A Pilot Study: Comparative Effects of Green Tea Extract and Duloxetine on Oxaliplatin-Induced Allodynia in a Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Protocol
2.3. Mechanical Sensitivity—Von Frey Filament Testing
2.4. Measurement of Serum Neurofilament Light
2.5. Drug Administration
2.6. Diet Intervention Administration
2.7. Statistical Analysis
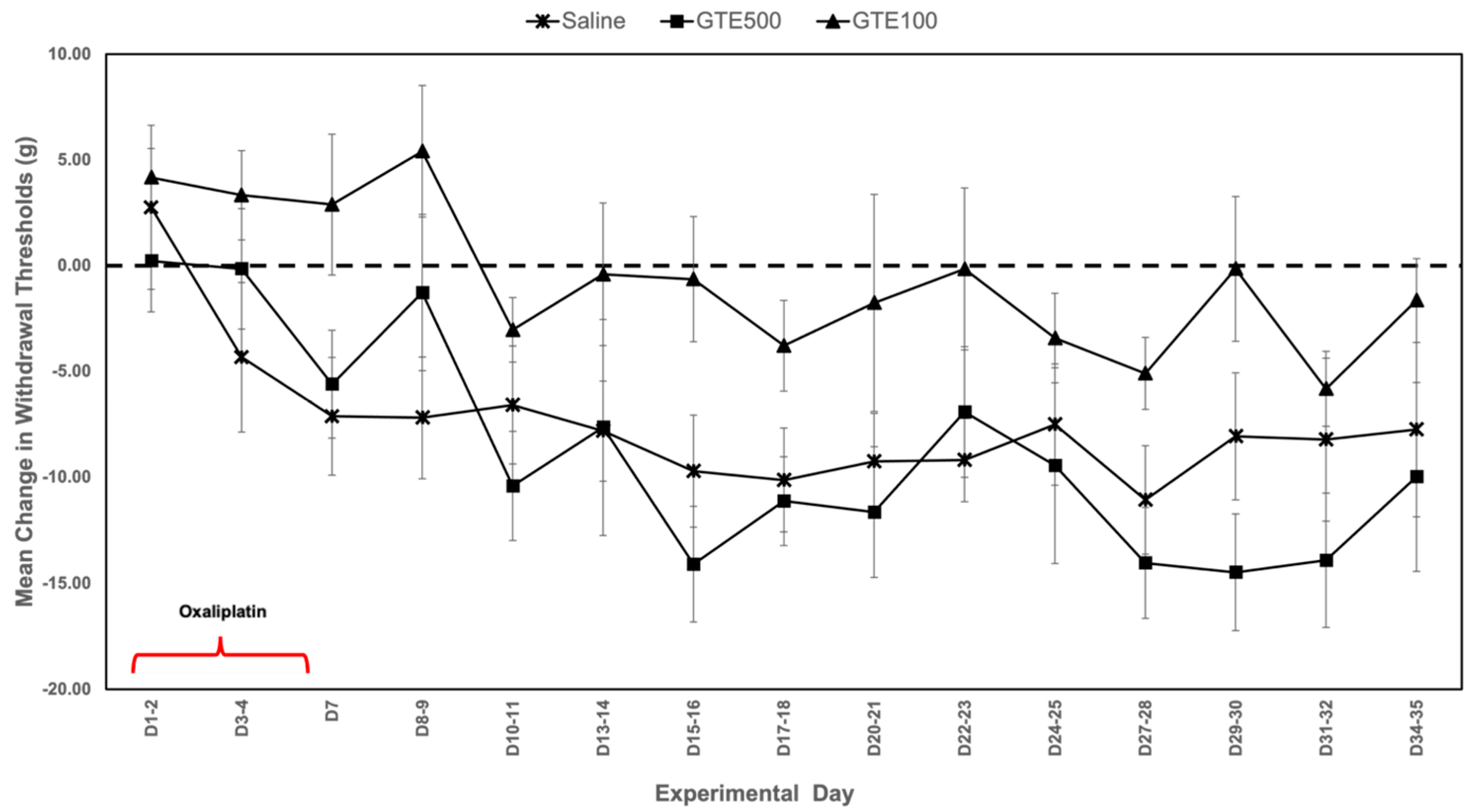
3. Results
3.1. Mechanical Sensitivity
3.2. Serum Neurofilament Light
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Beijers, A.J.; Mols, F.; Tjan-Heijnen, V.C.; Faber, C.G.; van de Poll-Franse, L.V.; Vreugdenhil, G. Peripheral neuropathy in colorectal cancer survivors: The influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol. 2015, 54, 463–469. [Google Scholar] [CrossRef]
- Pachman, D.R.; Qin, R.; Seisler, D.; Smith, E.M.; Kaggal, S.; Novotny, P.; Ruddy, K.J.; Lafky, J.M.; Ta, L.E.; Beutler, A.S.; et al. Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support. Care Cancer 2016, 24, 5059–5068. [Google Scholar] [CrossRef]
- Pachman, D.R.; Qin, R.; Seisler, D.K.; Smith, E.M.; Beutler, A.S.; Ta, L.E.; Lafky, J.M.; Wagner-Johnston, N.D.; Ruddy, K.J.; Dakhil, S.; et al. Clinical Course of Oxaliplatin-Induced Neuropathy: Results from the Randomized Phase III Trial N08CB (Alliance). J. Clin. Oncol. 2015, 33, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Cavaletti, G.; Antonacopoulou, A.; Genazzani, A.A.; Briani, C.; Bruna, J.; Terrazzino, S.; Velasco, R.; Alberti, P.; Campagnolo, M.; et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: Results from a prospective multicenter study. Cancer 2013, 119, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Qin, R.; Dakhil, S.R.; Fehrenbacher, L.; Flynn, K.A.; Atherton, P.; Seisler, D.; Qamar, R.; Lewis, G.C.; Grothey, A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J. Clin. Oncol. 2014, 32, 997–1005. [Google Scholar] [CrossRef]
- Tofthagen, C. Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin. J. Oncol. Nurs. 2010, 14, E22–E28. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C.; McAllister, R.D.; McMillan, S.C. Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clin. J. Oncol. Nurs. 2011, 15, 182–188. [Google Scholar] [CrossRef]
- Cavaletti, G.; Marmiroli, P. Management of Oxaliplatin-Induced Peripheral Sensory Neuropathy. Cancers 2020, 12, 1370. [Google Scholar] [CrossRef]
- Egashira, N. Pathological Mechanisms and Preventive Strategies of Oxaliplatin-Induced Peripheral Neuropathy. Front. Pain. Res. 2021, 2, 804260. [Google Scholar] [CrossRef]
- Kang, L.; Tian, Y.; Xu, S.; Chen, H. Oxaliplatin-induced peripheral neuropathy: Clinical features, mechanisms, prevention and treatment. J. Neurol. 2021, 268, 3269–3282. [Google Scholar] [CrossRef]
- Ventzel, L.; Jensen, A.B.; Jensen, A.R.; Jensen, T.S.; Finnerup, N.B. Chemotherapy-induced pain and neuropathy: A prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain 2016, 157, 560–568. [Google Scholar] [CrossRef]
- Wolf, S.L.; Barton, D.L.; Qin, R.; Wos, E.J.; Sloan, J.A.; Liu, H.; Aaronson, N.K.; Satele, D.V.; Mattar, B.I.; Green, N.B.; et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support. Care Cancer 2012, 20, 625–632. [Google Scholar] [CrossRef]
- Salat, K. Chemotherapy-induced peripheral neuropathy-part 2: Focus on the prevention of oxaliplatin-induced neurotoxicity. Pharmacol. Rep. 2020, 72, 508–527. [Google Scholar] [CrossRef]
- Sleczkowska, M.; Misra, K.; Santoro, S.; Gerrits, M.M.; Hoeijmakers, J.G.J.; PainNet Study, G. Ion Channel Genes in Painful Neuropathies. Biomedicines 2023, 11, 2680. [Google Scholar] [CrossRef]
- Postma, T.J.; Aaronson, N.K.; Heimans, J.J.; Muller, M.J.; Hildebrand, J.G.; Delattre, J.Y.; Hoang-Xuan, K.; Lanteri-Minet, M.; Grant, R.; Huddart, R.; et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Lavoie Smith, E.M.; Barton, D.L.; Qin, R.; Steen, P.D.; Aaronson, N.K.; Loprinzi, C.L. Assessing patient-reported peripheral neuropathy: The reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual. Life Res. 2013, 22, 2787–2799. [Google Scholar] [CrossRef]
- Ballarini, E.; Malacrida, A.; Rodriguez-Menendez, V.; Pozzi, E.; Canta, A.; Chiorazzi, A.; Monza, L.; Semperboni, S.; Meregalli, C.; Carozzi, V.A.; et al. Sodium-Calcium Exchanger 2: A Pivotal Role in Oxaliplatin Induced Peripheral Neurotoxicity and Axonal Damage? Int. J. Mol. Sci. 2022, 23, 10063. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Carozzi, V.A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Sandelius, Å.; Blennow, K.; et al. Neurofilament light chain as disease biomarker in a rodent model of chemotherapy induced peripheral neuropathy. Exp. Neurol. 2018, 307, 129–132. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Carozzi, V.A.; Blennow, K.; Zetterberg, H.; et al. Neurofilament light chain: A specific serum biomarker of axonal damage severity in rat models of Chemotherapy-Induced Peripheral Neurotoxicity. Arch. Toxicol. 2020, 94, 2517–2522. [Google Scholar] [CrossRef]
- Karteri, S.; Bruna, J.; Argyriou, A.A.; Mariotto, S.; Velasco, R.; Alemany, M.; Kalofonou, F.; Alberti, P.; Dinoto, A.; Velissaris, D.; et al. Prospectively assessing serum neurofilament light chain levels as a biomarker of paclitaxel-induced peripheral neurotoxicity in breast cancer patients. J. Peripher. Nerv. Syst. 2022, 27, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Huehnchen, P.; Schinke, C.; Bangemann, N.; Dordevic, A.D.; Kern, J.; Maierhof, S.K.; Hew, L.; Nolte, L.; Körtvelyessy, P.; Göpfert, J.C.; et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight 2022, 7, e154395. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, M.K.; Park, N.Y.; Hyun, J.W.; Lee, M.Y.; Kim, H.J.; Jung, S.K.; Cha, Y. Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci. Rep. 2020, 10, 7995. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef]
- Willis, W.; Westlund, K. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 1997, 14, 2–31. [Google Scholar] [CrossRef]
- Chappell, A.S.; Ossanna, M.J.; Liu-Seifert, H.; Iyengar, S.; Skljarevski, V.; Li, L.C.; Bennett, R.M.; Collins, H. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: A 13-week, randomized, placebo-controlled trial. Pain 2009, 146, 253–260. [Google Scholar] [CrossRef]
- Chappell, A.S.; Desaiah, D.; Liu-Seifert, H.; Zhang, S.; Skljarevski, V.; Belenkov, Y.; Brown, J.P. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain. Pract. 2011, 11, 33–41. [Google Scholar] [CrossRef]
- Lunn, M.P.; Hughes, R.A.; Wiffen, P.J. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst. Rev. 2009, CD007115. [Google Scholar] [CrossRef]
- Russell, I.J.; Mease, P.J.; Smith, T.R.; Kajdasz, D.K.; Wohlreich, M.M.; Detke, M.J.; Walker, D.J.; Chappell, A.S.; Arnold, L.M. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 2008, 136, 432–444. [Google Scholar] [CrossRef]
- Wernicke, J.F.; Pritchett, Y.L.; D’Souza, D.N.; Waninger, A.; Tran, P.; Iyengar, S.; Raskin, J. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006, 67, 1411–1420. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Goldstein, D.; Friedlander, M.; Kiernan, M.C. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve 2005, 32, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.A.; Smith, E.M.L.; Ayyash, N.; Toledo, J.; Rasheed, Z.; Holden, J.E. Effectiveness of Duloxetine on Oxaliplatin-induced Allodynia and Hyperalgesia in Rats. Biol. Res. Nurs. 2024, 26, 248–256. [Google Scholar] [CrossRef]
- Castellano-Tejedor, C. Non-Pharmacological Interventions for the Management of Chronic Health Conditions and Non-Communicable Diseases. Int. J. Environ. Res. Public Health 2022, 19, 8536. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Frangos, E.; Madian, N. Non-pharmacological Treatment of Pain: Grand Challenge and Future Opportunities. Front Pain Res. 2021, 2, 696783. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Smith, E.L. Promising Roles of Phytocompounds and Nutrients in Interventions to Mitigate Chemotherapy-Induced Peripheral Neuropathy. Semin. Oncol. Nurs. 2024, 40, 151713. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (-)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Szpakowski, P.; Krol, A.; Ksiazek-Winiarek, D.; Kobylecki, A.; Glabinski, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, L. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Imano, M.; Nishida, S.; Tsubaki, M.; Hashimoto, S.; Ito, A.; Satou, T. (-)-Epigallocatechin-3-gallate protects against neuronal cell death and improves cerebral function after traumatic brain injury in rats. Neuromolecular Med. 2011, 13, 300–309. [Google Scholar] [CrossRef]
- Machova Urdzikova, L.; Ruzicka, J.; Karova, K.; Kloudova, A.; Svobodova, B.; Amin, A.; Dubisova, J.; Schmidt, M.; Kubinova, S.; Jhanwar-Uniyal, M.; et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology 2017, 126, 213–223. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.T.; Jeon, E.K.; Won, H.S.; Cho, Y.S.; Ko, Y.H. Effect of green tea extracts on oxaliplatin-induced peripheral neuropathy in rats. BMC Complement. Altern. Med. 2012, 12, 124. [Google Scholar] [CrossRef]
- Essmat, A.; Hussein, M.S. Green tea extract for mild-to-moderate diabetic peripheral neuropathy A randomized controlled trial. Complement. Ther. Clin. Pract. 2021, 43, 101317. [Google Scholar] [CrossRef] [PubMed]
- Khalatbary, A.R.; Khademi, E. The green tea polyphenolic catechin epigallocatechin gallate and neuroprotection. Nutr. Neurosci. 2020, 23, 281–294. [Google Scholar] [CrossRef]
- Ding, M.L.; Ma, H.; Man, Y.G.; Lv, H.Y. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can. J. Physiol. Pharmacol. 2017, 95, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Totsch, S.K.; Waite, M.E.; Sorge, R.E. Dietary influence on pain via the immune system. Prog. Mol. Biol. Transl. Sci. 2015, 131, 435–469. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, J. Mechanisms of Pathological Axonal Degeneration. In Mechanisms of Axonal and Dendritic Remodelling in Health and Disease, 1st ed.; Tran, T., Yaron, A., Eds.; River Publishers: Abingdon, UK, 2024. [Google Scholar]
- Dixon, W.J. Staircase bioassay: The up-and-down method. Neurosci. Biobehav. Rev. 1991, 15, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Authier, N.; Balayssac, D.; Eschalier, A.; Coudore, F. Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain 2007, 128, 225–234. [Google Scholar] [CrossRef]
- Kim, W.; Chung, Y.; Choi, S.; Min, B.I.; Kim, S.K. Duloxetine Protects against Oxaliplatin-Induced Neuropathic Pain and Spinal Neuron Hyperexcitability in Rodents. Int. J. Mol. Sci. 2017, 18, 2626. [Google Scholar] [CrossRef]
- Ling, B.; Coudore-Civiale, M.A.; Balayssac, D.; Eschalier, A.; Coudore, F.; Authier, N. Behavioral and immunohistological assessment of painful neuropathy induced by a single oxaliplatin injection in the rat. Toxicology 2007, 234, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Taiyo. Sunphenon 90D (Green Teat Extract). Available online: https://sunphenon.com/about/ (accessed on 1 August 2024).
- Park, J.; Park, R.; Jang, M.; Park, Y.I. Therapeutic Potential of EGCG, a Green Tea Polyphenol, for Treatment of Coronavirus Diseases. Life 2021, 11, 197. [Google Scholar] [CrossRef]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Castaldelli-Maia, J.M.; Scomparini, L.B.; Andrade, A.G.; Bhugra, D.; de Toledo Ferraz Alves, T.C.; D’Elia, G. Perceptions of and attitudes toward antidepressants: Stigma attached to their use--a review. J. Nerv. Ment. Dis. 2011, 199, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Schnell, P.M.; Lustberg, M.B.; Henry, N.L. Adverse Events and Perception of Benefit from Duloxetine for Treating Aromatase Inhibitor-Associated Arthralgias. JNCI Cancer Spectr. 2021, 5, pkab018. [Google Scholar] [CrossRef] [PubMed]
- Elwadhi, D.; Cohen, A. Social inequalities in antidepressant treatment outcomes: A systematic review. Soc. Psychiatry Psychiatr. Epidemiol. 2020, 55, 1241–1259. [Google Scholar] [CrossRef]
- Wang, J.H.; Cheng, J.; Li, C.R.; Ye, M.; Ma, Z.; Cai, F. Modulation of Ca2+ signals by epigallocatechin-3-gallate(EGCG) in cultured rat hippocampal neurons. Int. J. Mol. Sci. 2011, 12, 742–754. [Google Scholar] [CrossRef]
- Deng, H.M.; Yin, S.T.; Yan, D.; Tang, M.L.; Li, C.C.; Chen, J.T.; Wang, M.; Ruan, D.Y. Effects of EGCG on voltage-gated sodium channels in primary cultures of rat hippocampal CA1 neurons. Toxicology 2008, 252, 1–8. [Google Scholar] [CrossRef]
- Wang, S.Y.; Calderon, J.; Kuo Wang, G. Block of neuronal Na+ channels by antidepressant duloxetine in a state-dependent manner. Anesthesiology 2010, 113, 655–665. [Google Scholar] [CrossRef]
- Stoetzer, C.; Papenberg, B.; Doll, T.; Volker, M.; Heineke, J.; Stoetzer, M.; Wegner, F.; Leffler, A. Differential inhibition of cardiac and neuronal Na+ channels by the selective serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine. Eur. J. Pharmacol. 2016, 783, 1–10. [Google Scholar] [CrossRef]
- Park, S.B.; Cetinkaya-Fisgin, A.; Argyriou, A.A.; Hoke, A.; Cavaletti, G.; Alberti, P. Axonal degeneration in chemotherapy-induced peripheral neurotoxicity: Clinical and experimental evidence. J. Neurol. Neurosurg. Psychiatry 2023, 94, 962–972. [Google Scholar] [CrossRef]
- Rice, A.S.C.; Finnerup, N.B.; Kemp, H.I.; Currie, G.L.; Baron, R. Sensory profiling in animal models of neuropathic pain: A call for back-translation. Pain 2018, 159, 819–824. [Google Scholar] [CrossRef]
- Gehr, N.L.; Mortensen, C.; Stage, T.B.; Pedersen, M.R.V.; Rafaelsen, S.R.; Madsen, J.S.; Olsen, D.A.; Timm, S.; Jensen, L.H.; Hansen, T.F.; et al. Neurofilament light chain as a marker for neuronal damage: Integrating in vitro studies and clinical findings in patients with oxaliplatin-induced neuropathy. Cancer Chemother. Pharmacol. 2025, 95, 53. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.E.; Boehmerle, W.; Huehnchen, P.; Stage, T.B. Neurofilament light chain as a biomarker of chemotherapy-induced peripheral neuropathy. Trends Pharmacol. Sci. 2024, 45, 872–879. [Google Scholar] [CrossRef]
- Pereira, A.F.; de Oliveira, F.F.B.; de Freitas Alves, B.W.; de Menezes, K.L.S.; de Mesquita, A.K.V.; Lisboa, M.R.P.; de Sousa, K.K.O.; Vale, M.L. Neurotoxic effect of oxaliplatin: Comparison with its oxalate-free analogue cis-[PtII(1R,2R-DACH)(3-acetoxy-1,1-cyclobutanedicarboxylato)] (LLC-1402) in mice. Toxicol. Appl. Pharmacol. 2018, 340, 77–84. [Google Scholar] [CrossRef]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-induced neurotoxicity and preventive strategies: Past, present, and future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef]
- Gamelin, L.; Boisdron-Celle, M.; Delva, R.; Guerin-Meyer, V.; Ifrah, N.; Morel, A.; Gamelin, E. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: A retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin. Cancer Res. 2004, 10, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Janle, E.M.; Morre, D.M.; Morre, D.J.; Zhou, Q.; Zhu, Y. Pharmacokinetics of green tea catechins in extract and sustained-release preparations. J. Diet. Suppl. 2008, 5, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, P.; Feng, K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur. J. Med. Chem. 2023, 250, 115197. [Google Scholar] [CrossRef]
- Cao, J.; Han, J.; Xiao, H.; Qiao, J.; Han, M. Effect of Tea Polyphenol Compounds on Anticancer Drugs in Terms of Anti-Tumor Activity, Toxicology, and Pharmacokinetics. Nutrients 2016, 8, 762. [Google Scholar] [CrossRef]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Nayerpour Dizaj, T.; Safaeian, A.; Bakhshi, A.; Abazari, O.; et al. Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene 2021, 787, 145638. [Google Scholar] [CrossRef]
- Chen, S.Z.; Zhen, Y.S. Molecular targets of tea polyphenols and its roles of anticancer drugs in experimental therapy. Yao Xue Xue Bao 2013, 48, 1–7. [Google Scholar]
- Chan, M.M.; Fong, D.; Soprano, K.J.; Holmes, W.F.; Heverling, H. Inhibition of growth and sensitization to cisplatin-mediated killing of ovarian cancer cells by polyphenolic chemopreventive agents. J. Cell Physiol. 2003, 194, 63–70. [Google Scholar] [CrossRef] [PubMed]

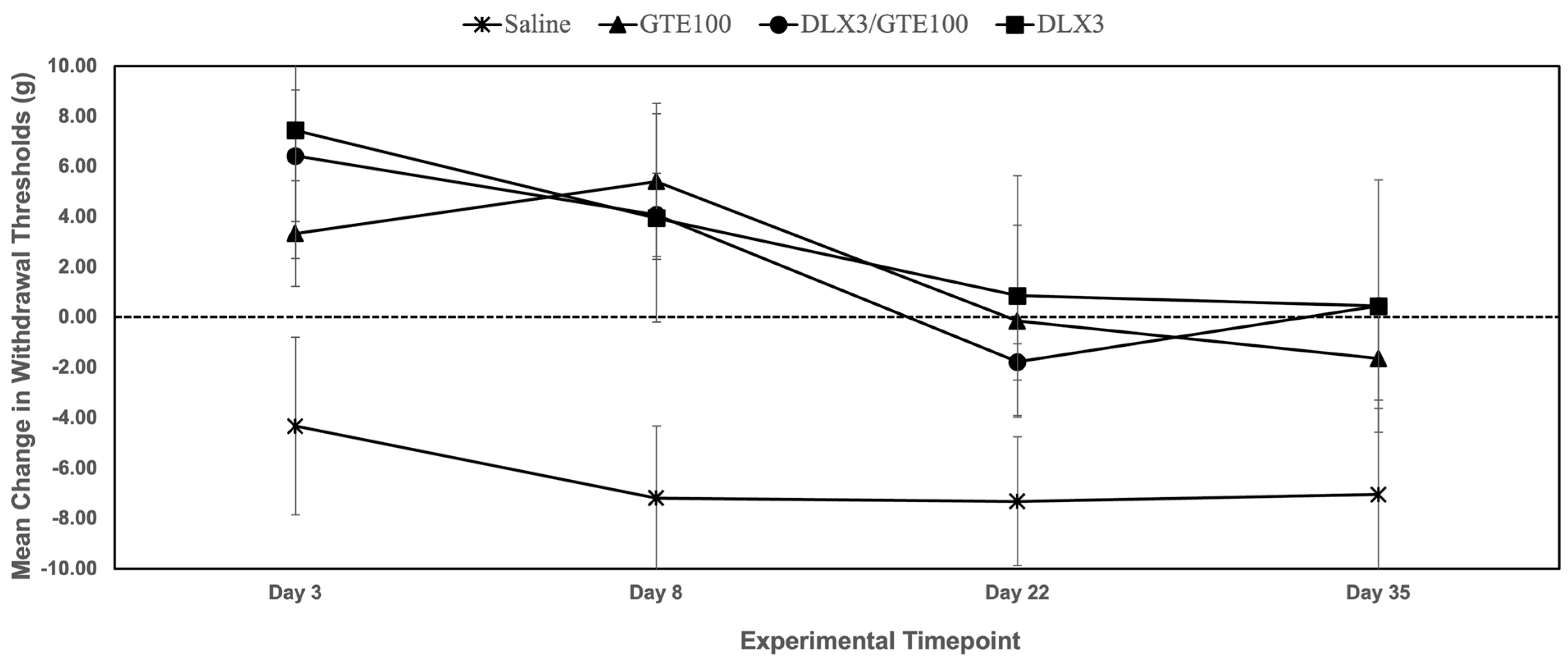

| Group | Day 3 | Day 8 | Day 22 | Day 35 |
|---|---|---|---|---|
| GTE 100 mg/kg | +3.33 ± 2.11 g (NS) | +5.41 ± 3.11 g (p = 0.01) * | −1.78 ± 0.73 g (NS) | −1.64 ± 1.98 g (NS) |
| DLX 3 mg/kg | +7.44 ± 5.10 g (NS) | +3.95 ± 4.15 g (p = 0.02) * | +0.86 ± 4.77 g (NS) | +0.45 ± 5.02 g (NS) |
| GTE 100 mg/kg + DLX 3 mg/kg | +6.43 ± 2.62 g (NS) | +4.08 ± 1.66 g (p = 0.01) * | −1.78 ± 0.73 g (NS) | +0.45 ± 0.20 g (NS) |
| Saline | −4.33 ± 3.53 g | −7.19 ± 2.87 g | −7.32 ± 2.56 g | −7.04 ± 3.75 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, M.; Totsch, S.; Li, P.; Odii, C.O.; Shelton, H.; Sorge, R.E.; Smith, E.M.L. A Pilot Study: Comparative Effects of Green Tea Extract and Duloxetine on Oxaliplatin-Induced Allodynia in a Murine Model. Metabolites 2025, 15, 680. https://doi.org/10.3390/metabo15100680
Daniel M, Totsch S, Li P, Odii CO, Shelton H, Sorge RE, Smith EML. A Pilot Study: Comparative Effects of Green Tea Extract and Duloxetine on Oxaliplatin-Induced Allodynia in a Murine Model. Metabolites. 2025; 15(10):680. https://doi.org/10.3390/metabo15100680
Chicago/Turabian StyleDaniel, Michael, Stacie Totsch, Peng Li, Chisom O. Odii, Heather Shelton, Robert E. Sorge, and Ellen Mary Lavoie Smith. 2025. "A Pilot Study: Comparative Effects of Green Tea Extract and Duloxetine on Oxaliplatin-Induced Allodynia in a Murine Model" Metabolites 15, no. 10: 680. https://doi.org/10.3390/metabo15100680
APA StyleDaniel, M., Totsch, S., Li, P., Odii, C. O., Shelton, H., Sorge, R. E., & Smith, E. M. L. (2025). A Pilot Study: Comparative Effects of Green Tea Extract and Duloxetine on Oxaliplatin-Induced Allodynia in a Murine Model. Metabolites, 15(10), 680. https://doi.org/10.3390/metabo15100680








