Maturation-Dependent Changes in Volatile Aroma Profile and β-Glucosidase Activity in Kozan Misket Orange (Citrus sinensis L.)
Abstract
1. Introduction
2. Materials and Methods
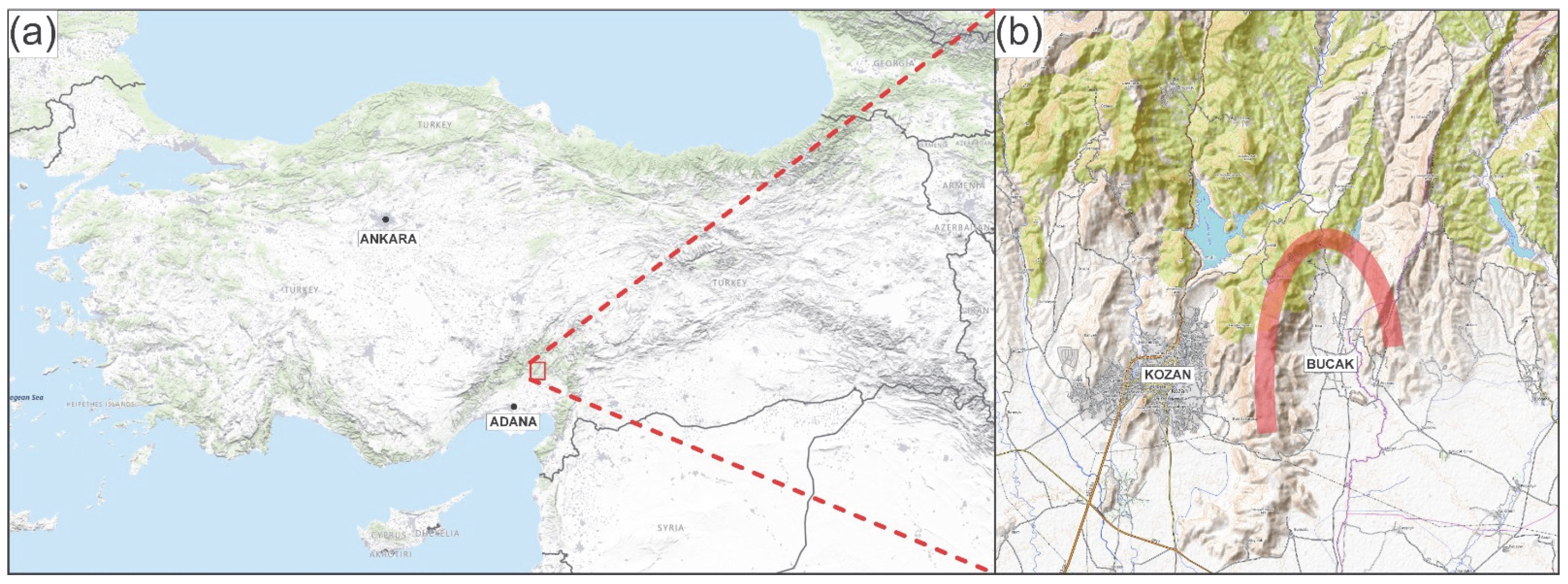
2.1. Plant Material
2.2. Fruit Quality Analysis
2.3. Extraction and Identification of Volatile Compounds
2.4. Extraction, Partial Purification, and Activity Assay of β-Glucosidase
2.5. Statistical Analysis
3. Results
3.1. Fruit Quality Parameters During Maturation
3.2. Variation in Volatile Compounds During Maturation
3.3. β-Glucosidase Activity During Maturation
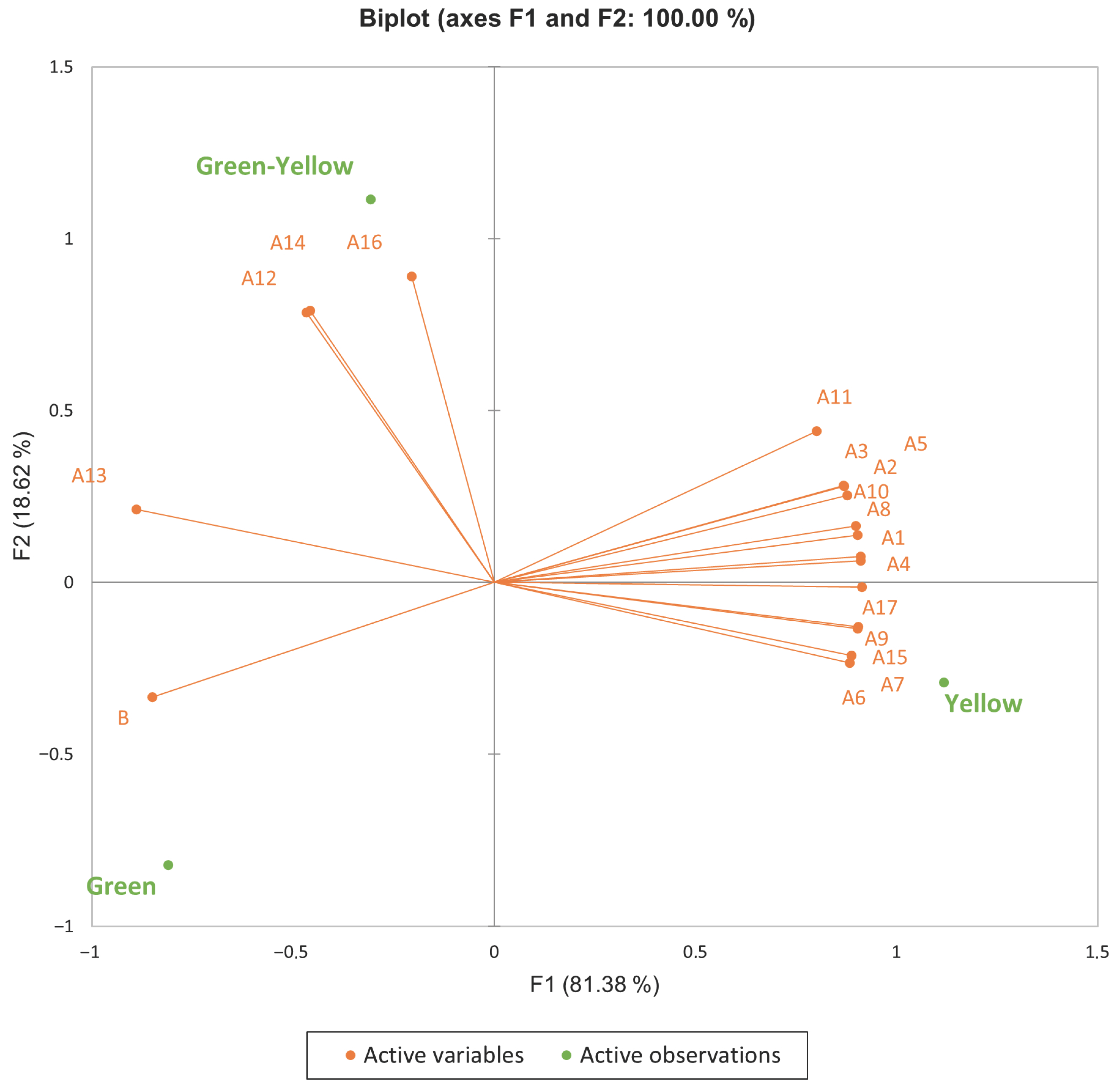
3.4. Multivariate Analysis (PCA)
4. Discussion
4.1. Fruit Quality Analysis of Kozan Misket Orange
4.2. Changes in Volatile Compounds During Maturation
4.3. Discussion on β-Glucosidase Activity During Maturation
4.4. Discussion of Multivariate Analysis (PCA)
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATÜ-BAP | Adana Alparslan Türkeş Science and Technology University—Scientific Research Projects Unit |
| AOAC | Association of Official Analytical Chemists |
| ANOVA | Analysis of Variance |
| DB-WAX | Polyethylene Glycol-Based Capillary GC Column (Brand: DB-Wax) |
| FPP | Farnesyl Diphosphate |
| GBVs | Glycosidically Bound Volatiles |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HS-SPME | Headspace Solid-Phase Microextraction |
| LRI | Linear Retention Index |
| MVA | Mevalonate Pathway |
| OAV | Odor Activity Value |
| OJ | Orange Juice |
| pNP | p-Nitrophenol |
| PMSF | Phenylmethylsulfonyl Fluoride |
| PTFE | Polytetrafluoroethylene |
| PVPP | Polyvinylpolypyrrolidone |
| SD | Standard Deviation |
| SSC | Soluble Solids Content |
| TA | Titratable Acidity |
| TOJ | Threshold in Orange Juice matrix |
| U | Enzyme Unit |
References
- Selli, S. Comparison of sugar and organic acid contents of TURKISH oranges juices. Online J. Sci. Technol. 2017, 7, 43–46. [Google Scholar]
- Biçgel, N.K. Pastörizasyon Sıcaklığının Valensiya ve Kozan Misket Portakallarından Üretilen Meyve Sularının Kalitesi Üzerine Etkisi. Master’s Thesis, Çukurova University, Adana, Turkey, 2008. [Google Scholar]
- Erdoğan, M. Enzim Uygulaması ve Pastörizasyon Işleminin Kozan Misket Portakalından Elde Edilen Meyve Suyunun Aroma ve Aroma Aktif Bileşikleri Üzerine Etkileri. Master’s Thesis, Nevşehir Hacı Bektaş Veli Üniversitesi, Nevşehir, Turkey, 2019. [Google Scholar]
- MoIT–DGIZ. Kozan OSB 1/5000 1/1000 Ölçekli Revizyon İmar Planı Açıklama Raporu; Republic of Türkiye Ministry of Industry and Technology, Directorate General for Industrial Zones [Sanayi ve Teknoloji Bakanlığı Sanayi Bölgeleri Genel Müdürlüğü]: Adana, Turkey, 2021.
- USDA. Citrus: World Markets and Trade; U.S. Department of Agriculture, Foreign Agricultural Service: Washington, DC, USA, 2025.
- Hou, J.; Liang, L.; Wang, Y. Volatile composition changes in navel orange at different growth stages by HS-SPME–GC–MS. Food Res. Int. 2020, 136, 109333. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- El-Otmani, M.; Zacarias, L. Citrus postharvest physiology and technology. In Postharvest Physiology and Technology: Tropical and Subtropical Fruits; CAB International: Wallingford, UK, 2014; pp. 17–33. [Google Scholar]
- Ren, J.N.; Yang, Z.Y.; Tai, Y.N.; Dong, M.; He, M.M.; Fan, G. Characteristics of beta-glucosidase from oranges during maturation and its relationship with changes in bound volatile compounds. J. Sci. Food Agric. 2015, 95, 2345–2352. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Vare, T.B.; Joshi, R.S.; Giri, A.P. Aroma Alchemy: Uridine diphosphate-dependent glycosyltransferases mediated regulation of fruit aroma and flavor biosynthesis. Phytochem. Rev. 2024, 1–24. [Google Scholar] [CrossRef]
- de Morais Souto, B.; Barbosa, M.F.; Sales, R.M.M.; Moura, S.C.; Araújo, A.d.R.B.; Quirino, B.F. The potential of β-glucosidases for aroma and flavor improvement in the food industry. Microbe 2023, 1, 100004. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Rambla, J.L.; Gonzalez-Mas, M.C.; Pons, C.; Bernet, G.P.; Asins, M.J.; Granell, A. Fruit volatile profiles of two citrus hybrids are dramatically different from those of their parents. J. Agric. Food Chem. 2014, 62, 11312–11322. [Google Scholar] [CrossRef]
- Lecas, M.; Gunata, Z.Y.; Sapis, J.-C.; Bayonove, C.L. Purification and partial characterization of β-glucosidase from grape. Phytochemistry 1991, 30, 451–454. [Google Scholar] [CrossRef]
- Whitaker, J.R. Principles of Enzymology for the Food Sciences; Routledge: London, UK, 2018. [Google Scholar]
- Riou, C.; Salmon, J.M.; Vallier, M.J.; Gunata, Z.; Barre, P. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant beta-glucosidase from Aspergillus oryzae. Appl. Env. Microbiol. 1998, 64, 3607–3614. [Google Scholar] [CrossRef]
- Mazzuca, S.; Spadafora, A.; Innocenti, A.M. Cell and tissue localization of β-glucosidase during the ripening of olive fruit (Olea europaea) by in situ activity assay. Plant Sci. 2006, 171, 726–733. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 57–61. [Google Scholar]
- Davies, F.S.; Albrigo, L.G. Crop Production Science in Horticulture 2: Citrus; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic-Amst. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Gupta, A.K.; Pathak, U.; Tongbram, T.; Medhi, M.; Terdwongworakul, A.; Magwaza, L.S.; Mditshwa, A.; Chen, T.; Mishra, P. Emerging approaches to determine maturity of citrus fruit. Crit. Rev. Food Sci. Nutr. 2022, 62, 5245–5266. [Google Scholar] [CrossRef] [PubMed]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- European, U. Reglamento de Ejecución (UE) No 543/2011 de la Comisión de 7 de Junio de 2011 por el que se Establecen las Disposiciones de Aplicación del REGLAMENTO (CE) No 1234/2007 del Consejo en lo que Respecta a los Sectores de las Frutas y Hortalizas y de las Frutas y Hortalizas Transformadas; European Union: Brussels, Belgium, 2011; pp. 71–75. [Google Scholar]
- Plotto, A.; Margaría, C.A.; Goodner, K.L.; Goodrich, R.; Baldwin, E.A. Odour and flavor thresholds for key aroma components in an orange juice matrix: Terpenes and aldehydes. Flavour. Frag. J. 2004, 19, 491–498. [Google Scholar] [CrossRef]
- Perez-Cacho, P.R.; Rouseff, R.L. Fresh squeezed orange juice odor: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 681–695. [Google Scholar] [CrossRef]
- Hognadottir, A.; Rouseff, R.L. Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 998, 201–211. [Google Scholar] [CrossRef]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic-Amst. 2018, 233, 238–248. [Google Scholar] [CrossRef]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Saïdani Tounsi, M. Changes of peel essential oil composition of four Tunisian citrus during fruit maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.; Xue, S.; Ren, J.; Fan, G.; Yang, J.; Huang, L.; Li, G.; Cheng, Y.; Wang, Q.; Yang, Y. Mechanism and Multilayer Perceptron prediction model of the removal of α-terpineol, terpinen-4-ol and carvone from pasteurized citrus juices by β-cyclodextrin encapsulation. Front. Nutr. 2025, 12, 1557934. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter: Zeist, The Netherlands, 2011. [Google Scholar]
- Sawamura, M.; Thi Minh Tu, N.; Onishi, Y.; Ogawa, E.; Choi, H.S. Characteristic odor components of Citrus reticulata Blanco (ponkan) cold-pressed oil. Biosci. Biotechnol. Biochem. 2004, 68, 1690–1697. [Google Scholar] [CrossRef]
- Xu, M.L.; Jiang, Y.F.; Chen, S.M.; Chen, F.D.; Chen, F. Herbivory-Induced Emission of Volatile Terpenes in Functions as an Indirect Defense against Larvae by Attracting Natural Enemies. J. Agric. Food Chem. 2021, 69, 9743–9753. [Google Scholar] [CrossRef]
- Elston, A.; Lin, J.; Rouseff, R. Determination of the role of valencene in orange oil as a direct contributor to aroma quality. Flavour. Frag. J. 2005, 20, 381–386. [Google Scholar] [CrossRef]
- Klesk, K.; Qian, M. Aroma extract dilution analysis of cv. Marion (Rubus spp. hyb) and cv. Evergreen (R. laciniatus L.) blackberries. J. Agric. Food Chem. 2003, 51, 3436–3441. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Plotto, A.; Margaría, C.A.; Goodner, K.L.; Baldwin, E.A. Odour and flavour thresholds for key aroma components in an orange juice matrix: Esters and miscellaneous compounds. Flavour. Frag. J. 2008, 23, 398–406. [Google Scholar] [CrossRef]
- Sharon-Asa, L.; Shalit, M.; Frydman, A.; Bar, E.; Holland, D.; Or, E.; Lavi, U.; Lewinsohn, E.; Eyal, Y. Citrus fruit flavor and aroma biosynthesis: Isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J. 2003, 36, 664–674. [Google Scholar] [CrossRef]
- Matsui, K.; Engelberth, J. Green leaf volatiles—The forefront of plant responses against biotic attack. Plant Cell Physiol. 2022, 63, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Qian, R.; Wang, D.; Liu, L.; Sun, C.; Lin, X. Lipid-derived aldehydes: New key mediators of plant growth and stress responses. Biology 2022, 11, 1590. [Google Scholar] [CrossRef]
- Maoz, I.; Lewinsohn, E.; Gonda, I. Amino acids metabolism as a source for aroma volatiles biosynthesis. Curr. Opin. Plant Biol. 2022, 67, 102221. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Castellari, M.; López-Tamames, E.; Galassi, S.; Buxaderas, S. Characterisation of volatile compounds of fruit juices and nectars by HS/SPME and GC/MS. Food Chem. 2004, 87, 627–637. [Google Scholar] [CrossRef]
- Obenland, D.; Collin, S.; Mackey, B.; Sievert, J.; Fjeld, K.; Arpaia, M.L. Determinants of flavor acceptability during the maturation of navel oranges. Postharvest Biol. Technol. 2009, 52, 156–163. [Google Scholar] [CrossRef]
- Ren, J.N.; Tai, Y.N.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Orruño, E.; Apenten, R.O.; Zabetakis, I. The role of β-glucosidase in the biosynthesis of 2, 5-dimethyl-4-hydroxy-3 (2H)-furanone in strawberry (Fragaria × ananassa cv. Elsanta). Flavour. Frag. J. 2001, 16, 81–84. [Google Scholar] [CrossRef]
- Barbagallo, R.; Palmeri, R.; Fabiano, S.; Rapisarda, P.; Spagna, G. Characteristic of β-glucosidase from Sicilian blood oranges in relation to anthocyanin degradation. Enzym. Microb. Technol. 2007, 41, 570–575. [Google Scholar] [CrossRef]
- Burns, J.K.; Baldwin, E.A. Glycosidase activities in grapefruit flavedo, albedo and juice vesicles during maturation and senescence. Physiol. Plant. 1994, 90, 37–44. [Google Scholar] [CrossRef]

| Maturation Stage | pH (Mean ± SD) | Titratable Acidity (g Citric Acid/100 mL ± SD) | Brix (%) (Mean ± SD) | SSC/TA Ratio |
|---|---|---|---|---|
| Green | 3.22 ± 0.01 c | 1.18 ± 0.01 a | 10.87 ± 0.10 b | 9.21 c |
| Green-Yellow | 3.45 ± 0.01 b | 0.96 ± 0.01 b | 10.33 ± 0.15 c | 10.76 b |
| Yellow | 3.50 ± 0.01 a | 0.77 ± 0.03 c | 12.00 ± 0.10 a | 15.58 a |
| Chemical Class | Green (µg/L) | Green-Yellow (µg/L) | Yellow (µg/L) |
|---|---|---|---|
| Monoterpenes | 39,431.51 ± 2962.02 a | 45,215.47 ± 2255.85 ab | 50,878.07 ± 3421.31 b |
| Sesquiterpenes | 0.00 ± 0.00 a | 81.54 ± 15.13 b | 778.20 ± 21.04 c |
| Aromatic Hydrocarbons | 2995.88 ± 263.66 a | 3302.12 ± 195.05 ab | 4709.54 ± 788.29 b |
| Aldehydes | 246.01 ± 45.16 a | 473.24 ± 46.36 b | 323.38 ± 43.96 a |
| Higher Alcohols | 295.75 ± 18.60 b | 540.67 ± 8.11 c | 193.74 ± 10.28 a |
| Total Volatiles | 42,969.14 ± 3289.44 a | 49,613.04 ± 2520.50 ab | 56,882.94 ± 4284.88 b |
| LRI | Compound | Green (µg/L) | Green-Yellow (µg/L) | Yellow (µg/L) | Significance |
|---|---|---|---|---|---|
| 1171 | δ-3-Carene | 134.97 ± 11.82 a | 184.35 ± 28.81 a | 286.98 ± 32.59 b | ** |
| 1197 | D-Limonene | 38,268.14 ± 2858.08 a | 43,747.03 ± 2143.37 a | 49,012.73 ± 3048.85 b | ** |
| 1207 | Β-Phellandrene | 156.29 ± 14.36 a | 173.72 ± 14.97 a | 188.61 ± 24.86 a | ns |
| 1250 | γ-Terpinene | 32.89 ± 13.66 a | 47.64 ± 17.32 a | 76.58 ± 23.64 a | ns |
| 1268 | Trans-isolimonene | 37.48 ± 2.44 a | 50.34 ± 1.99 b | 61.44 ± 4.44 c | *** |
| 1274 | α-Terpinolene | 175.11 ± 30.90 a | 174.39 ± 20.73 a | 294.04 ± 83.98 a | ns |
| 1277 | Trans-geranylacetylene | 17.40 ± 1.64 a | 20.25 ± 1.75 a | 140.97 ± 22.48 b | *** |
| 1279 | β-Ocimene | 175.15 ± 9.74 a | 200.30 ± 8.83 b | 238.27 ± 34.85 b | * |
| 1286 | 1,3,8-p-Menthatriene | 21.01 ± 1.40 a | 31.81 ± 1.40 a | 108.19 ± 102.53 a | ns |
| 1296 | Neo-Allo-Ocimene | 137.86 ± 10.06 a | 171.80 ± 0.57 b | 217.89 ± 6.60 c | *** |
| 1407 | Limonene oxide | 22.13 ± 0.00 a | 46.12 ± 0.74 b | 56.10 ± 3.73 c | *** |
| 1545 | Linalool | 74.51 ± 2.39 b | 141.08 ± 7.42 c | 49.44 ± 2.37 a | *** |
| 1609 | 4-Terpineol | 118.87 ± 2.36 b | 117.26 ± 3.65 b | 53.23 ± 14.78 a | *** |
| 1690 | α-terpineol | 59.71 ± 3.16 b | 109.38 ± 4.30 c | 41.98 ± 5.04 a | *** |
| 1707 | Valencene | 0.00 ± 0.00 a | 81.54 ± 15.13 b | 692.43 ± 7.83 c | *** |
| 1284 | Octanal | 179.82 ± 37.71 a | 381.09 ± 42.76 b | 189.41 ± 21.34 a | *** |
| 1495 | Decanal | 66.19 ± 7.45 a | 67.90 ± 1.56 a | 73.09 ± 14.17 a | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yabacı Karaoğlan, S. Maturation-Dependent Changes in Volatile Aroma Profile and β-Glucosidase Activity in Kozan Misket Orange (Citrus sinensis L.). Metabolites 2025, 15, 689. https://doi.org/10.3390/metabo15110689
Yabacı Karaoğlan S. Maturation-Dependent Changes in Volatile Aroma Profile and β-Glucosidase Activity in Kozan Misket Orange (Citrus sinensis L.). Metabolites. 2025; 15(11):689. https://doi.org/10.3390/metabo15110689
Chicago/Turabian StyleYabacı Karaoğlan, Selin. 2025. "Maturation-Dependent Changes in Volatile Aroma Profile and β-Glucosidase Activity in Kozan Misket Orange (Citrus sinensis L.)" Metabolites 15, no. 11: 689. https://doi.org/10.3390/metabo15110689
APA StyleYabacı Karaoğlan, S. (2025). Maturation-Dependent Changes in Volatile Aroma Profile and β-Glucosidase Activity in Kozan Misket Orange (Citrus sinensis L.). Metabolites, 15(11), 689. https://doi.org/10.3390/metabo15110689







