Shift in Metabolite Profiling and Mineral Composition of Edible Halophytes Cultivated Hydroponically Under Increasing Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Setting
2.2. Measurements
2.2.1. HPLC Analysis of Sugars and Organic Acids
2.2.2. HPLC Analysis of Carotenoids and Chlorophylls
2.2.3. Spectrophotometry-Based Protein Estimation
2.2.4. Spectrometry-Based Elemental Analysis
2.2.5. Biometric Measurements
2.3. Statistical Analysis
3. Results
3.1. Metabolites
3.2. Biomass and Mineral Elements
3.3. Correlation and Principal Component Analysis
4. Discussion
4.1. Differential Metabolic Responses of Halophytes to Increased Salinity
4.2. Ion Regulation and Biomass Allocation Strategies in Halophytes Under Increased Salinity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DW | Dry weight |
| FW | Fresh weight |
References
- Kim, J.; Yu, J.M.; Jang, W.; Lee, J.; Kim, H.; Kim, H.; Lee, J.E.; Ding, X.; Zhang, K.H.L.; Kwak, S.K.; et al. Enhancing Water Oxidation Catalysis by Controlling Metal Cation Distribution in Layered Double Hydroxides. Adv. Funct. Mater. 2024, 34, 2470010. [Google Scholar] [CrossRef]
- Naresh, R.; Jadav, S.K.; Singh, M.; Patel, A.; Singh, B.; Beese, S.; Pandey, S.K. Role of Hydroponics in Improving Water-Use Efficiency and Food Security. Int. J. Environ. Clim. Change 2024, 14, 608–633. [Google Scholar] [CrossRef]
- Banboukian, A.; Chen, Y.; Thomas, V.M. The Challenges of Controlled Environment Hydroponic Farming: A Life Cycle Assessment of Lettuce. Int. J. Life Cycle Assess. 2025, 30, 1691–1704. [Google Scholar] [CrossRef]
- Sousa, R.D.; Bragança, L.; Da Silva, M.V.; Oliveira, R.S. Challenges and Solutions for Sustainable Food Systems: The Potential of Home Hydroponics. Sustainability 2024, 16, 817. [Google Scholar] [CrossRef]
- Bwambale, E.; Abagale, F.K.; Anornu, G.K. Smart Irrigation Monitoring and Control Strategies for Improving Water Use Efficiency in Precision Agriculture: A Review. Agric. Water Manag. 2022, 260, 107324. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Zhang, Y.; Tong, Y. Effects of Nutrient Solution Recycling on Water and Nutrient Consumption Patterns and Lettuce Growth. Sci. Hortic. 2025, 341, 113976. [Google Scholar] [CrossRef]
- Nazir, M.; Roy, K.; Saha, A.; Saha, D. A Sustainable Holistic Approach of Hydroponic Farming for Reclaiming, and Rehabilitating Wastewater: A Review. Water Air Soil Pollut. 2024, 235, 445. [Google Scholar] [CrossRef]
- Gruda, N.S.; Dong, J.; Li, X. From Salinity to Nutrient-Rich Vegetables: Strategies for Quality Enhancement in Protected Cultivation. Crit. Rev. Plant Sci. 2024, 43, 327–347. [Google Scholar] [CrossRef]
- Martins, T.S.; Da-Silva, C.J.; Shabala, S.; Striker, G.G.; Carvalho, I.R.; De Oliveira, A.C.B.; Do Amarante, L. Understanding Plant Responses to Saline Waterlogging: Insights from Halophytes and Implications for Crop Tolerance. Planta 2024, 259, 24. [Google Scholar] [CrossRef]
- Bisht, D.; Mishra, S.; Bihani, S.C.; Seth, T.; Srivastava, A.K.; Pandey, G.K. Salt Stress Tolerance and Calcium Signalling Components: Where We Stand and How Far We Can Go? J. Plant Growth Regul. 2025, 44, 1429–1447. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Antinutrients in Halophyte-Based Crops. Front. Biosci. Landmark Ed. 2024, 29, 323. [Google Scholar] [CrossRef]
- Khare, T.; Jamla, M.; Mathur, V.; Kumar, V. Exploring Halobiome Resources for Developing Salt-Tolerant Crops: A Perspective Review. J. Plant Growth Regul. 2024, 43, 2137–2164. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.; Rasheed, A.; Ahmed, M.Z.; Abbas, S. Exploring the Potentials of Halophytes in Addressing Climate Change-Related Issues: A Synthesis of Their Biological, Environmental, and Socioeconomic Aspects. World 2024, 5, 36–57. [Google Scholar] [CrossRef]
- Jang, J.E.; Jeong, H.J.; Seol, J.; Kang, E.S.; Lee, S.W.; Kim, H.B.; Son, D.C. Plantago coronopus (Plantaginaceae), a New Invasive Alien Plant in the Republic of Korea. Korean J. Pl. Taxon 2025, 55, 29–35. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. The Medicinal Potential of Plants from the Genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Custódio, L.; Rodrigues, M.J.; Neng, N.R.; Nogueira, J.M.F.; Carlier, J.; Costa, M.C.; Varela, J.; Barreira, L. Profiling of Antioxidant Potential and Phytoconstituents of Plantago coronopus. Braz. J. Biol. 2016, 77, 632–641. [Google Scholar] [CrossRef]
- He, J.; You, X.; Qin, L. High Salinity Reduces Plant Growth and Photosynthetic Performance but Enhances Certain Nutritional Quality of C4 Halophyte Portulaca Oleracea L. Grown Hydroponically Under LED Lighting. Front. Plant Sci. 2021, 12, 651341. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; AlJuhaimi, F.; Özcan, M.M.; Uslu, N.; Karrar, E. Determination of the Distribution of Bioactive Compounds, Antioxidant Activities, Polyphenols and Macro and Microelement Contents in Different Parts of Wild and Cultivated Purslane (Portulaca oleracea L.) Plants. Food Meas. 2025, 19, 3714–3724. [Google Scholar] [CrossRef]
- Al Abide, N.M. Comparative Anatomical Study of Some Species of Genus Salsola L. (Chenopodiaceae) in Iraq. djps 2018, 14, 24–33. [Google Scholar] [CrossRef]
- Murshid, S.S.A.; Atoum, D.; Abou-Hussein, D.R.; Abdallah, H.M.; Hareeri, R.H.; Almukadi, H.; Edrada-Ebel, R. Genus Salsola: Chemistry, Biological Activities and Future Prospective—A Review. Plants 2022, 11, 714. [Google Scholar] [CrossRef]
- Levengood, H.; Smith, L.; Gillis, S.; Zhou, Y.; Zhang, C. Plantago Species Are Emerging Model Organisms for Functional Genomics and Stress Biology. Plant Cell Rep. 2025, 44, 142. [Google Scholar] [CrossRef] [PubMed]
- Fekete, R.; Haszonits, G.; Schmidt, D.; Bak, H.; Vincze, O.; Süveges, K.; Molnár, V.A. Rapid Continental Spread of a Salt-Tolerant Plant along the European Road Network. Biol. Invasions 2021, 23, 2661–2674. [Google Scholar] [CrossRef]
- Iqbal, U.; Arif, M.S.; Sharif, M.; Wahab, A.; Ahmad, M.; Yousuf, M.; Rafiq, S.; Abid, S. Phytoremediation Capacity of Saltwort (Salsola imbricata Forssk.) Determined by Tissue Organization and Physio-Biochemical Traits under Arid Saline Environments. Water Air Soil Pollut. 2025, 236, 203. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, R.K.; Thushar, S. Proximate Composition and Nutritional Values of Selected Wild Plants of the United Arab Emirates. Molecules 2023, 28, 1504. [Google Scholar] [CrossRef]
- Margaryan, G.; Singh, A.; Khachatryan, H.; Rajput, V.D.; Minkina, T.; Petropoulos, D.; Kriemadis, A.; Alexiou, A.; Elshikh, M.S.; Mustafa, A.E.-Z.M.A.; et al. Unveiling the Salinity Tolerance Potential of Armenian Dandur (Portulaca oleracea L.) Genotypes: Enhancing Sustainable Agriculture and Food Security. J. King Saud Univ.-Sci. 2024, 36, 103332. [Google Scholar] [CrossRef]
- Kudirka, G.; Viršilė, A.; Laužikė, K.; Sutulienė, R.; Samuolienė, G. Photosynthetic Photon Flux Density Effects on Portulaca Olearacea in Controlled-Environment Agriculture. Plants 2023, 12, 3622. [Google Scholar] [CrossRef]
- Shahid, S.A.; Alkandari, A.J. Halophytic Crops as a Solution for Food Security, Land Rehabilitation, and Mitigating Future Water Crises by Utilizing Marginal Quality Waters. In Halophytes vis-à-vis Saline Agriculture; Dagar, J.C., Gupta, S.R., Kumar, A., Eds.; Springer Nature: Singapore, 2024; pp. 441–478. ISBN 9789819731565. [Google Scholar]
- Quiroz, I.V.; Sánchez Oropeza, D.V.; Trujillo Lira, M.F.; López Pérez, M.A.; García, C.R.; Maya, S.R. Conservation and Reuse of Water in Agriculture: Biotechnological Techniques for Efficient Use. In Soil Improvement and Water Conservation Biotechnology; Quiroz, I.V., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2025; pp. 297–321. ISBN 9789815322439. [Google Scholar]
- Alghamdi, S. Drought and Salinity Effects on Plant Growth: A Comprehensive Review. SABRAO J. Breed. Genet. 2024, 56, 2231–2340. [Google Scholar] [CrossRef]
- Ikram, M.; Batool, M.; Ullah, M.; Khalid, B.; El-Badri, A.M.; Mohamed, I.A.A.; Zhang, L.; Kuai, J.; Xu, Z.; Zhao, J.; et al. Molecular Alchemy: Converting Stress into Resilience via Secondary Metabolites and Calcium Signaling in Rice. Rice 2025, 18, 32. [Google Scholar] [CrossRef]
- Goura, K.; Legrifi, I.; Kallali, N.S.; Taoussi, M.; Kenfaoui, J.; Meddich, A.; Esmaeel, Q.; Ait Barka, E.; Lahlali, R. Beyond Survival: The Role of Secondary Metabolites in Plant Defense Mechanisms. J. Crop Health 2025, 77, 121. [Google Scholar] [CrossRef]
- Samuolienė, G.; Pukalskas, A.; Gudžinskaitė, I.; Viršilė, A. The Harnessing of Controlled Environment Agriculture Technologies for Phytochemical and Mineral Element Enrichment in Mesembryanthemum crystallinum. Horticulturae 2025, 11, 229. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of Organic Acids in Fruits and Vegetables by Liquid Chromatography with Tandem-Mass Spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Sander, L.C.; Rimmer, C.A.; Wilson, W.B. Characterization of Triacontyl (C-30) Liquid Chromatographic Columns. J. Chromatogr. A 2020, 1614, 460732. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Araújo, G.C.L.; Gonzalez, M.H.; Ferreira, A.G.; Nogueira, A.R.A.; Nóbrega, J.A. Effect of Acid Concentration on Closed-Vessel Microwave-Assisted Digestion of Plant Materials. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 2121–2132. [Google Scholar] [CrossRef]
- Barbosa, J.T.P.; Santos, C.M.M.; Peralva, V.N.; Flores, E.M.M.; Korn, M.; Nóbrega, J.A.; Korn, M.G.A. Microwave-Assisted Diluted Acid Digestion for Trace Elements Analysis of Edible Soybean Products. Food Chem. 2015, 175, 212–217. [Google Scholar] [CrossRef]
- Mendis, C.L.; Padmathilake, R.E.; Attanayake, R.N.; Perera, D. Learning from Salicornia: Physiological, Biochemical, and Molecular Mechanisms of Salinity Tolerance. Int. J. Mol. Sci. 2025, 26, 5936. [Google Scholar] [CrossRef]
- Hurrah, I.M.; Mohiuddin, T.; Mandal, S.; Kumar, V.; Gupta, A. Crosstalk and Interaction among Salt Stress Tolerance Pathways. In Exogenous Priming and Engineering of Plant Metabolic and Regulatory Genes; Elsevier: Amsterdam, The Netherlands, 2025; pp. 513–529. ISBN 978-0-443-13490-6. [Google Scholar]
- Iqbal, S.; Baloch, H.; Hafeez, M.B.; Zahra, N.; Fatima, E.M.; Raza, A.; Raza, S.; Saddiq, M.S. Salt Tolerance in Quinoa Genotypes: Ion-Specific Adaptations and Growth Performance under Hydroponic Conditions. Agrociencia Urug. 2025, 29, e1538. [Google Scholar] [CrossRef]
- Puccinelli, M.; Marchioni, I.; Botrini, L.; Carmassi, G.; Pardossi, A.; Pistelli, L. Growing Salicornia europaea L. with Saline Hydroponic or Aquaculture Wastewater. Horticulturae 2024, 10, 196. [Google Scholar] [CrossRef]
- Gil, R.; Lull, C.; Boscaiu, M.; Bautista, I.; Lidón, A.; Vicente, O. Soluble Carbohydrates as Osmolytes in Several Halophytes from a Mediterranean Salt Marsh. Not. Bot. Horti Agrobot. Cluj 2011, 39, 09. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, Metabolomics, and Ionomics Perspectives of Salinity Tolerance in Halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gao, Z.; Li, L.; Li, C.; Yan, H.; Xiao, B.; Ma, Y.; Wang, H.; Yang, C.; Xun, H. Adaptive Strategy of the Perennial Halophyte Grass Puccinellia tenuiflora to Long-Term Salinity Stress. Plants 2024, 13, 3445. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Agarwal, P.; Chittora, A.; Bhawsar, A.; Thomas, T. Aeluropus Lagopoides: An Important Halophyte with Key Physiological and Molecular Mechanisms for Salinity Tolerance and a Unique Genetic Resource for Developing Climate Resilient Crops. J. Plant Res. 2025, 138, 535–554. [Google Scholar] [CrossRef]
- Sharma, A.; Taybi, T. Differences in the Temporal Kinetics of the Metabolic Responses to Salinity Between the Salt-Tolerant Thellungiella salsuginea and the Salt-Sensitive Arabidopsis thaliana Reveal New Insights in Salt Tolerance Mechanisms. Int. J. Mol. Sci. 2025, 26, 5141. [Google Scholar] [CrossRef]
- Wang, P.; Lei, X.; Lü, J.; Gao, C. Overexpression of the ThTPS Gene Enhanced Salt and Osmotic Stress Tolerance in Tamarix Hispida. J. For. Res. 2022, 33, 299–308. [Google Scholar] [CrossRef]
- Cui, Y.-N.; Yan, S.-J.; Zhang, Y.-N.; Wang, R.; Song, L.-L.; Ma, Y.; Guo, H.; Yang, P.-Z. Physiological, Metabolome and Gene Expression Analyses Reveal the Accumulation and Biosynthesis Pathways of Soluble Sugars and Amino Acids in Sweet Sorghum under Osmotic Stresses. Int. J. Mol. Sci. 2024, 25, 8942. [Google Scholar] [CrossRef]
- Athar, H.-R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt Stress Proteins in Plants: An Overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Dissanayake, B.M.; Staudinger, C.; Munns, R.; Taylor, N.L.; Millar, A.H. Distinct Salinity-Induced Changes in Wheat Metabolic Machinery in Different Root Tissue Types. J. Proteom. 2022, 256, 104502. [Google Scholar] [CrossRef]
- Wan, Q.; Hongbo, S.; Zhaolong, X.; Jia, L.; Dayong, Z.; Yihong, H. Salinity Tolerance Mechanism of Osmotin and Osmotin-like Proteins: A Promising Candidate for Enhancing Plant Salt Tolerance. Curr. Genom. 2017, 18, 553–556. [Google Scholar] [CrossRef]
- Fitzner, M.; Schreiner, M.; Baldermann, S. The Interaction of Salinity and Light Regime Modulates Photosynthetic Pigment Content in Edible Halophytes in Greenhouse and Indoor Farming. Front. Plant Sci. 2023, 14, 1105162. [Google Scholar] [CrossRef]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as New Model Plant Species for Salt Tolerance Strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Lee, G.; Carrow, R.N.; Duncan, R.R.; Eiteman, M.A.; Rieger, M.W. Synthesis of Organic Osmolytes and Salt Tolerance Mechanisms in Paspalum vaginatum. Environ. Exp. Bot. 2008, 63, 19–27. [Google Scholar] [CrossRef]
- Zhou, B.N.; Mao, L.; Hua, Z.Z.; Lu, J.G. Effects on photochemical fluorescence properties under salt-alkaline stresses about Sinocalycanthus chinensis. Acta Agric. Zhejiangensis 2021, 33, 1416–1425. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Oyedeji, S.; Joseph, G.G.; Adedibu, P.A.; Krishnamurthy, R. Sodium chloride stress induced differential growth, biomass yield, and phytochemical composition responses in the halophytic grass Aeluropus lagopoides (L.). West Afr. J. Appl. Ecol. 2020, 28, 31–40. [Google Scholar]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.A.; Babar, M.A. Role of Sugars, Amino Acids and Organic Acids in Improving Plant Abiotic Stress Tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
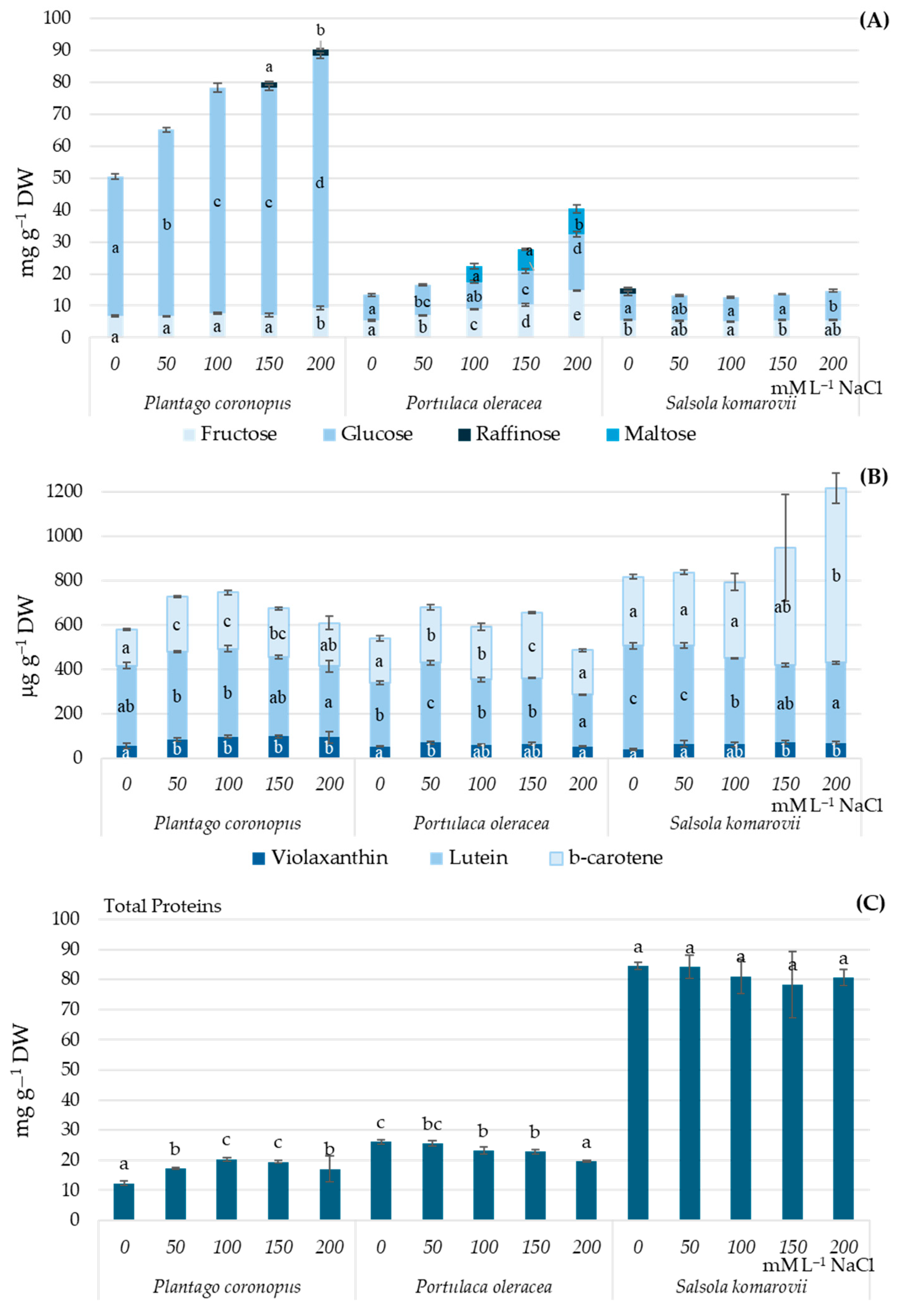

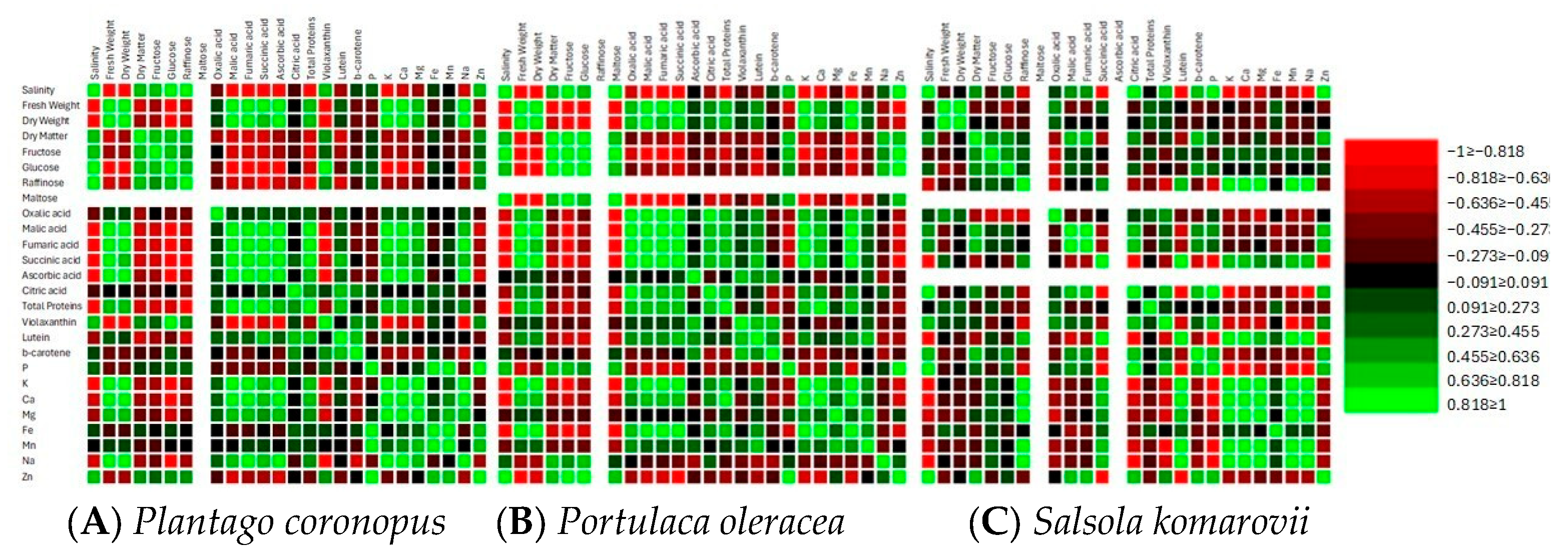
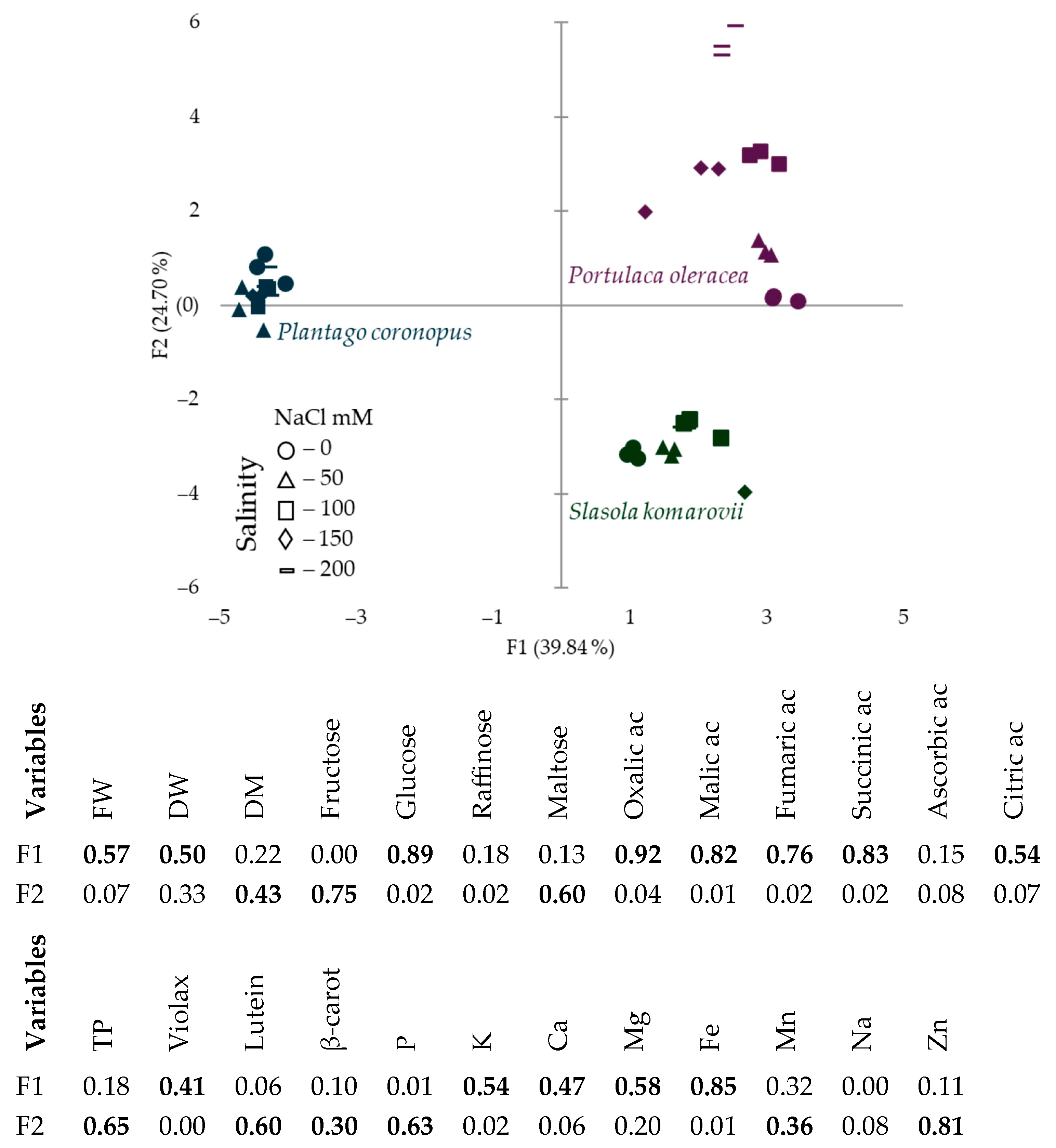
| NaCl mM·L−1 | Oxalic Acid | Malic Acid | Fumaric Acid | Succinic Acid | Ascorbic Acid | Citric Acid |
|---|---|---|---|---|---|---|
| Plantago coronopus | ||||||
| 0 | 0.029a | 44.5d | 52.6d | 1.22c | 0.361d | 3.82a |
| 50 | 0.025a | 38.3c | 41.4c | 1.17bc | 0.223c | 3.95a |
| 100 | 0.029a | 30.0b | 29.8b | 1.13b | 0.054b | 4.42b |
| 150 | 0.025a | 28.8b | 28.6b | 0.96a | 0.000a | 3.85a |
| 200 | 0.026a | 23.6a | 22.1a | 0.91a | 0.000a | 3.66a |
| Portulaca oleracea | ||||||
| 0 | 19.0b | 10.8b | 10.9b | 0.625c | 0.000a | 8.71b |
| 50 | 21.4b | 10.2b | 10.0b | 0.612c | 0.124b | 8.34b |
| 100 | 15.1a | 5.51a | 5.75a | 0.523b | 0.132b | 6.45ab |
| 150 | 12.8a | 4.88a | 5.36a | 0.487ab | 0.092ab | 5.71a |
| 200 | 14.4a | 4.08a | 4.50a | 0.425a | 0.000a | 7.11ab |
| Salsola komarovii | ||||||
| 0 | 14.7a | 4.91a | 4.36a | 0.502c | n.d | 3.89a |
| 50 | 18.5c | 3.91ab | 3.82ab | 0.493c | n.d | 4.32a |
| 100 | 19.4c | 4.80ab | 4.19ab | 0.462b | n.d | 5.51b |
| 150 | 17.9bc | 5.86b | 4.66b | 0.416a | n.d | 5.96bc |
| 200 | 15.8ab | 5.86b | 4.55b | 0.408a | n.d | 6.83c |
| NaCl | 0 | 50 | 100 | 150 | 200 | 0 | 50 | 100 | 150 | 200 | 0 | 50 | 100 | 150 | 200 | Macro elements | ||
| P | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | 0.52 | 0.76 | 1.3 | 0.8 | 1.31 | 0.4 | 0.53 | 0.6 | 0.7 | 0.8 | |||
| K | 35 | 25 | 20 | 20 | 19 | 52.4 | 47.9 | 38 | 30 | 29.6 | 41.9 | 37.8 | 35 | 35 | 35 | |||
| Ca | 22.3 | 13 | 11 | 11 | 8.2 | 5.67 | 3.79 | 3.4 | 2.4 | 2.67 | 15 | 8.49 | 5.7 | 4.7 | 5 | |||
| Mg | 3.45 | 2.1 | 2.2 | 2.2 | 1.9 | 4.89 | 5.1 | 5.4 | 4.4 | 4.85 | 3.93 | 3.26 | 3.1 | 3.1 | 3.3 | |||
| Fe | 162 | 167 | 194 | 185 | 169 | 56.1 | 47.6 | 36 | 33 | 24.3 | 35.5 | 51.3 | 29 | 33 | 26 | Micro elements | ||
| Mn | 115 | 99 | 112 | 120 | 101 | 208 | 274 | 237 | 188 | 196 | 136 | 111 | 106 | 100 | 107 | |||
| Na | 5172 | 689 | 665 | 116 | 103 | 1659 | 1471 | 131 | 804 | 3875 | 1961 | 1112 | 679 | 670 | 551 | |||
| Zn | 37.1 | 35 | 46 | 53 | 51 | 49.4 | 101 | 148 | 107 | 216 | 21.9 | 20.5 | 23 | 26 | 26 | |||
| Plantago coronopus | Portulaca oleracea | Salsola komarovii | ||||||||||||||||
| ANOVA between salinity | a | ab | b | bc | c | d | e | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuolienė, G.; Pukalskas, A.; Viršilė, A. Shift in Metabolite Profiling and Mineral Composition of Edible Halophytes Cultivated Hydroponically Under Increasing Salinity. Metabolites 2025, 15, 724. https://doi.org/10.3390/metabo15110724
Samuolienė G, Pukalskas A, Viršilė A. Shift in Metabolite Profiling and Mineral Composition of Edible Halophytes Cultivated Hydroponically Under Increasing Salinity. Metabolites. 2025; 15(11):724. https://doi.org/10.3390/metabo15110724
Chicago/Turabian StyleSamuolienė, Giedrė, Audrius Pukalskas, and Akvilė Viršilė. 2025. "Shift in Metabolite Profiling and Mineral Composition of Edible Halophytes Cultivated Hydroponically Under Increasing Salinity" Metabolites 15, no. 11: 724. https://doi.org/10.3390/metabo15110724
APA StyleSamuolienė, G., Pukalskas, A., & Viršilė, A. (2025). Shift in Metabolite Profiling and Mineral Composition of Edible Halophytes Cultivated Hydroponically Under Increasing Salinity. Metabolites, 15(11), 724. https://doi.org/10.3390/metabo15110724








