Plasma and Milk Variables Classify Diet, Dry Period Length, and Lactation Week of Dairy Cows Using a Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Body Weight (BW), Milk Yield, and Composition
2.2. Blood Sampling and Analysis
2.3. Data Imputation and Processing
2.4. Classification of Diet, Dry Period Length, and Lactation Week
2.5. Importance of Features for Classification
2.6. Software and Data
3. Results
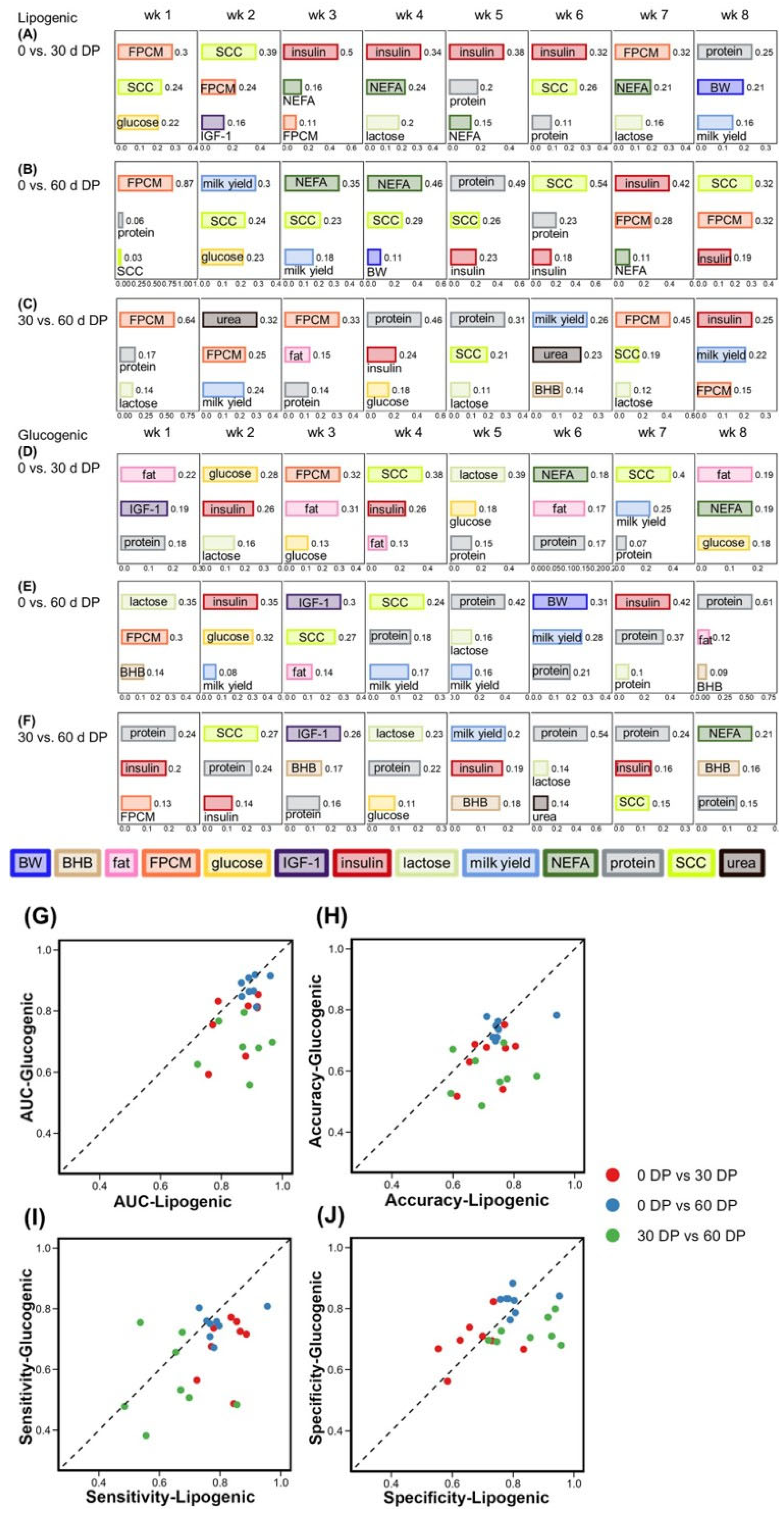
3.1. Diet Classification
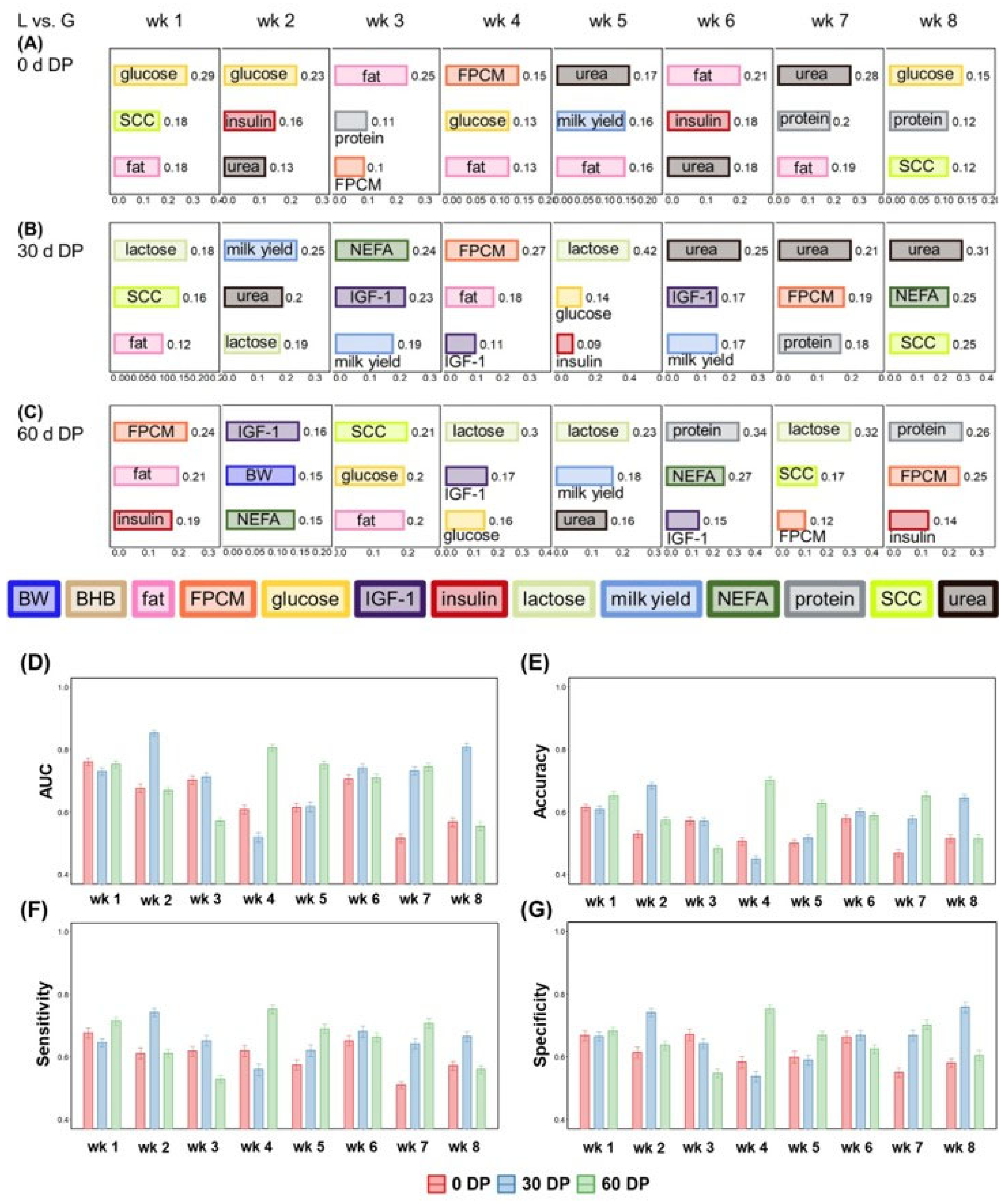
3.2. Dry Period Length Classification
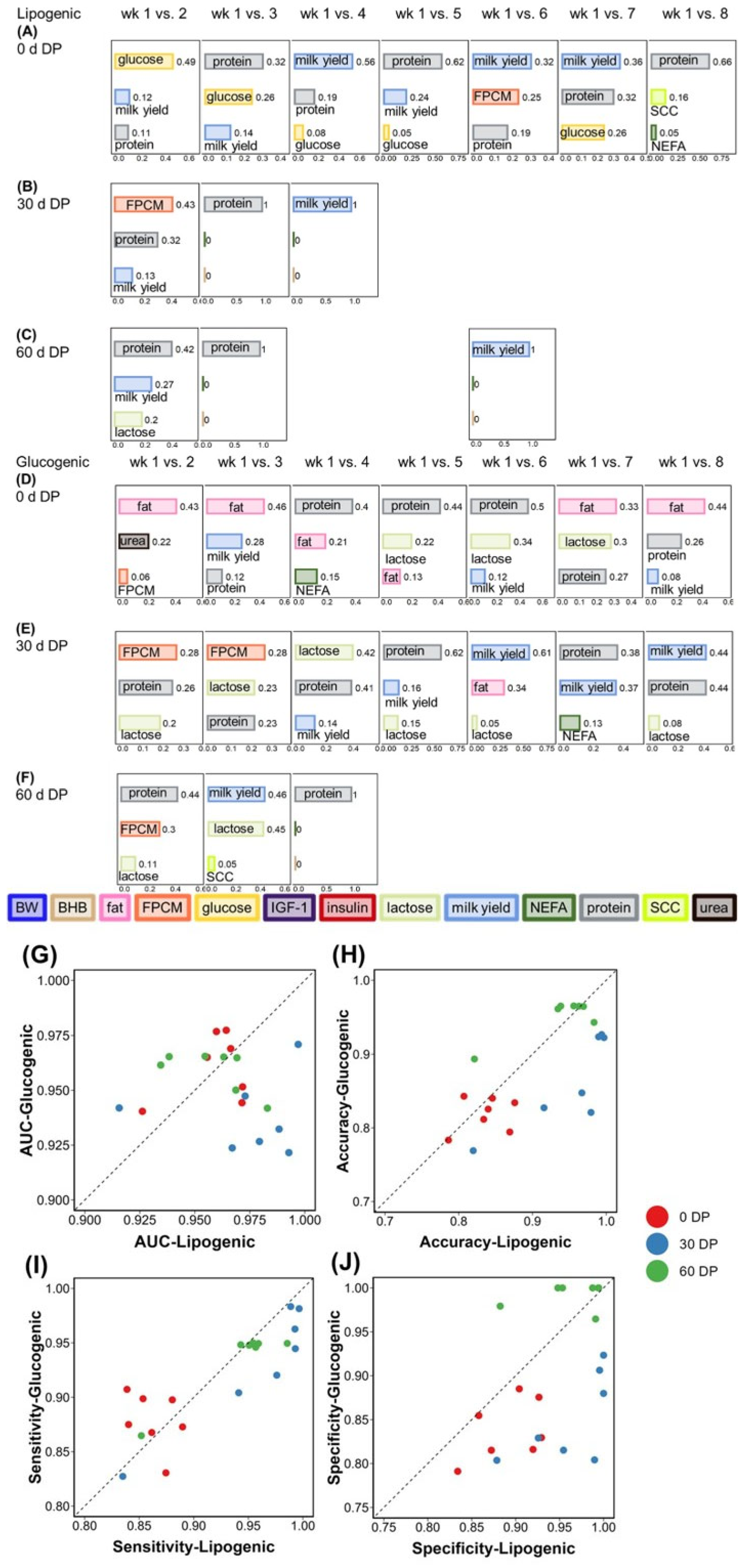
3.3. Lactation Week Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Knegsel, A.T.M.; van den Brand, H.; Dijkstra, J.; Kemp, B. Effects of Dietary Energy Source on Energy Balance, Metabolites and Reproduction Variables in Dairy Cows in Early Lactation. Theriogenology 2007, 68, S274–S280. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of Organic Nutrient Metabolism during Transition from Late Pregnancy to Early Lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef]
- Benedet, A.; Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Variation of Blood Metabolites of Brown Swiss, Holstein-Friesian, and Simmental Cows. Animals 2020, 10, 271. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Hou, X.; Hu, C.; Zhang, L.; Wang, S.; Zhang, Q.; Shi, K. Multi-Channel Metabolomics Analysis Identifies Novel Metabolite Biomarkers for the Early Detection of Fatty Liver Disease in Dairy Cows. Cells 2022, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Kumprechtová, D.; Chabrillat, T.; Guillaume, S.; Kerros, S.; Kadek, R.; Indrová, E.; Illek, J. Effect of Plant Bioactive Compounds Supplemented in Transition Dairy Cows on the Metabolic and Inflammatory Status. Molecules 2022, 27, 6092. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of Hyperketonemia in Early Lactation Dairy Cows on Health and Production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Sturm, V.; Efrosinin, D.; Öhlschuster, M.; Gusterer, E.; Drillich, M.; Iwersen, M. Combination of Sensor Data and Health Monitoring for Early Detection of Subclinical Ketosis in Dairy Cows. Sensors 2020, 20, 1484. [Google Scholar] [CrossRef]
- Collard, B.L.; Boettcher, P.J.; Dekkers, J.C.M.; Petitclerc, D.; Schaeffer, L.R. Relationships Between Energy Balance and Health Traits of Dairy Cattle in Early Lactation. J. Dairy Sci. 2000, 83, 2683–2690. [Google Scholar] [CrossRef]
- Rastani, R.R.; Grummer, R.R.; Bertics, S.J.; Gümen, A.; Wiltbank, M.C.; Mashek, D.G.; Schwab, M.C. Reducing Dry Period Length to Simplify Feeding Transition Cows: Milk Production, Energy Balance, and Metabolic Profiles. J. Dairy Sci. 2005, 88, 1004–1014. [Google Scholar] [CrossRef]
- Lu, J.; Antunes Fernandes, E.; Páez Cano, A.E.; Vinitwatanakhun, J.; Boeren, S.; van Hooijdonk, T.; van Knegsel, A.; Vervoort, J.; Hettinga, K.A. Changes in Milk Proteome and Metabolome Associated with Dry Period Length, Energy Balance, and Lactation Stage in Postparturient Dairy Cows. J. Proteome Res. 2013, 12, 3288–3296. [Google Scholar] [CrossRef]
- van Knegsel, A.T.M.; Remmelink, G.J.; Jorjong, S.; Fievez, V.; Kemp, B. Effect of Dry Period Length and Dietary Energy Source on Energy Balance, Milk Yield, and Milk Composition of Dairy Cows. J. Dairy Sci. 2014, 97, 1499–1512. [Google Scholar] [CrossRef]
- van Knegsel, A.T.M.; van den Brand, H.; Dijkstra, J.; van Straalen, W.M.; Jorritsma, R.; Tamminga, S.; Kemp, B. Effect of Glucogenic vs. Lipogenic Diets on Energy Balance, Blood Metabolites, and Reproduction in Primiparous and Multiparous Dairy Cows in Early Lactation. J. Dairy Sci. 2007, 90, 3397–3409. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Simões, P.; Bexiga, R.; Silva, E.; Mateus, L.; Fernandes, T.; Alves, S.P.; Bessa, R.J.B.; Lopes-da-Costa, L. Effects of Feeding Rumen-Protected Linseed Fat to Postpartum Dairy Cows on Plasma n-3 Polyunsaturated Fatty Acid Concentrations and Metabolic and Reproductive Parameters. J. Dairy Sci. 2022, 105, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Alstrup, L.; Nielsen, M.O.; Lund, P.; Sehested, J.; Larsen, M.K.; Weisbjerg, M.R. Milk Yield, Feed Efficiency and Metabolic Profiles in Jersey and Holstein Cows Assigned to Different Fat Supplementation Strategies. Livest. Sci. 2015, 178, 165–176. [Google Scholar] [CrossRef]
- de Souza, J.; Strieder-Barboza, C.; Contreras, G.A.; Lock, A.L. Effects of Timing of Palmitic Acid Supplementation during Early Lactation on Nutrient Digestibility, Energy Balance, and Metabolism of Dairy Cows. J. Dairy Sci. 2019, 102, 274–287. [Google Scholar] [CrossRef]
- Chen, J.; Gross, J.J.; van Dorland, H.A.; Remmelink, G.J.; Bruckmaier, R.M.; Kemp, B.; van Knegsel, A.T.M. Effects of Dry Period Length and Dietary Energy Source on Metabolic Status and Hepatic Gene Expression of Dairy Cows in Early Lactation. J. Dairy Sci. 2015, 98, 1033–1045. [Google Scholar] [CrossRef]
- Steeneveld, W.; Schukken, Y.H.; van Knegsel, A.T.M.; Hogeveen, H. Effect of Different Dry Period Lengths on Milk Production and Somatic Cell Count in Subsequent Lactations in Commercial Dutch Dairy Herds. J. Dairy Sci. 2013, 96, 2988–3001. [Google Scholar] [CrossRef]
- Andrée O’Hara, E.; Omazic, A.; Olsson, I.; Båge, R.; Emanuelson, U.; Holtenius, K. Effects of Dry Period Length on Milk Production and Energy Balance in Two Cow Breeds. Animal 2018, 12, 508–514. [Google Scholar] [CrossRef]
- Caraviello, D.Z.; Weigel, K.A.; Craven, M.; Gianola, D.; Cook, N.B.; Nordlund, K.V.; Fricke, P.M.; Wiltbank, M.C. Analysis of Reproductive Performance of Lactating Cows on Large Dairy Farms Using Machine Learning Algorithms. J. Dairy Sci. 2006, 89, 4703–4722. [Google Scholar] [CrossRef]
- Ghaffari, M.H.; Jahanbekam, A.; Sadri, H.; Schuh, K.; Dusel, G.; Prehn, C.; Adamski, J.; Koch, C.; Sauerwein, H. Metabolomics Meets Machine Learning: Longitudinal Metabolite Profiling in Serum of Normal versus Overconditioned Cows and Pathway Analysis. J. Dairy Sci. 2019, 102, 11561–11585. [Google Scholar] [CrossRef]
- Frizzarin, M.; O’Callaghan, T.F.; Murphy, T.B.; Hennessy, D.; Casa, A. Application of Machine-Learning Methods to Milk Mid-Infrared Spectra for Discrimination of Cow Milk from Pasture or Total Mixed Ration Diets. J. Dairy Sci. 2021, 104, 12394–12402. [Google Scholar] [CrossRef] [PubMed]
- Becchi, P.P.; Rocchetti, G.; Lucini, L. Advancing Dairy Science through Integrated Analytical Approaches Based on Multi-Omics and Machine Learning. Curr. Opin. Food Sci. 2025, 63, 101289. [Google Scholar] [CrossRef]
- Khanashyam, A.C.; Jagtap, S.; Agrawal, T.K.; Thorakkattu, P.; Malav, O.P.; Trollman, H.; Hassoun, A.; Ramesh, B.; Manoj, V.; Rathnakumar, K.; et al. Applications of Artificial Intelligence in the Dairy Industry: From Farm to Product Development. Comput. Electron. Agric. 2025, 238, 110879. [Google Scholar] [CrossRef]
- Wang, X.; Jahagirdar, S.; Bakker, W.; Lute, C.; Kemp, B.; Knegsel, A.V.; Saccenti, E. Discrimination of Lipogenic or Glucogenic Diet Effects in Early-Lactation Dairy Cows Using Plasma Metabolite Abundances and Ratios in Combination with Machine Learning. Metabolites 2024, 14, 230. [Google Scholar] [CrossRef]
- Van Es, A.J.H. Feed Evaluation for Dairy Cows. Livest. Prod. Sci. 1975, 2, 95–107. [Google Scholar] [CrossRef]
- Tamminga, S.; Van Straalen, W.M.; Subnel, A.P.J.; Meijer, R.G.M.; Steg, A.; Wever, C.J.G.; Blok, M.C. The Dutch Protein Evaluation System: The DVE/OEB-System. Livest. Prod. Sci. 1994, 40, 139–155. [Google Scholar] [CrossRef]
- Data Science and Machine Learning: Confusion Matrix. 2023. Available online: https://manisha-sirsat.blogspot.com/2019/04/confusion-matrix.html (accessed on 22 February 2023).
- CVB (Centraal Veevoederbureau), Veevoedertabel. Gegevens van Chemische Samenstelling, Verteerbaarheid en Voederwaarde van Voedermiddelen; CVB: Lelystad, The Netherlands, 2005.
- Graber, M.; Kohler, S.; Müller, A.; Burgermeister, K.; Kaufmann, T.; Bruckmaier, R.M.; van Dorland, H.A. Identification of Plasma and Hepatic Parameters Related to Metabolic Robustness in Dairy Cows. J. Anim. Physiol. Anim. Nutr. 2012, 96, 75–84. [Google Scholar] [CrossRef]
- Vicari, T.; van den Borne, J.J.G.C.; Gerrits, W.J.J.; Zbinden, Y.; Blum, J.W. Postprandial Blood Hormone and Metabolite Concentrations Influenced by Feeding Frequency and Feeding Level in Veal Calves. Domest. Anim. Endocrinol. 2008, 34, 74–88. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Bühlmann, P. MissForest—Non-parametric missing value imputation for mixed-type. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Søreide, K. Receiver-Operating Characteristic Curve Analysis in Diagnostic, Prognostic and Predictive Biomarker Research. J. Clin. Pathol. 2009, 62, 1–5. [Google Scholar] [CrossRef]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. Xgboost: Extreme Gradient Boosting; xgboost 2023.
- Husson, F.; Le, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017; ISBN 978-0-429-22543-7. [Google Scholar]
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix. Corrplot 2010. Available online: https://cran.r-project.org/web/packages/corrplot/corrplot.pdf (accessed on 22 February 2023).
- Vossebeld, F.; van Knegsel, A.T.M.; Saccenti, E. Phenotyping Metabolic Status of Dairy Cows Using Clustering of Time Profiles of Energy Balance Peripartum. J. Dairy Sci. 2022, 105, 4565–4580. [Google Scholar] [CrossRef]
- Kok, A.; Chen, J.; Kemp, B.; van Knegsel, A.T.M. Review: Dry Period Length in Dairy Cows and Consequences for Metabolism and Welfare and Customised Management Strategies. Animal 2019, 13, s42–s51. [Google Scholar] [CrossRef]
- van Knegsel, A.T.M.; van den Brand, H.; Dijkstra, J.; Tamminga, S.; Kemp, B. Effect of Dietary Energy Source on Energy Balance, Production, Metabolic Disorders and Reproduction in Lactating Dairy Cattle. Reprod. Nutr. Dev. 2005, 45, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Karis, P.; Jaakson, H.; Ling, K.; Bruckmaier, R.M.; Gross, J.J.; Pärn, P.; Kaart, T.; Ots, M. Body Condition and Insulin Resistance Interactions with Periparturient Gene Expression in Adipose Tissue and Lipid Metabolism in Dairy Cows. J. Dairy Sci. 2020, 103, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- van Knegsel, A.T.M. Energy Partitioning in Dairy Cows: Effects of Lipogenic and Glucogenic Diets on Energy Balance, Metabolites and Reproduction Variables in Early Lactation. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2007. [Google Scholar]
- Mirzaei-Alamouti, H.; Panahiha, P.; Patra, A.K.; Mansouryar, M. Effects of Prepartum Diet Grain Type and Postpartum Starch Level on Milk Production, Milk Composition, and Plasma Metabolites of Primiparous and Multiparous Holstein Cows. Anim. Feed Sci. Technol. 2022, 291, 115393. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Lock, A.; Mann, G.E.; Sinclair, K.D.; Webb, R. Nutrition, Metabolism, and Fertility in Dairy Cows: 1. Dietary Energy Source and Ovarian Function. J. Dairy Sci. 2008, 91, 3814–3823. [Google Scholar] [CrossRef]
- Salazar, J.H. Overview of Urea and Creatinine. Lab. Med. 2014, 45, e19–e20. [Google Scholar] [CrossRef]
- Carbone, J.W.; McClung, J.P.; Pasiakos, S.M. Skeletal Muscle Responses to Negative Energy Balance: Effects of Dietary Protein. Adv. Nutr. 2012, 3, 119–126. [Google Scholar] [CrossRef]
- van Knegsel, A.T.M.; van den Brand, H.; Dijkstra, J.; van Straalen, W.M.; Heetkamp, M.J.W.; Tamminga, S.; Kemp, B. Dietary Energy Source in Dairy Cows in Early Lactation: Energy Partitioning and Milk Composition. J. Dairy Sci. 2007, 90, 1467–1476. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Hodson, L.; Guiducci, L.; La Rosa, F.; Salvadori, P.A.; Burchielli, S.; Iozzo, P. The Role of Glucose, Insulin and NEFA in Regulating Tissue Triglyceride Accumulation: Substrate Cooperation in Adipose Tissue versus Substrate Competition in Skeletal Muscle. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 956–963. [Google Scholar] [CrossRef]
- Andrée O’Hara, E.; Båge, R.; Emanuelson, U.; Holtenius, K. Effects of Dry Period Length on Metabolic Status, Fertility, Udder Health, and Colostrum Production in 2 Cow Breeds. J. Dairy Sci. 2019, 102, 595–606. [Google Scholar] [CrossRef]
- Wittrock, J.A.M.; Duffield, T.F.; LeBlanc, S.J. Short Communication: Validation of a Point-of-Care Glucometer for Use in Dairy Cows. J. Dairy Sci. 2013, 96, 4514–4518. [Google Scholar] [CrossRef]
- Byrd, M.K.H.; Arneson, A.G.; Soffa, D.R.; Stewart, J.W.; Rhoads, M.L. Human Continuous Glucose Monitors for Measurement of Glucose in Dairy Cows. JDS Commun. 2021, 3, 78–83. [Google Scholar] [CrossRef]
- Ferreira, C.; Todorovic, M.; Sugrue, P.; Teixeira, S.; Galvin, P. Review: Emerging Sensors and Instrumentation Systems for Bovine Health Monitoring. Animal 2025, 19, 101527. [Google Scholar] [CrossRef] [PubMed]
- Pattamanont, P.; Marcondes, M.I.; Clay, J.S.; Bach, A.; De Vries, A. Piecewise Modeling of the Associations between Dry Period Length and Milk, Fat, and Protein Yield Changes in the Subsequent Lactation. J. Dairy Sci. 2021, 104, 486–500. [Google Scholar] [CrossRef]
- de Vries, R.; van Knegsel, A.; Johansson, M.; Lindmark-Månsson, H.; van Hooijdonk, T.; Holtenius, K.; Hettinga, K. Effect of Shortening or Omitting the Dry Period of Holstein-Friesian Cows on Casein Composition of Milk. J. Dairy Sci. 2015, 98, 8678–8687. [Google Scholar] [CrossRef] [PubMed]
- Cermakova, J.; Kudrna, V.; Simeckova, M.; Vyborna, A.; Dolezal, P.; Illek, J. Comparison of Shortened and Conventional Dry Period Management Strategies. J. Dairy Sci. 2014, 97, 5623–5636. [Google Scholar] [CrossRef] [PubMed]
- Norouzi Ebdalabadi, M.; Valizadah, R.; Moussavi, A.H.; Danesh Mesgaran, M.; Tahmoorespour, M.; Ehsani, A. Effects of Timing to Start Lipogenic Diet on Productive and Reproductive Responses in Periparturient Dairy Cows. Livest. Sci. 2014, 162, 104–114. [Google Scholar] [CrossRef]
- Li, X.P.; Tan, Z.L.; Li, Z.C.; Gao, S.; Yi, K.L.; Zhou, C.S.; Tang, S.X.; Han, X.F. Metabolomic Changes in the Liver Tissues of Cows in Early Lactation Supplemented with Dietary Rumen-Protected Glucose during the Transition Period. Anim. Feed Sci. Technol. 2021, 281, 115093. [Google Scholar] [CrossRef]
- Wang, Y.P.; Cai, M.; Hua, D.K.; Zhang, F.; Jiang, L.S.; Zhao, Y.G.; Wang, H.; Nan, X.M.; Xiong, B.H. Metabolomics Reveals Effects of Rumen-Protected Glucose on Metabolism of Dairy Cows in Early Lactation. Anim. Feed Sci. Technol. 2020, 269, 114620. [Google Scholar] [CrossRef]
- Madley-Dowd, P.; Hughes, R.; Tilling, K.; Heron, J. The Proportion of Missing Data Should Not Be Used to Guide Decisions on Multiple Imputation. J. Clin. Epidemiol. 2019, 110, 63–73. [Google Scholar] [CrossRef] [PubMed]
- De Hond, A.A.H.; Shah, V.B.; Kant, I.M.J.; Van Calster, B.; Steyerberg, E.W.; Hernandez-Boussard, T. Perspectives on Validation of Clinical Predictive Algorithms. Npj Digit. Med. 2023, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Pencina, M.; Thakur, A.; Zhu, T.; Clifton, D.; Shah, N.H. External Validation of AI Models in Health Should Be Replaced with Recurring Local Validation. Nat. Med. 2023, 29, 2686–2687. [Google Scholar] [CrossRef] [PubMed]

| Composition | Glucogenic | Lipogenic |
|---|---|---|
| Ingredient | ||
| Grass silage | 338 | 338 |
| Corn silage | 227 | 227 |
| Soybean meal | 46 | 46 |
| Rapeseed meal | 36 | 36 |
| Rapeseed straw | 10 | 10 |
| Wheat straw | 5 | 5 |
| Concentrate | 338 | 338 |
| Chemical composition | ||
| DM (g/kg of product) | 561 | 566 |
| CP | 167 | 169 |
| Crude fat | 31 | 37 |
| NDF | 318 | 389 |
| ADF | 182 | 224 |
| Starch | 215 | 106 |
| Sugars | 82 | 85 |
| Ash | 76 | 80 |
| DVE | 87 | 84 |
| OEB | 17 | 17 |
| NE (MJ/kg of DM) | 6.55 | 6.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jahagirdar, S.; Kemp, B.; Gross, J.J.; Bruckmaier, R.M.; Saccenti, E.; van Knegsel, A. Plasma and Milk Variables Classify Diet, Dry Period Length, and Lactation Week of Dairy Cows Using a Machine Learning Approach. Metabolites 2025, 15, 698. https://doi.org/10.3390/metabo15110698
Wang X, Jahagirdar S, Kemp B, Gross JJ, Bruckmaier RM, Saccenti E, van Knegsel A. Plasma and Milk Variables Classify Diet, Dry Period Length, and Lactation Week of Dairy Cows Using a Machine Learning Approach. Metabolites. 2025; 15(11):698. https://doi.org/10.3390/metabo15110698
Chicago/Turabian StyleWang, Xiaodan, Sanjeevan Jahagirdar, Bas Kemp, Josef J. Gross, Rupert M. Bruckmaier, Edoardo Saccenti, and Ariette van Knegsel. 2025. "Plasma and Milk Variables Classify Diet, Dry Period Length, and Lactation Week of Dairy Cows Using a Machine Learning Approach" Metabolites 15, no. 11: 698. https://doi.org/10.3390/metabo15110698
APA StyleWang, X., Jahagirdar, S., Kemp, B., Gross, J. J., Bruckmaier, R. M., Saccenti, E., & van Knegsel, A. (2025). Plasma and Milk Variables Classify Diet, Dry Period Length, and Lactation Week of Dairy Cows Using a Machine Learning Approach. Metabolites, 15(11), 698. https://doi.org/10.3390/metabo15110698








