Geographical Variation Shapes Nutritional Metabolite Profile and Food Functionality of Houttuynia cordata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Analysis of LC-MS/MS Platform
2.2.1. Sample Preparation and Extraction
2.2.2. UPLC Conditions
2.2.3. ESI-Q TRAP-MS/MS
2.2.4. Selection of Differential Metabolites with KEGG Annotation and Enrichment Analysis
2.3. RNA-Sequencing
2.3.1. RNA Extraction and Detection
2.3.2. mRNA Library Construction
2.3.3. Sequencing Run
2.3.4. Gene Function Annotation and Differential Analysis
2.4. Pharmacological Efficiency Analysis of Metabolites
2.4.1. Prediction of Targets
2.4.2. Analysis of Protein–Protein Interaction Network
2.4.3. GO and KEGG Enrichment Analysis
2.4.4. Network of Efficacious Metabolites–Target–Pathway–Disease Interactions
2.5. Molecular Docking Simulation
2.6. Data Statistics
3. Results
3.1. General Overview of Metabolites
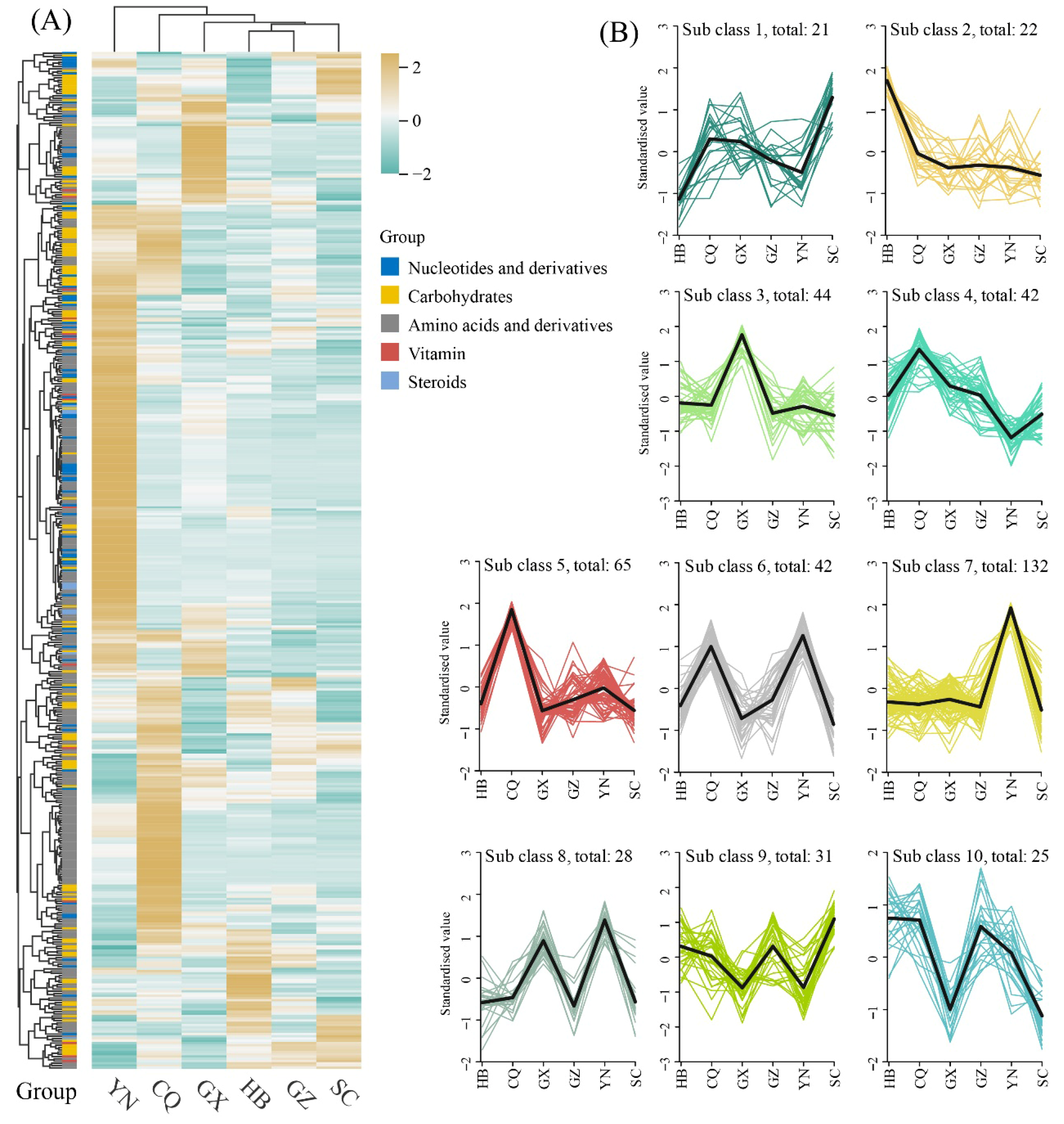
3.2. Global Profiling of Differential Metabolites
3.3. Comparative Analysis of DAMs Across Different Comparison Groups
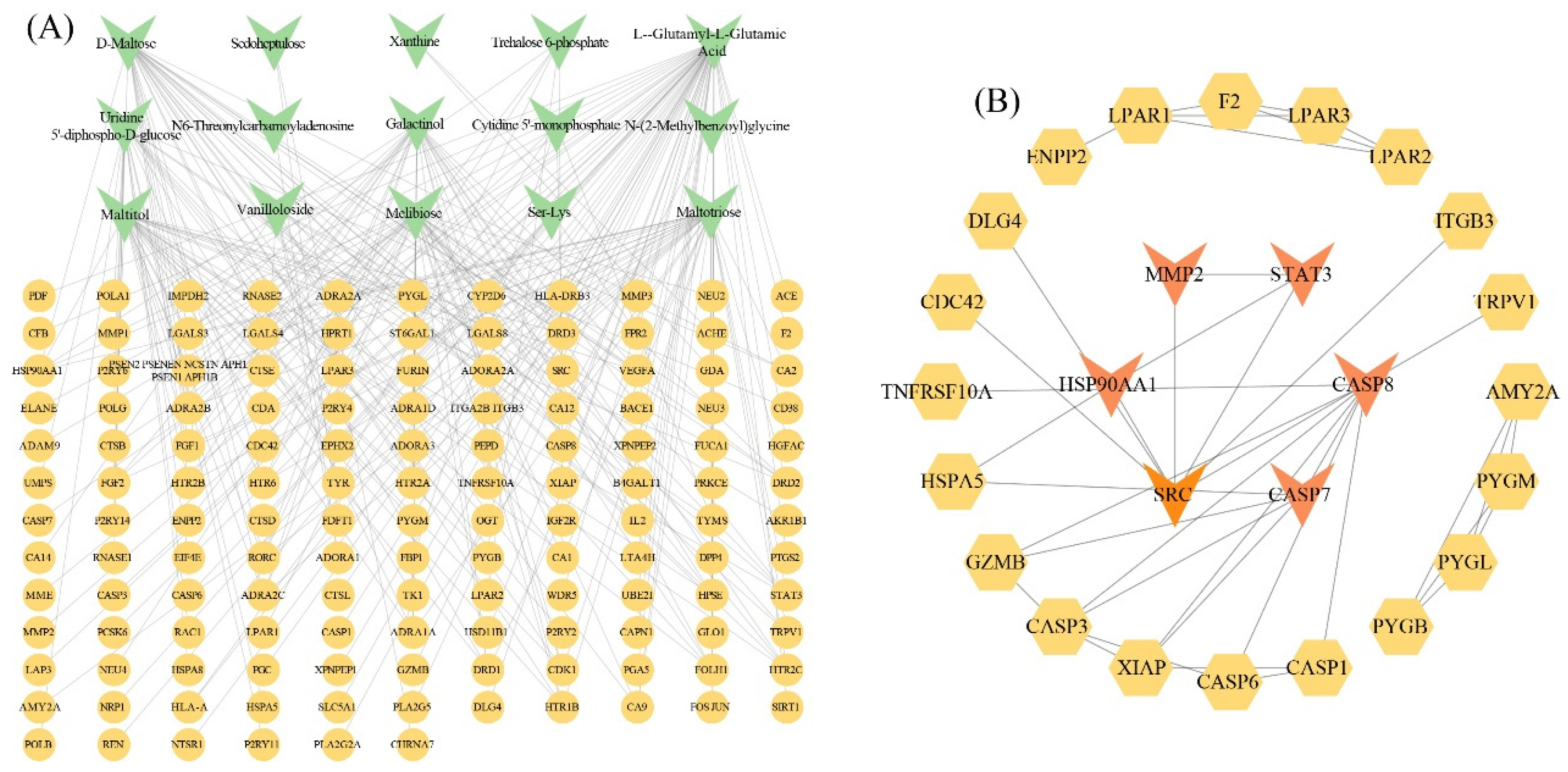
3.4. Analysis of Putative Targets for Metabolites
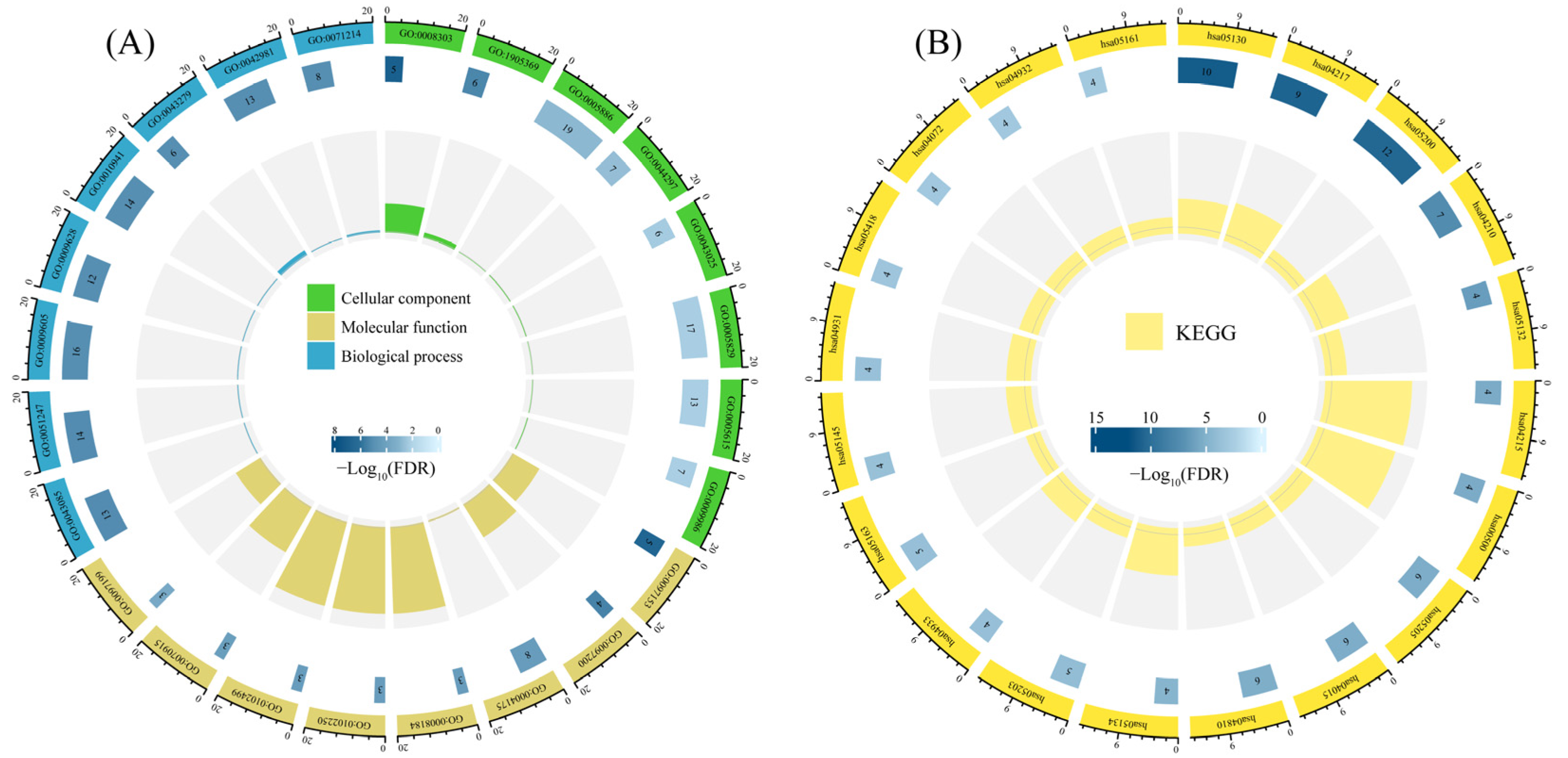
3.5. Enrichment Analysis of Candidate Targets
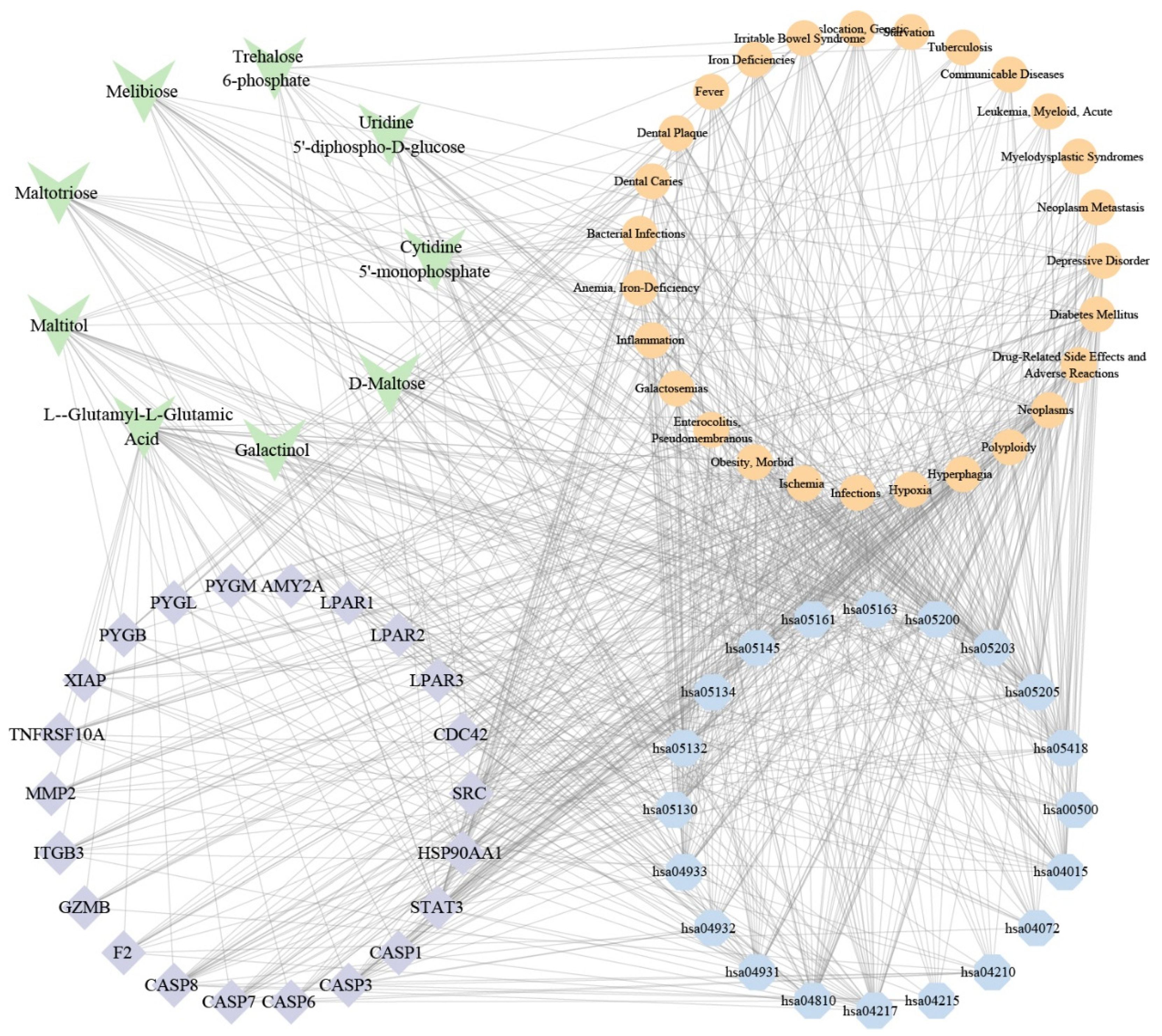
3.6. Combined Analysis
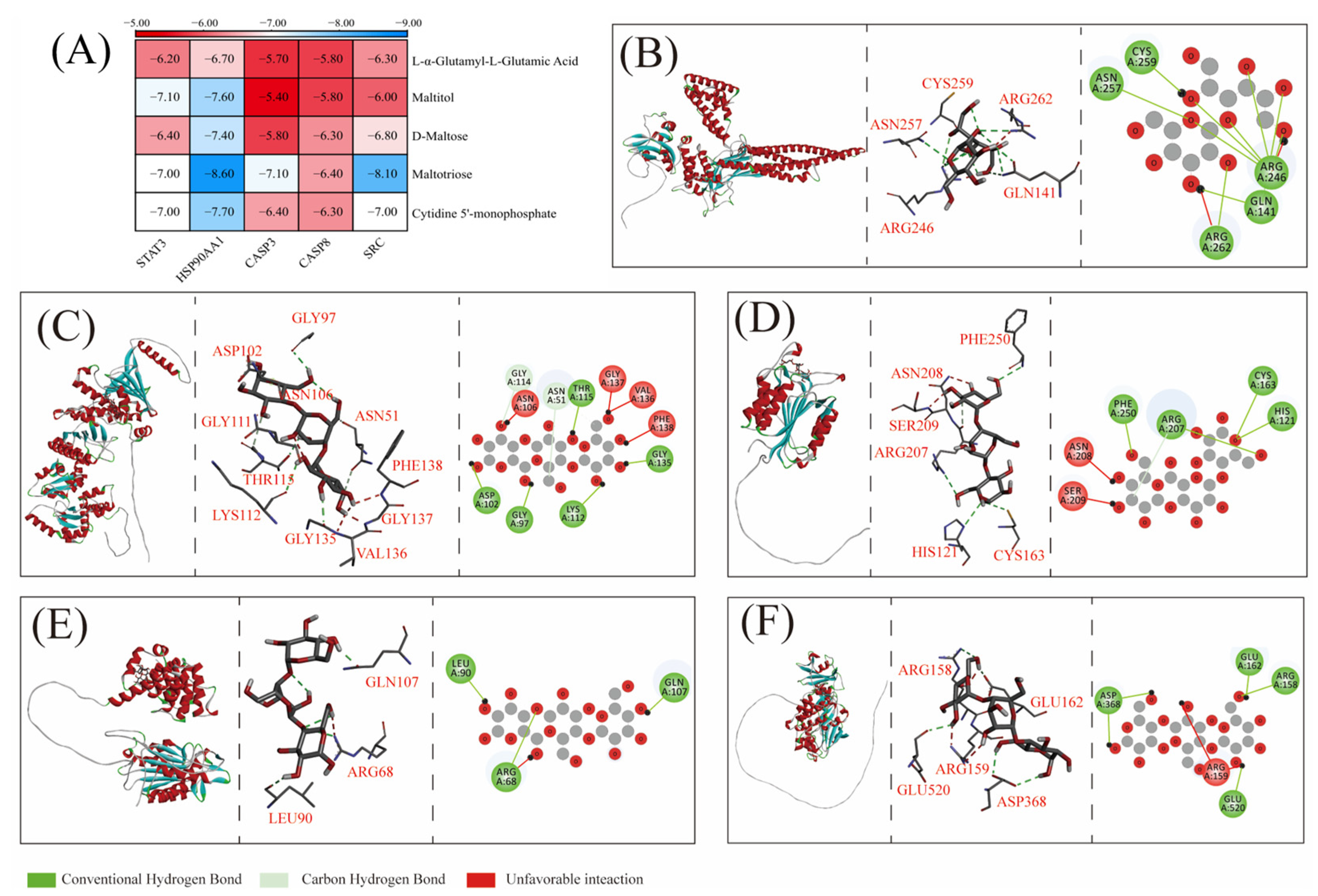
3.7. Molecular Docking
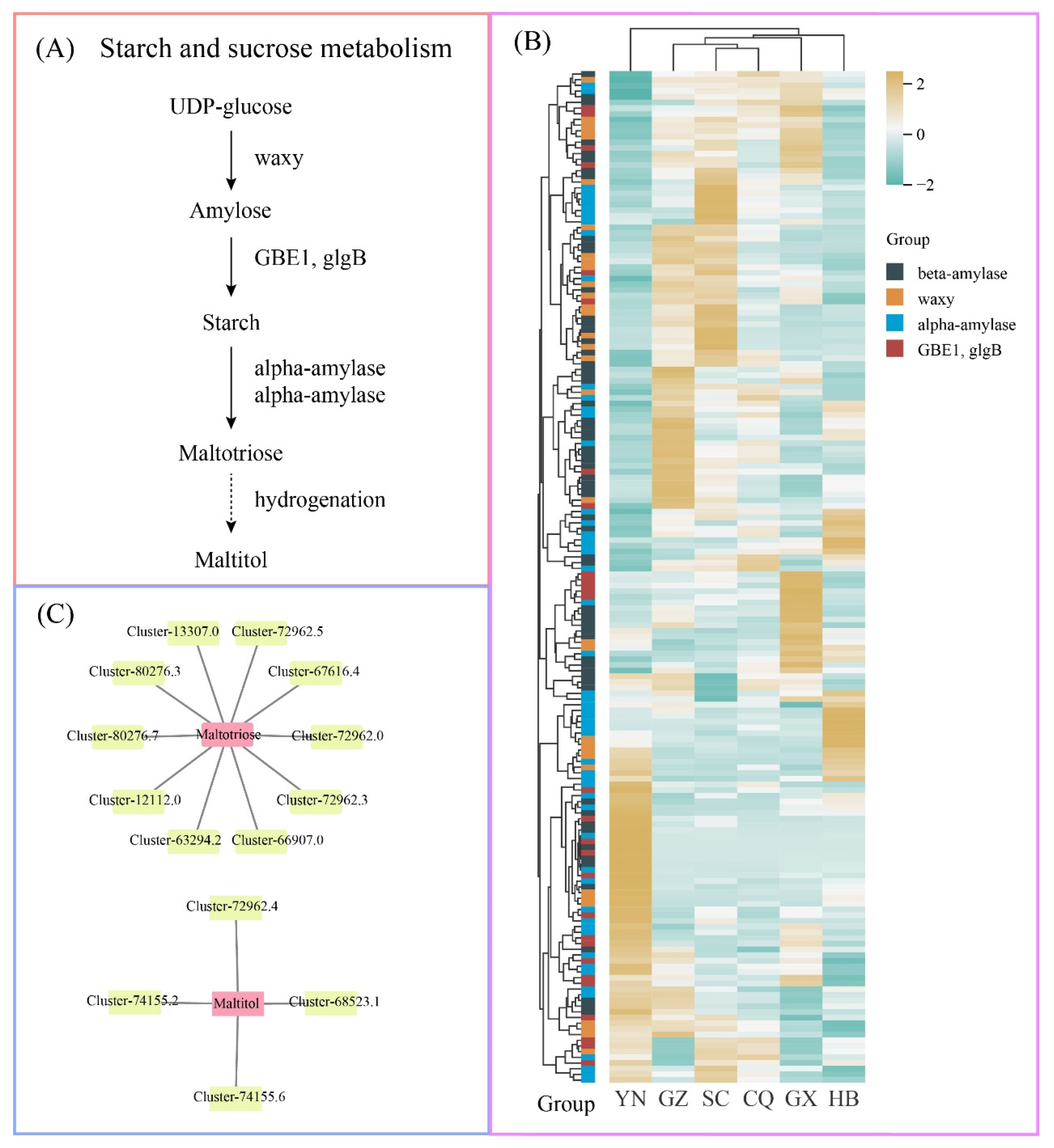
3.8. Metabolic Pathway Analysis of Key Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, S.; Zha, L.; Peng, Y.; Luo, W.; Chen, K.; Li, X.; Huang, D.; Yin, D. Quality and metabolomics analysis of Houttuynia cordata based on HS-SPME/GC-MS. Molecules 2022, 27, 3921. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Jiang, X.; Du, B.; Wang, Q.; Ma, Y.; Liu, M.; Mao, Y.; Yang, J.; Li, F.; et al. Uncovering nutritional metabolites and candidate genes involved in flavonoid metabolism in Houttuynia cordata through combined metabolomic and transcriptomic analyses. Plant Physiol. Biochem. 2023, 203, 108059. [Google Scholar] [CrossRef]
- Jin, L.; Yang, J.; Liu, C.; He, M.; Yan, H. Complete plastome of Houttuynia cordata (Saururaceae), a medicinal and edible plant. Mitochondrial DNA Part B Resour. 2019, 4, 3208–3209. [Google Scholar] [CrossRef]
- Choeisoongnern, T.; Chaiyasut, C.; Sivamaruthi, B.S.; Makhamrueang, N.; Peerajan, S.; Sirilun, S.; Sittiprapaporn, P. Bacteriocin-Producing enterococcus faecium OV3-6 as a Bio-Preservative agent to produce fermented Houttuynia cordata Thunb. Beverages: A preliminary study. Foods 2023, 12, 3520. [Google Scholar] [CrossRef]
- Laldinsangi, C. The therapeutic potential of Houttuynia cordata: A current review. Heliyon 2022, 8, e10386. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia cordata Thunb: An ethnopharmacological review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, Q.; Yang, J.; Du, B.; Yuan, Z.; Liu, H.; Yuan, J.; Zhang, Y.; Chen, L.; Liu, L. Deep integration of metabolome and transcriptome characterizes alkaloid metabolism in Houttuynia cordata. Genomics 2024, 116, 110881. [Google Scholar] [CrossRef] [PubMed]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Houdková, M.; Ventura, K.; Bajerová, P. Chemical composition and determination of the antibacterial activity of essential oils in liquid and vapor phases extracted from two different southeast ssian Herbs-Houttuynia cordata (Saururaceae) and Persicaria odorata (Polygonaceae). Molecules 2020, 25, 2432. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Chen, Y.; Liu, Q.; Zhou, S.; Li, N.; Wu, Y.; Yuan, J. Houttuynia cordata thunb. alleviates inflammatory bowel disease by modulating intestinal microenvironment: A research review. Front. Immunol. 2023, 14, 1306375. [Google Scholar] [CrossRef]
- Rafiq, S.; Hao, H.; Ijaz, M.; Raza, A. Pharmacological effects of Houttuynia cordata Thunb (H. cordata): A comprehensive review. Pharmaceuticals 2022, 15, 1079. [Google Scholar] [CrossRef]
- Wang, J.H.; Bose, S.; Shin, N.R.; Chin, Y.W.; Choi, Y.H.; Kim, H. Pharmaceutical impact of Houttuynia cordata and metformin combination on High-Fat-Diet-Induced metabolic disorders: Link to intestinal microbiota and metabolic endotoxemia. Front. Endocrinol. 2018, 9, 620. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, B.; Parihar, N.; Ilaweibaphyrnai, M.; Panda, S.R.; Alexander, A.; Chella, N.; Murty, U.; Naidu, V.; Kumar, G.J.; et al. Nutraceutical prospects of Houttuynia cordata against the infectious viruses. Food Biosci. 2022, 50, 101977. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ji, P.; Li, C.; Wu, F.; Hua, Y.; Wei, Y.; Cao, Y. Research progress on the chemical constituents and pharmacological effects of Houttuynia cordata Thunb and a predictive analysis of quality markers. Curr. Issues Mol. Biol. 2024, 47, 18. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Orozco, A.; Carranza-Becerra, L.; Delgadillo-Ruiz, L.; Bollaín y Goytia, J.J.; Gaytán-Saldaña, N.A.; Mandujano-García, C.D.; Delgadillo-Ruiz, E.; Michel-López, C.Y.; Huerta-García, J.; Valladares-Carranza, B.; et al. Environmental heterogeneity drives secondary metabolite diversity from mesquite pods in semiarid regions. Ecologies 2025, 6, 19. [Google Scholar] [CrossRef]
- Xiong, Q.; Li, L. Cultivation techniques and management of Houttuynia cordata L. in Guizhou area. J. Chang. Veg. 2017, 8, 77–82. [Google Scholar]
- Luo, Q.; Meng, P.H.; Jiang, D.W.; Han, Z.M.; Wang, Z.H.; Tan, G.F.; Zhang, J. Comprehensive assessment of Houttuynia cordata Thunb.; an important medicinal plant and vegetable. Agronomy 2022, 12, 2582. [Google Scholar] [CrossRef]
- Wei, L.; Wu, X.J. Genetic variation and population differentiation in a medical herb Houttuynia cordata in China revealed by Inter-Simple Sequence Repeats (ISSRs). Int. J. Mol. Sci. 2012, 13, 8159–8170. [Google Scholar] [CrossRef]
- Liu, L.; Guan, L.L.; Zhao, H.X.; Huang, Y.; Mou, Q.Y.; Liu, K.; Chen, T.T.; Wang, X.Y.; Zhang, Y.; Wei, B.; et al. Modeling habitat suitability of Houttuynia cordata Thunb (Ceercao) using Maxent under climate change in China. Ecol. Inform. 2021, 63, 101324. [Google Scholar] [CrossRef]
- Fang, H.; Yang, Z.; Yang, L. Protective effect of Houttuynia cordata extract on propofol-induced injury of rat hippocampal neurons by regulating PI3K/Akt and Toll-like receptor 4/NF-κB signaling pathway. Neuroreport 2021, 32, 577–582. [Google Scholar] [CrossRef]
- Hou, S.; Huang, P.; Yao, Z. Ethnobotany study on wild edible plants used by the Tujia ethnic group in Laifeng, southwest Hubei, China. J. Ethnobiol. Ethnomed. 2024, 20, 94. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, C.; Fisher, D.; Hien, N.T.T.; Musabaev, E.; Pronyuk, K.; Xia, Y.; Zhu, Z.; Wang, Y.; Dang, Y.; et al. Antiviral treatment for viral pneumonia: Current drugs and natural compounds. Virol. J. 2025, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Kirbis, B.S.; Mula, Z.; Zhang, W.; Kuang, Y.; Huang, Q.; Yin, L. Ethnobotanical study on medicinal plants used by Bulang people in Yunnan, China. J. Ethnobiol. Ethnomed. 2023, 19, 38. [Google Scholar] [CrossRef]
- Ding, F.; Yu, Y.; Zhang, Y.; Wei, S.; Han, J.; Li, Z.; Jiang, H.; Ryu, D.; Park, W.; Ha, K.; et al. Harnessing nutrients and natural products for sustainable drug development against aging. Front. Pharmacol. 2025, 16, 1579266. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia, T.; Rea, G.; Antonacci, A.; Giardi, M.T. Healthy and adverse effects of plant-derived functional metabolites: The need of revealing their content and bioactivity in a complex food matrix. Crit. Rev. Food Sci. Nutr. 2013, 53, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.; Tao, L.; Wei, X.; Gao, L.; Gao, Y.; Suo, J.; Yu, W.; Hu, Y.; Yang, B.; et al. Ethylene treatment promotes umami taste-active amino acids accumulation of Torreya grandis nuts post-harvest by comparative chemical and transcript analyses. Food Chem. 2023, 408, 135214. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; He, Z.; Du, R. Plant-Derived as alternatives to Animal-Derived bioactive peptides: A review of the preparation, bioactivities, Structure-Activity relationships, and applications in chronic diseases. Nutrients 2024, 16, 3277. [Google Scholar] [CrossRef]
- Haferkamp, I.; Fernie, A.R.; Neuhaus, H.E. Adenine nucleotide transport in plants: Much more than a mitochondrial issue. Trends Plant Sci. 2011, 16, 507–515. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Zrig, A.; Alsherif, E.A.; Aloufi, A.S.; Korany, S.M.; Selim, S.; Almuhayawi, M.S.; Tarabulsi, M.K.; Nhs, M.; Albasri, H.M.; Bouqellah, N.A. The biomass and health-enhancing qualities of lettuce are amplified through the inoculation of arbuscular mycorrhizal fungi. BMC Plant Biol. 2025, 25, 521. [Google Scholar] [CrossRef]
- Kaur, S.; Seem, K.; Ali, A.; Jaiswal, S.; Gumachanamardi, P.; Kaur, G.; Singh, N.; Touthang, L.; Singh, S.K.; Bhardwaj, R.; et al. A comprehensive review on nutritional, nutraceutical, and industrial perspectives of perilla (Perilla frutscens L.) seeds—An orphan oilseed crop. Heliyon 2024, 10, e33281. [Google Scholar] [CrossRef]
- Jang, W.Y.; Hwang, J.Y.; Cho, J.Y. Ginsenosides from Panax ginseng as key modulators of NF-κB signaling are powerful Anti-Inflammatory and anticancer agents. Int. J. Mol. Sci. 2023, 24, 6119. [Google Scholar] [CrossRef]
- Adiguna, S.P.; Panggabean, J.A.; Atikana, A.; Untari, F.; Izzati, F.; Bayu, A.; Rosyidah, A.; Rahmawati, S.I.; Putra, M.Y. Antiviral activities of andrographolide and its derivatives: Mechanism of action and delivery system. Pharmaceuticals 2021, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chao, L.K.; Lin, L.Y.; Wu, C.S.; Chu, L.P.; Huang, C.H.; Chen, H.C. Analysis of volatile compounds from different parts of Houttuynia cordata Thunb. Molecules 2022, 27, 8893. [Google Scholar] [CrossRef] [PubMed]
- Bongioanni, A.; Bueno, M.S.; Mezzano, B.A.; Longhi, M.R.; Garnero, C. Amino acids and its pharmaceutical applications: A mini review. Int. J. Pharm. 2022, 613, 121375. [Google Scholar] [CrossRef] [PubMed]
- Minchin, S.; Lodge, J. Understanding biochemistry: Structure and function of nucleic acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; Iwouno, J.O.; Ofoedu, E.O.; Ogueke, C.C.; Igwe, V.S.; Agunwah, I.M.; Ofoedum, A.F.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Revisiting food-sourced vitamins for consumer diet and health needs: A perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ 2021, 9, e11940. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar]
- He, Y.; Junker, R.R.; Xiao, J.; Lasky, J.R.; Cao, M.; Asefa, M.; Swenson, N.G.; Xu, G.; Yang, J.; Sedio, B.E. Genetic and environmental drivers of intraspecific variation in foliar metabolites in a tropical tree community. New Phytol. 2025, 246, 2551–2564. [Google Scholar] [CrossRef]
- Murai, T.; Naeve, S.; Annor, G.A. Regional variability in sugar and amino acid content of U.S. soybeans and the impact of autoclaving on reducing sugars and free lysine. Foods 2024, 13, 1884. [Google Scholar] [CrossRef]
- Bao, J.; Malunga, L.N. Editorial: Compositional diversity in cereals in relation to their nutritional quality and health benefits. Front. Nutr. 2022, 8, 819923. [Google Scholar] [CrossRef]
- Yang, Y.; Marczak, E.D.; Usui, H.; Kawamura, Y.; Yoshikawa, M. Antihypertensive properties of spinach leaf protein digests. J. Agric. Food Chem. 2004, 52, 2223–2225. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Velitchkova, M.; Stefanov, M.; Popova, A.V. Effect of low light on photosynthetic performance of tomato plants-Ailsa craig and carotenoid mutant Tangerine. Plants 2023, 12, 3000. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An updated pharmacological review and therapeutic perspectives. Oxidative Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef]
- Valdés-González, J.A.; Sánchez, M.; Moratilla-Rivera, I.; Iglesias, I.; Gómez-Serranillos, M.P. Immunomodulatory, anti-inflammatory, and anti-cancer properties of ginseng: A pharmacological update. Molecules 2023, 28, 3863. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, G.; Li, X.; Tao, H.; Li, L.; He, Y.; Zhang, X.; Wang, F.; Hong, G.; Zhu, Y. Metabolomics reveals nutritional diversity among six coarse cereals and antioxidant activity analysis of grain Sorghum and sweet Sorghum. Antioxidants 2022, 11, 1984. [Google Scholar] [CrossRef]
- Castro-Alves, V.; Kalbina, I.; Nilsen, A.; Aronsson, M.; Rosenqvist, E.; Jansen, M.A.K.; Qian, M.; Öström, Å.; Hyötyläinen, T.; Strid, Å. Integration of non-target metabolomics and sensory analysis unravels vegetable plant metabolite signatures associated with sensory quality: A case study using dill (Anethum graveolens). Food Chem. 2021, 344, 128714. [Google Scholar] [CrossRef]
- Iqrar, U.; Javaid, H.; Ashraf, N.; Ahmad, A.; Latief, N.; Shahid, A.A.; Ahmad, W.; Ijaz, B. Structural and functional analysis of pullulanase type 1 (PulA) from Geobacillus thermopakistaniensis. Mol. Biotechnol. 2020, 62, 370–379. [Google Scholar] [CrossRef]
- Kato, A.; Kataoka, H.; Yano, S.; Hayashi, K.; Hayashi, N.; Tanaka, M.; Naitoh, I.; Ban, T.; Miyabe, K.; Kondo, H.; et al. Maltotriose conjugation to a chlorin derivative enhances the antitumor effects of photodynamic therapy in peritoneal dissemination of pancreatic Cancer. Mol. Cancer Ther. 2017, 16, 1124–1132. [Google Scholar] [CrossRef]
- Zhu, J.P.; Ma, Y.R.; Teng, Y.; Chen, J.; Banwell, M.G.; Lan, P. Emulsifying properties of an homologous series of medium- and long-Chain d-Maltotriose esters and their impacts on the viabilities of selected cell lines. J. Agric. Food Chem. 2020, 68, 9004–9013. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.; Lee, S.J. Global transcriptional regulator TrmB family members in prokaryotes. J. Microbiol. 2016, 54, 639–645. [Google Scholar] [CrossRef]
- Lira, S.M.; Dionísio, A.P.; Holanda, M.O.; Marques, C.G.; Silva, G.S.D.; Correa, L.C.; Santos, G.B.M.; Abreu, F.A.P.; Magalhães, F.E.A.; Rebouças, E.L.; et al. Metabolic profile of pitaya (Hylocereus polyrhizus (F.A.C. Weber) Britton & Rose) by UPLC-QTOF-MSE and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Int. 2020, 127, 108701. [Google Scholar] [CrossRef] [PubMed]
- Campus, G.; Cagetti, M.G.; Sale, S.; Petruzzi, M.; Solinas, G.; Strohmenger, L.; Lingström, P. Six months of high-dose xylitol in high-risk caries subjects—A 2-year randomised, clinical trial. Clin. Oral Investig. 2013, 17, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Söderling, E.; Pienihäkkinen, K. Effects of xylitol chewing gum and candies on the accumulation of dental plaque: A systematic review. Clin. Oral Investig. 2022, 26, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ohba, R.; Ueda, S. Production of maltose and maltotriose from starch and pullulan by a immobilized multienzyme of pullulanase and β-amylase. Biotechnol. Bioeng. 1980, 22, 2137–2154. [Google Scholar] [CrossRef]
- De Godoy, V.R.; Muller, G.; Stambuk, B. Efficient maltotriose fermentation through hydrolysis mediated by the intracellular invertase of Saccharomyces cerevisiae. BMC Proc. 2014, 8 (Suppl. 4), P181. [Google Scholar] [CrossRef]
- Qu, J.; Xu, S.; Zhang, Z.; Chen, G.; Zhong, Y.; Liu, L.; Zhang, R.; Xue, J.; Guo, D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci. Rep. 2018, 8, 12736. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Bimo Setiarto, R.H.; Isra, M.; Andrianto, D.; Widhyastuti, N.; Masrukhin. Improvement of prebiotic properties and resistant starch content of corn flour (Zea mays L.) momala gorontalo using physical, chemical and enzymatic modification. Trop. Life Sci. Res. 2023, 34, 255–278. [Google Scholar] [CrossRef]
- Zmasek, C.M.; Godzik, A. Phylogenomic analysis of glycogen branching and debranching enzymatic duo. BMC Evol. Biol. 2014, 14, 183. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.; Jo, M.; Jeon, J.S. Exploration of sugar and starch metabolic pathway crucial for pollen fertility in Rice. Int. J. Mol. Sci. 2022, 23, 14091. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoo, S.H. Effects of combined α-amylase and endo-xylanase treatments on the properties of fresh and frozen doughs and final breads. Polymers 2020, 12, 1349. [Google Scholar] [CrossRef]
- Zhang, Q.; Pritchard, J.; Mieog, J.; Byrne, K.; Colgrave, M.L.; Wang, J.R.; Ral, J.F. Over-expression of a wheat late maturity alpha-amylase type 1 impact on starch properties during grain development and germination. Front. Plant Sci. 2022, 13, 811728. [Google Scholar] [CrossRef]
- Li, E.; Wu, A.C.; Li, J.; Liu, Q.; Gilbert, R.G. Improved understanding of rice amylose biosynthesis from advanced starch structural characterization. Rice 2015, 8, 20. [Google Scholar] [CrossRef]
- Duan, Y.; Jin, L. Genome-wide identification and expression profiling of the α-amylase (AMY) gene family in potato. Genes 2024, 15, 793. [Google Scholar] [CrossRef]
- Bera, S.; Sabikhi, L.; Singh, A.K. Assessment of malting characteristics of different Indian barley cultivars. J. Food Sci. Technol. 2018, 55, 704–711. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Francisco, P.; Wong, R.; Zhang, D.; Smith, S.M. Inhibition of Arabidopsis chloroplast β-amylase BAM3 by maltotriose suggests a mechanism for the control of transitory leaf starch mobilisation. PloS One 2017, 12, e0172504. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fu, X.; Zhu, J.; Gui, Y.; Huang, H.; Liao, Y.; Mao, Y.; Tian, H.; Liu, L. Geographical Variation Shapes Nutritional Metabolite Profile and Food Functionality of Houttuynia cordata. Metabolites 2025, 15, 701. https://doi.org/10.3390/metabo15110701
Zhang Y, Fu X, Zhu J, Gui Y, Huang H, Liao Y, Mao Y, Tian H, Liu L. Geographical Variation Shapes Nutritional Metabolite Profile and Food Functionality of Houttuynia cordata. Metabolites. 2025; 15(11):701. https://doi.org/10.3390/metabo15110701
Chicago/Turabian StyleZhang, Yuanyuan, Xuelang Fu, Jinqun Zhu, Yu Gui, Huilin Huang, Yangye Liao, Yanping Mao, Hui Tian, and Lei Liu. 2025. "Geographical Variation Shapes Nutritional Metabolite Profile and Food Functionality of Houttuynia cordata" Metabolites 15, no. 11: 701. https://doi.org/10.3390/metabo15110701
APA StyleZhang, Y., Fu, X., Zhu, J., Gui, Y., Huang, H., Liao, Y., Mao, Y., Tian, H., & Liu, L. (2025). Geographical Variation Shapes Nutritional Metabolite Profile and Food Functionality of Houttuynia cordata. Metabolites, 15(11), 701. https://doi.org/10.3390/metabo15110701





