2.1. Sample Collection and Preparation
2.2.2. UPLC-QTOF-MS Conditions
2.2.3. Data Processing
2.3. Determination of Anti-Hyperuricemia Activities on HK-2 Cells Induced by Adenosine with Xanthine Oxidase
2.3.1. Sample Preparation for Determination of Anti-Hyperuricemia Activities on HK-2 Cells
2.3.2. Cell Culture and Model Construction
2.3.3. Uric Acid Quantification
2.3.4. Statistical Analysis
2.4. Anti-HUA Mechanism of Chicory Leaves Based on Non-Targeted Cell Metabolomics
2.4.1. Sample Preparation for Non-Targeted Cell Metabolomics
2.4.2. UPLC-Q-TOF-MS Conditions
2.4.3. Data Acquisition and Processing for Non-Targeted Metabolomics
3. Results
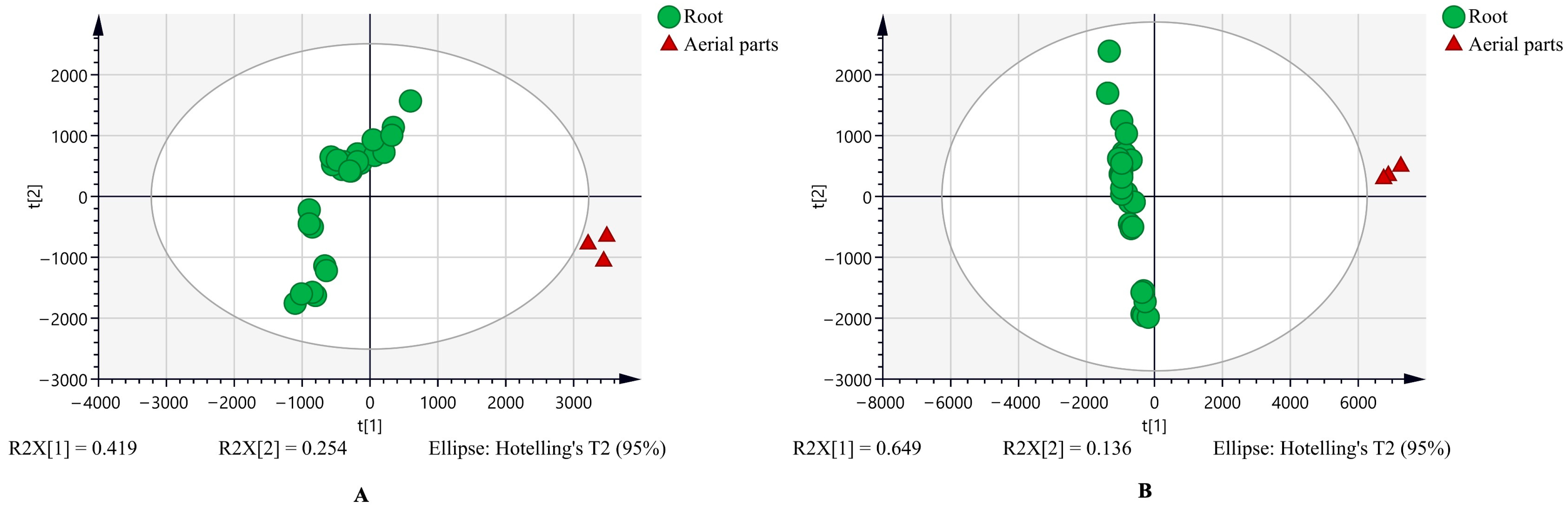
3.1. The Untargeted Metabolome and the Characteristic Metabolites Between Chicory Root and Leaf Extracts
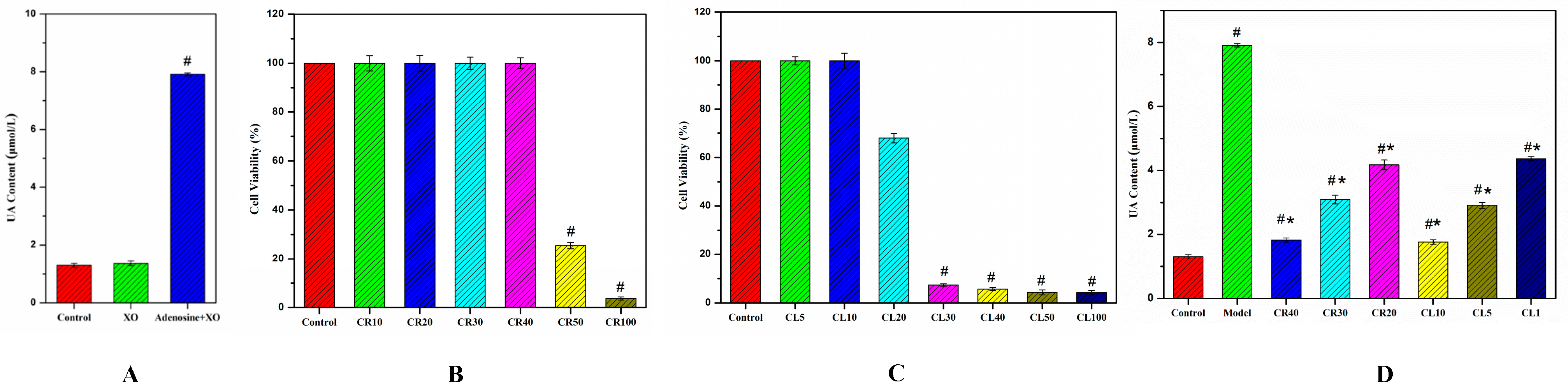
3.2. Construction of Hyperuricemia Cell Model
3.3. Anti-Hyperuricemia Effects of Chicory on the UA Levels
3.4. Untargeted Metabolomics Analysis of Cell Metabolome Treated by Chicory Root
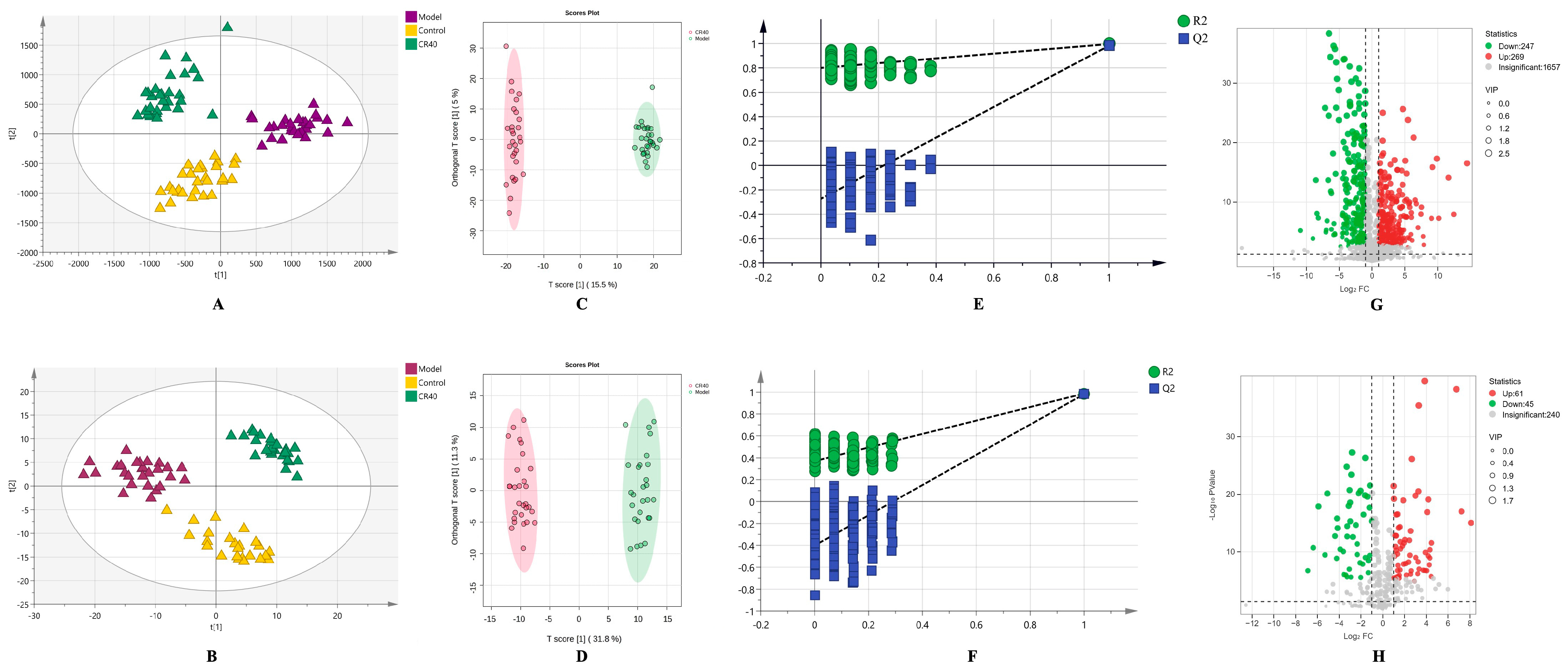
3.4.1. Multivariate Statistical Analysis
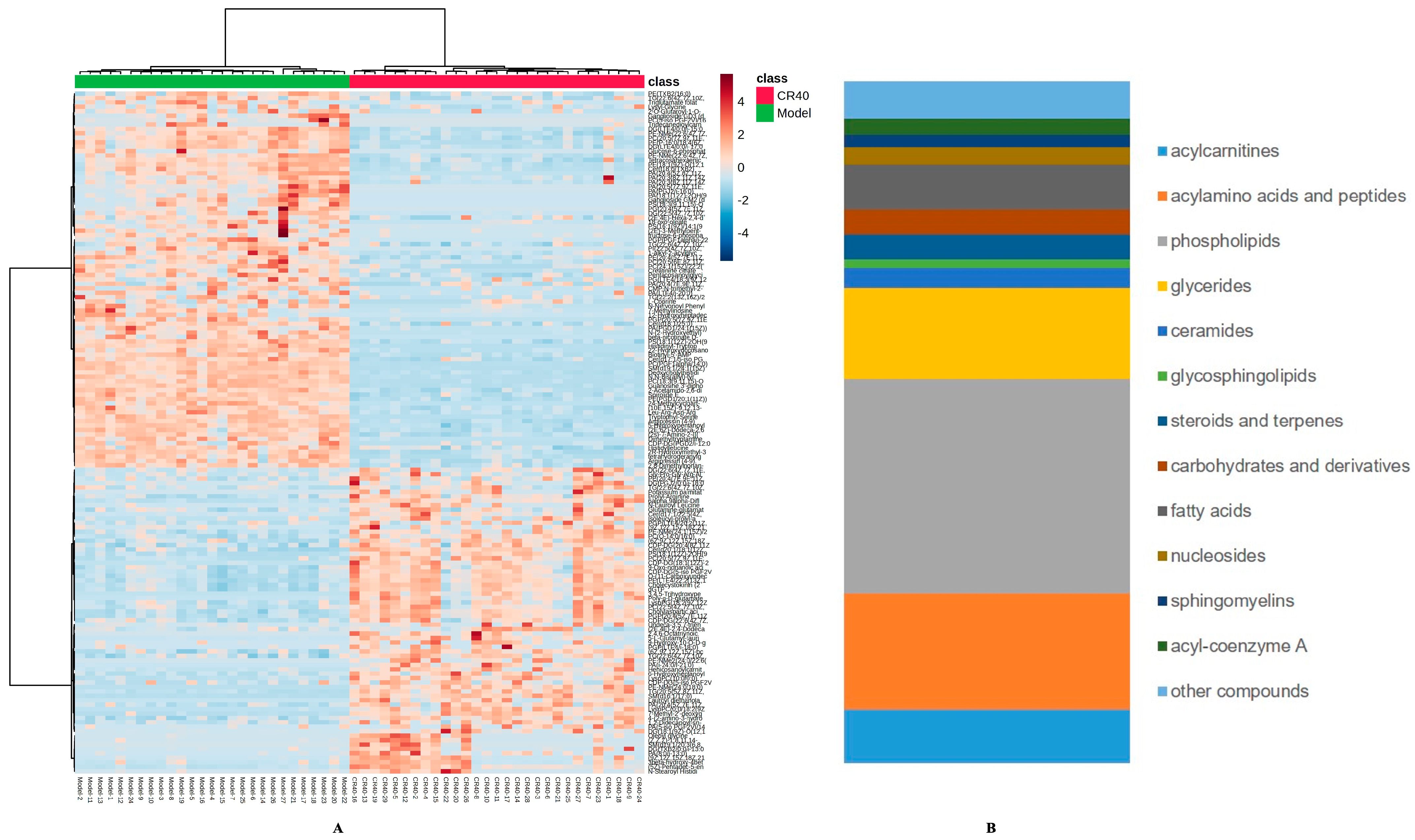
3.4.2. Identification of Differential Metabolites Between the Model and CR40 Groups
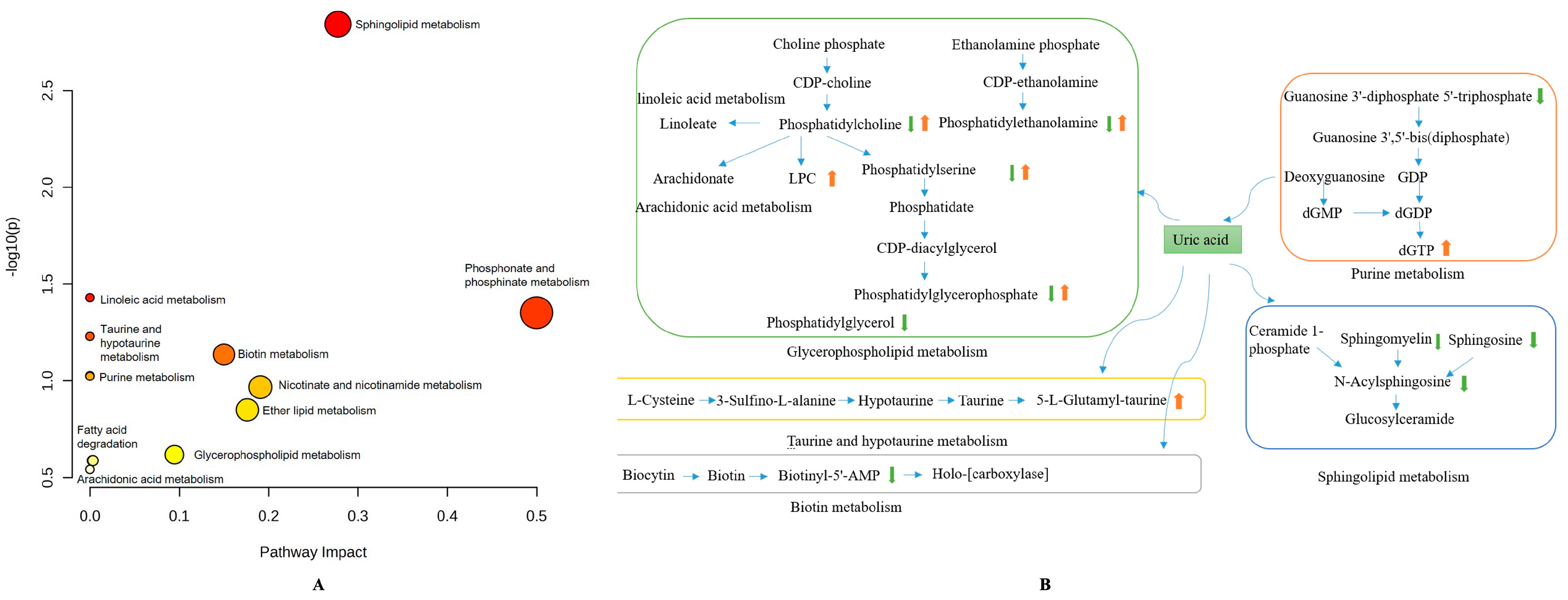
3.4.3. Metabolic Pathways Analysis in HUA Cell Model by Chicory Root
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Shen, Z.; Zhu, B.; Zhang, H.; Zhang, X.; Ding, X. Demographic, regional and temporal trends of hyperuricemia epidemics in mainland China from 2000 to 2019: A systematic review and meta-analysis. Glob. Health Action 2021, 14, 1874652. [Google Scholar] [CrossRef]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zeng, C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167–3181. [Google Scholar] [PubMed]
- Bardin, T.; Richette, P. Impact of comorbidities on gout and hyperuricemia: An update on prevalence and treatment options. BMC Med. 2017, 15, 123. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Zhang, Z.; Mu, R.; Song, J.; Yang, Z.; Li, R.; Zhang, J.; Zhu, X.; Gong, M.; et al. Integrating metabolomics with network pharmacology to reveal the mechanism of Poria cocos in hyperuricemia treatment. J. Ethnopharmacol. 2025, 337, 118977. [Google Scholar] [CrossRef] [PubMed]
- Pascart, T.; Richette, P. Investigational drugs for hyperuricemia, an update on recent developments. Expert. Opin. Investig. Drugs 2018, 5, 437–444. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, C.B.; Chang, C.J.; Lin, Y.J.; Wang, C.W.; Chi, C.C.; Lu, C.W.; Chen, W.T.; Pan, R.Y.; Su, S.C.; et al. Hypersensitivity and Cardiovascular Risks Related to Allopurinol and Febuxostat Therapy in Asians: A Population-Based Cohort Study and Meta-Analysis. Clin. Pharmacol. Ther. 2019, 2, 391–401. [Google Scholar] [CrossRef]
- Stamp, L.K.; Barclay, M.L. How to prevent allopurinol hypersensitivity reactions? Rheumatology 2018, 57, i35–i41. [Google Scholar] [CrossRef]
- Hang, N.T.; Thi Tu Uyen, T.; Van Phuong, N. Green extraction of apigenin and luteolin from celery seed using deep eutectic solvent. J. Pharm. Biomed. Anal. 2022, 207, 114406. [Google Scholar] [CrossRef]
- Pouille, C.L.; Ouaza, S.; Roels, E.; Behra, J.; Tourret, M.; Molinié, R.; Fontaine, J.X.; Mathiron, D.; Gagneul, D.; Taminiau, B.; et al. Chicory: Understanding the Effects and Effectors of This Functional Food. Nutrients 2022, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jang, T.W.; Song, P.H.; Choi, S.H.; Ku, S.K.; Song, C.H. Combination Effects of Metformin and a Mixture of Lemon Balm and Dandelion on High-Fat Diet-Induced Metabolic Alterations in Mice. Antioxidants 2022, 11, 580. [Google Scholar] [CrossRef]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Medical importance of Cichorium intybus—A review. J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Bahmani, M.; Shahinfard, N.; Rafieian-Kopaei, M.; Saki, K.; Shahsavari, S.; Taherikalani, M.; Ghafourian, S.; Baharvand-Ahmadi, B. Chicory: A review on ethnobotanical effects of Cichorium intybus L. J. Chem. Pharm. Sci. 2015, 4, 672–682. [Google Scholar]
- Bian, M.; Wang, J.; Wang, Y.; Nie, A.; Zhu, C.; Sun, Z.; Zhou, Z.; Zhang, B. Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. Biomed. Pharmacother. 2020, 131, 110719. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, B.; Liu, X.; Jin, R.; Zhu, W. Effects of chicory inulin on serum metabolites of uric acid, lipids, glucose, and abdominal fat deposition in quails induced by purine-rich diets. J. Med. Food 2014, 11, 1214–1221. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 6, 353–367. [Google Scholar] [CrossRef]
- Pang, X.; Guo, Z.; Ao, L.; Yang, Y.; Liu, C.; Gu, Z.; Xin, Y.; Li, M.; Zhang, L. Integrated cell metabolomics and serum metabolomics to reveal the mechanism of hypouricemic effect of Inonotus hispidus. J. Funct. Foods 2023, 105, 105572. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Rong, Y. Fermentation mechanism of ginkgo rice wine using an ultra-high-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry based metabolomics method. J. Food Compos. Anal. 2022, 105, 104230. [Google Scholar] [CrossRef]
- Peng, A.; Lin, L.; Zhao, M.; Sun, B. Identifying mechanisms underlying the amelioration effect of Chrysanthemum morifolium Ramat. ‘Boju’ extract on hyperuricemia using biochemical characterization and UPLC-ESI-QTOF/MS-based metabolomics. Food Funct. 2019, 12, 8042–8055. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.A.; Lindon, J.C.; Cloarec, O.; Antti, H.; Charuel, C.; Hanton, G.; Provost, J.P.; Le Net, J.L.; Baker, D.; Walley, R.J.; et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 2006, 7087, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Slupsky, C.M.; Rankin, K.N.; Wagner, J.; Fu, H.; Chang, D.; Weljie, A.M.; Saude, E.J.; Lix, B.; Adamko, D.J.; Shah, S.; et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal. Chem. 2007, 18, 6995–7004. [Google Scholar] [CrossRef]
- Liu, P.; Yang, J.; Jin, M.; Hu, P.; Zhu, Y.; Tang, Y.; Chen, Y.; Xu, X.; He, H. Alterations in the gut microbiome and metabolism profiles reveal the possible molecular mechanism of renal injury induced by hyperuricemia in a mouse model of renal insufficiency. Ren. Fail. 2024, 2, 2387429. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Yan, F.; He, Y.; Ji, A.; Liu, Z.; Li, M.; Ji, X.; Li, C. Kidney and plasma metabolomics provide insights into the molecular mechanisms of urate nephropathy in a mouse model of hyperuricemia. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 6, 166374. [Google Scholar] [CrossRef]
- Yu, H.; Huang, L.; Gui, L.; Wu, Z.; Luo, H.; Xu, M.; Zhang, Y.; Qian, Y.; Cao, W.; Liu, L.; et al. Luteolin ameliorates hyperuricemic nephropathy by activating urate excretion and Nrf2/HO-1/NQO1 antioxidant pathways in mice. Food Sci. Nutr. 2024, 10, 8053–8066. [Google Scholar] [CrossRef]
- Papetti, A.; Maietta, M.; Corana, F.; Marrubini, G.; Gazzzni, G. Polyphenolic profile of green/red spotted Italian Cichorium intybus salads by RP-HPLC-PDA-ESI-MSn. J. Food Compos. Anal. 2017, 63, 189–197. [Google Scholar] [CrossRef]
- Jiang, Y.; Lin, Y.; Hu, Y.J.; Song, X.J.; Pan, H.H.; Zhang, H.J. Caffeoylquinic acid derivatives rich extract from Gnaphalium pensylvanicum willd. Ameliorates hyperuricemia and acute gouty arthritis in animal model. BMC Complement. Altern. Med. 2017, 1, 320. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2021, 4, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Battista, N.; Bari, M.; Bisogno, T. N-Acyl Amino Acids: Metabolism, Molecular Targets, and Role in Biological Processes. Biomolecules 2019, 12, 822. [Google Scholar] [CrossRef]
- Xiang, L.; Ru, Y.; Shi, J.; Wang, L.; Zhao, H.; Huang, Y.; Cai, Z. Derivatization of N-Acyl Glycines by 3-Nitrophenylhydrazine for Targeted Metabolomics Analysis and Their Application to the Study of Diabetes Progression in Mice. Anal. Chem. 2023, 4, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.R.; Ueno, S.; Chen, M.X.; Harvey, J.; Dowell, S.J.; Irving, A.J.; Brown, A.J. N-Palmitoylglycine and other N-acylamides activate the lipid receptor G2A/GPR132. Pharmacol. Res. Perspect. 2019, 6, e00542. [Google Scholar] [CrossRef]
- Steiber, A.; Kerner, J.; Hoppel, C.L. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Asp. Med. 2004, 5–6, 455–473. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J.A.; Ranea-Robles, P. Metabolic interactions between peroxisomes and mitochondria with a special focus on acylcarnitine metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 5, 165720. [Google Scholar] [CrossRef]
- Wang, F.; Sun, L.; Zong, G.; Gao, X.; Zhang, H.; Xiong, Q.; Huo, S.; Niu, Z.; Sun, Q.; Zeng, R.; et al. Associations of Amino Acid and Acylcarnitine Profiles with Incident Hyperuricemia in Middle-Aged and Older Chinese Individuals. Arthritis Care Res. 2020, 9, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, L.; Sun, Q.; Liang, L.; Gao, X.; Li, R.; Pan, A.; Li, H.; Deng, Y.; Hu, F.B.; et al. Associations of Plasma Amino Acid and Acylcarnitine Profiles with Incident Reduced Glomerular Filtration Rate. Clin. J. Am. Soc. Nephrol. 2018, 4, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, L.; Gao, X.; Zhang, H.; Yao, P.; Hu, Y.; Ma, Y.; Wang, F.; Jin, Q.; Li, H.; et al. Early Prediction of Developing Type 2 Diabetes by Plasma Acylcarnitines: A Population-Based Study. Diabetes Care 2016, 9, 1563–1570. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Liu, X. Hyperuricemia impairs endothelial function through SMS2-dependent activation of the endoplasmic reticulum stress response. Toxicol. In Vitro 2026, 110, 106133. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Chen, T.; Huang, B.; Liu, Y.; Chen, J. Untargeted metabolomics reveal the therapeutic effects of Ermiao wan categorized formulas on rats with hyperuricemia. J. Ethnopharmacol. 2021, 281, 114545. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Ma, L.; Ge, Y.; Wang, X.M. The effect of lipid metabolism disorder on patients with hyperuricemia using Multi-Omics analysis. Sci. Rep. 2023, 1, 18211. [Google Scholar] [CrossRef]
- Wu, X.; You, C. The biomarkers discovery of hyperuricemia and gout: Proteomics and metabolomics. PeerJ 2023, 11, e14554. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 5, 1149. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Nam, Y.; Park, Y.S.; Lee, H.S.; Hong, S.A.; Kim, B.K.; Park, E.S.; Chung, Y.H.; Jeong, J.H. Protective effect of phosphatidylcholine on lipopolysaccharide-induced acute inflammation in multiple organ injury. Korean J. Physiol. Pharmacol. 2013, 3, 209–216. [Google Scholar] [CrossRef] [PubMed]
| No. | Tentative Compound Identity | Score b | F Score b | Fold Change c |
|---|---|---|---|---|
| 1 | 3-galactosyllactose | 41.8 | 25.1 | 57.16 |
| 2 | Maltooctaose | 47.0 | 44.8 | Infinity d |
| 3 | Xyloglucan heptasaccharide | 47.0 | 44.8 | Infinity |
| 4 | α-L-arabinofuranosyl-(1->3)-β-D-xylopyranosyl-(1->4)-D-xylose | 39.9 | 7.74 | 113.22 |
| 5 | β-D-xylopyranosyl-(1->5)- α-L-arabinofuranosyl-(1->3)-L-arabinose | 56.0 | 93.6 | 36.68 |
| 6 | 3-β-glucosylcellotriose | 55.9 | 84.5 | 7.91 |
| 7 | Maltohexaose | 51.4 | 62.4 | 6.44 |
| 8 | Cellopentaose | 52.8 | 68.1 | 9.26 |
| 9 | Cellobiose | 44.0 | 21.6 | 311.06 |
| 10 | Lactulose | 41.8 | 13.4 | 10.35 |
| 11 | Kaempferol-3-glucoside-7-rhamnoside | 39.6 | 8.60 | 64.12 |
| 12 | Kaempferol-7-O-neohesperidoside | 40.0 | 7.13 | 27.28 |
| 13 | Kaempferide-3-[rhamnopyranosyl-(1->6)-glucoside]-7-rhamnoside | 44.1 | 31.8 | 72.26 |
| 14 | Kaempferol-7-sophoroside | 39.0 | 8.51 | 3.56 |
| 15 | Kaempferol-3-[glucosyl-(1->3)-rhamnosyl-(1->2)-[rhamnosyl-(1->6)-galactoside]] | 36.1 | 0.86 | Infinity |
| 16 | Apigenin a | 40.0 | 1.41 | 94.98 |
| 17 | Apigenin-7-(6″-O-alpha-rhamnosyl-β-glucoside) | 37.0 | 2.53 | 54.50 |
| 18 | Apigenin-7-glucuronide a | 39.6 | 4.01 | 38.34 |
| 19 | Luteolin a | 40.5 | 6.00 | 247.53 |
| 20 | Luteolin-7-glucoside a | 38.0 | 8.46 | 552.55 |
| 21 | Luteolin-7-glucuronide a | 42.3 | 23.2 | 182.87 |
| 22 | Luteolin-7-galactoside | 40.3 | 6.25 | 58.45 |
| 23 | Luteolin-3′-(3″-acetylglucuronide) | 36.9 | 2.27 | 30.67 |
| 24 | 6-hydroxyluteolin | 44.9 | 33.2 | 2.18 |
| 25 | 3,5,7-trihydroxy-4′-methoxy-8-prenylflavone-3-[rhamnosyl-(1->6)-galactoside]-7-galactoside | 42.9 | 26.8 | 8929.80 |
| 26 | Delphinidin | 29.1 | 8.44 | 18.38 |
| 27 | Peonidin-acetyl-3,5-diglucoside | 41.9 | 31.1 | Infinity |
| 28 | Pelargonidin-3-(6″-malonylglucoside) | 39.0 | 16.7 | 56,924.90 |
| 29 | Cyaniding-5-O-β-D-glucoside | 35.8 | 1.91 | 2115.68 |
| 30 | Isorhamnetin-3-rutinoside-4′-rhamnoside | 39.9 | 16.1 | Infinity |
| 31 | Chicoric acid a | 42.4 | 31.8 | 604,942 |
| 32 | Tartaric acid a | 41.2 | 9.95 | 74.82 |
| 33 | Caftaric acid a | 51.7 | 64.7 | 29.28 |
| 34 | 2-O-p-Coumaroyltartronic acid | 52.3 | 69.8 | 24.90 |
| 35 | 1,4-dicaffeoylquinic acid a | 45.7 | 49.9 | 19.00 |
| 36 | 1,5-dicaffeoylquinic acid a | 37.2 | 4.21 | 6.47 |
| 37 | 6′-O-trans-caffeoyl-caryoptosidic acid | 41.1 | 22.5 | 11.54 |
| 38 | Ferulic acid-4-O-glucuronide | 49.9 | 54.2 | 2.97 |
| 39 | Isoferulic acid-3-O-glucuronide | 43.9 | 24.2 | 3.12 |
| 40 | 3-O-caffeoyl-1-O-methylquinic acid | 40.1 | 5.31 | 12.32 |
| 41 | 3-O-(E)-feruloylquinic acid a | 41.6 | 16.0 | 2.55 |
| 42 | 4-feruloyl-1,5-quinolactone | 37.8 | 2.97 | 6.52 |
| 43 | Lactucin a | 48.6 | 48.1 | 38.00 |
| 44 | 8-deoxylactucin a | 44.3 | 27.6 | 108.45 |
| 45 | Lactupicrin a | 42.2 | 30.5 | 8.06 |
| 46 | 11b,13-dihydrolactucopicrin a | 47.4 | 42.3 | 99.43 |
| 47 | Cichorioside B/F/G/H/I | 45.2 | 28.5 | 8.79 |
| 48 | Cichorioside B/F/G/H/I | 42.4 | 17.2 | 40.60 |
| 49 | Cichorioside B/F/G/H/I | 42.0 | 16.7 | 6.78 |
| 50 | Cichorioside J | 38.9 | 4.08 | 39.49 |
| 51 | Cichorioside K | 37.2 | 4.71 | 46.57 |
| 52 | Cichoriin | 45.0 | 31.9 | 12.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shen, S.; Zhao, Q.; Shen, X.; Zhuo, Q. Plant Metabolome Between Root and Aerial Parts of Cichorium intybus L. and Anti-Hyperuricemia Mechanisms Based on Cell Metabolomics. Metabolites 2025, 15, 727. https://doi.org/10.3390/metabo15110727
Wang J, Shen S, Zhao Q, Shen X, Zhuo Q. Plant Metabolome Between Root and Aerial Parts of Cichorium intybus L. and Anti-Hyperuricemia Mechanisms Based on Cell Metabolomics. Metabolites. 2025; 15(11):727. https://doi.org/10.3390/metabo15110727
Chicago/Turabian StyleWang, Jingbo, Shi Shen, Qi Zhao, Xin Shen, and Qin Zhuo. 2025. "Plant Metabolome Between Root and Aerial Parts of Cichorium intybus L. and Anti-Hyperuricemia Mechanisms Based on Cell Metabolomics" Metabolites 15, no. 11: 727. https://doi.org/10.3390/metabo15110727
APA StyleWang, J., Shen, S., Zhao, Q., Shen, X., & Zhuo, Q. (2025). Plant Metabolome Between Root and Aerial Parts of Cichorium intybus L. and Anti-Hyperuricemia Mechanisms Based on Cell Metabolomics. Metabolites, 15(11), 727. https://doi.org/10.3390/metabo15110727






