Multi-Omics Insights into Altered Flavonoid Metabolism Underlying Skin Color Variation in a Bud Mutant of Vitis vinifera Zaoheibao
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Metabolite Profiling and Data Analysis
2.3. Transcriptome Sequencing and Analysis
2.4. Statistical Analysis and Data Visualization
3. Results
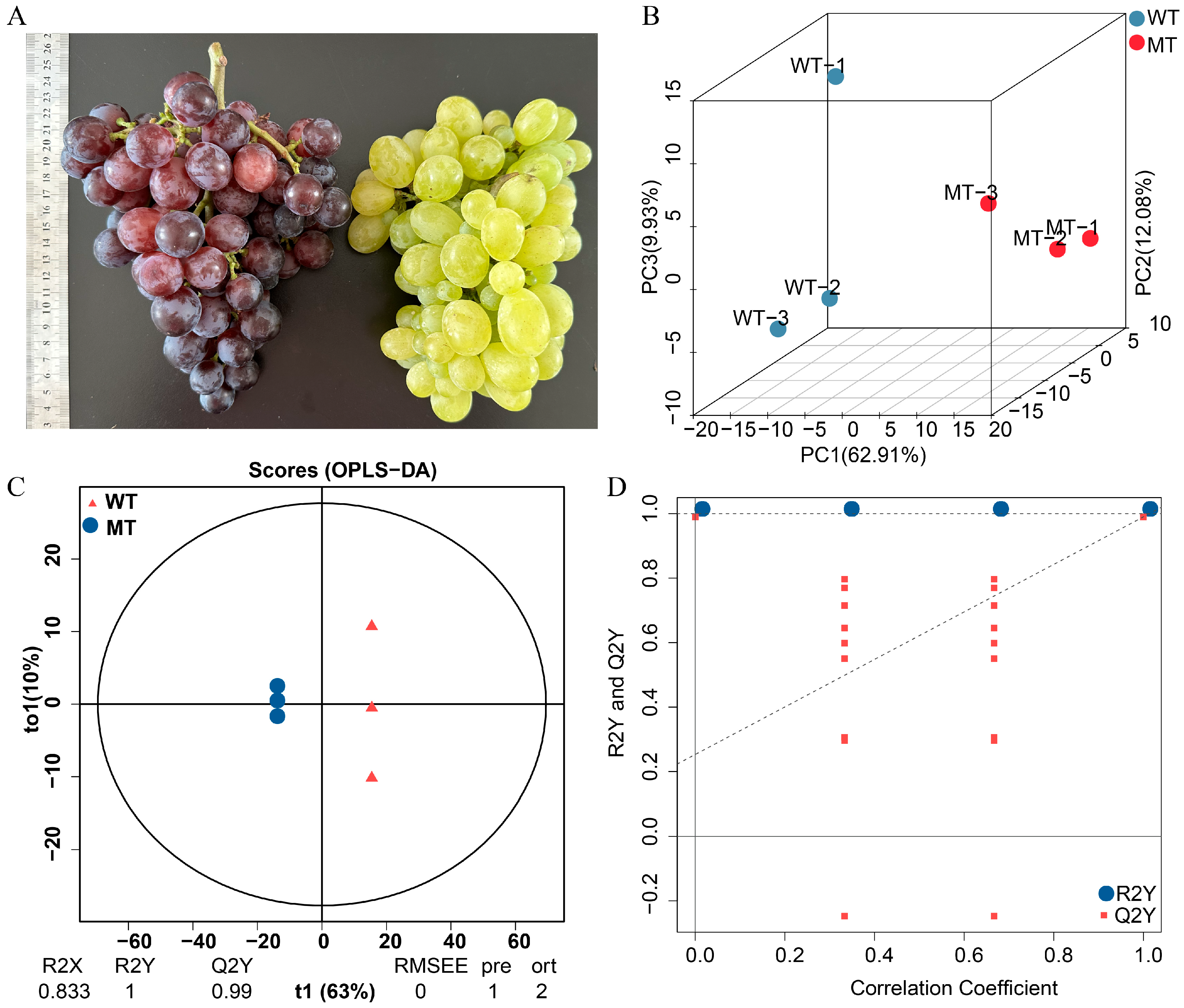
3.1. Phenotypic and Metabolomic Separation Between WT and MT Zaoheibao Grape Peels
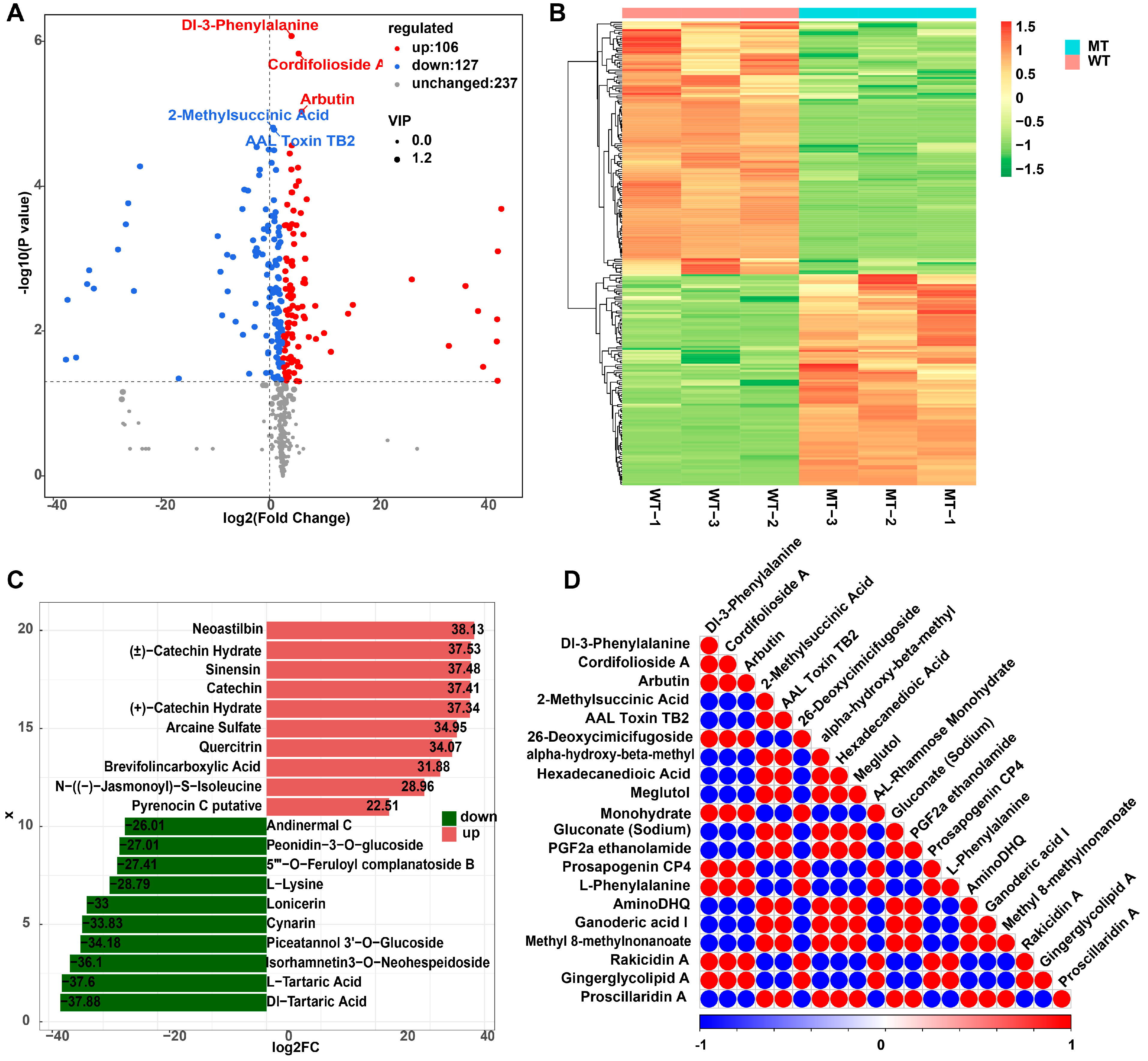
3.2. Differential Metabolite Analysis Between WT and MT Peels
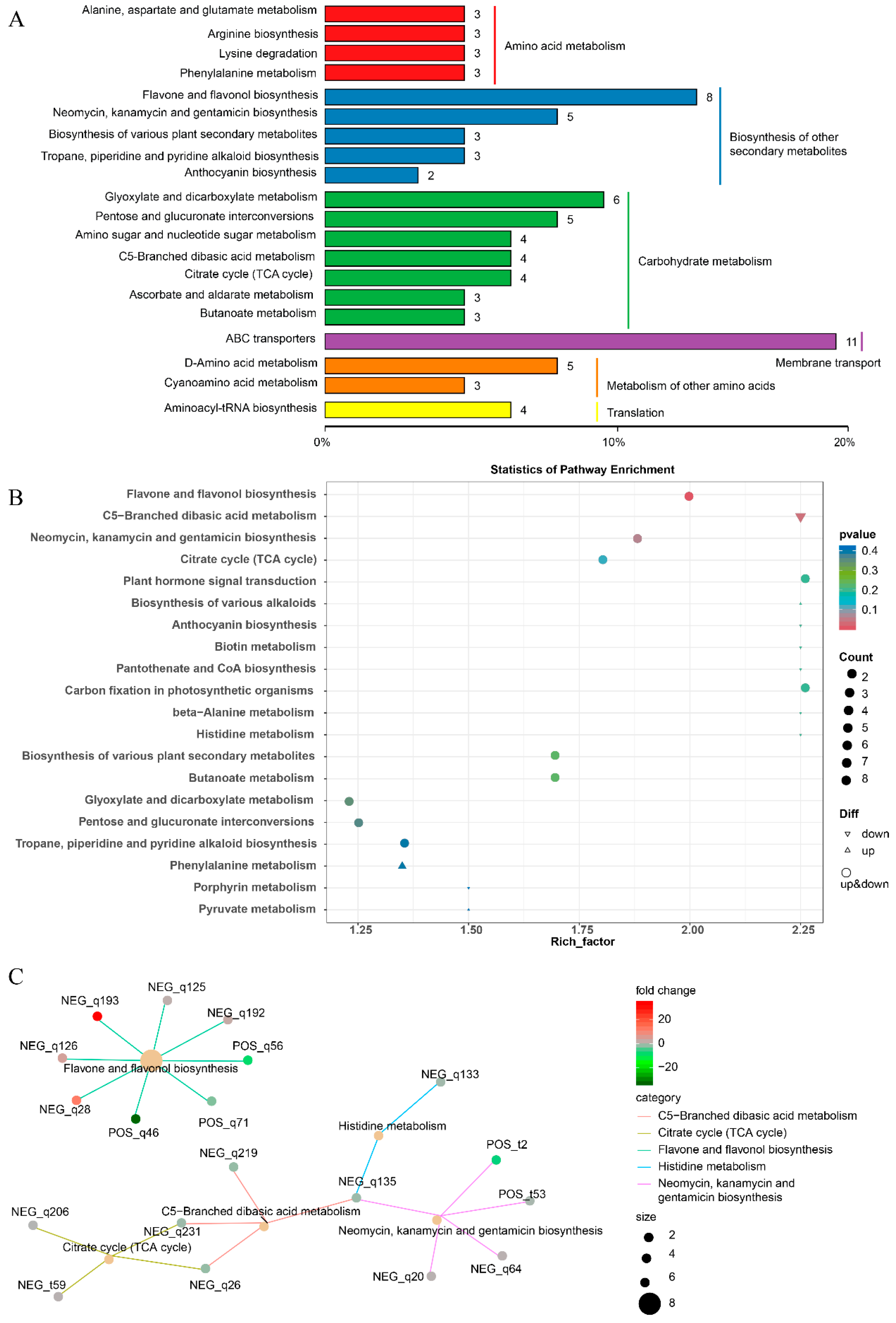
3.3. KEGG Pathway Enrichment Analysis of DAM
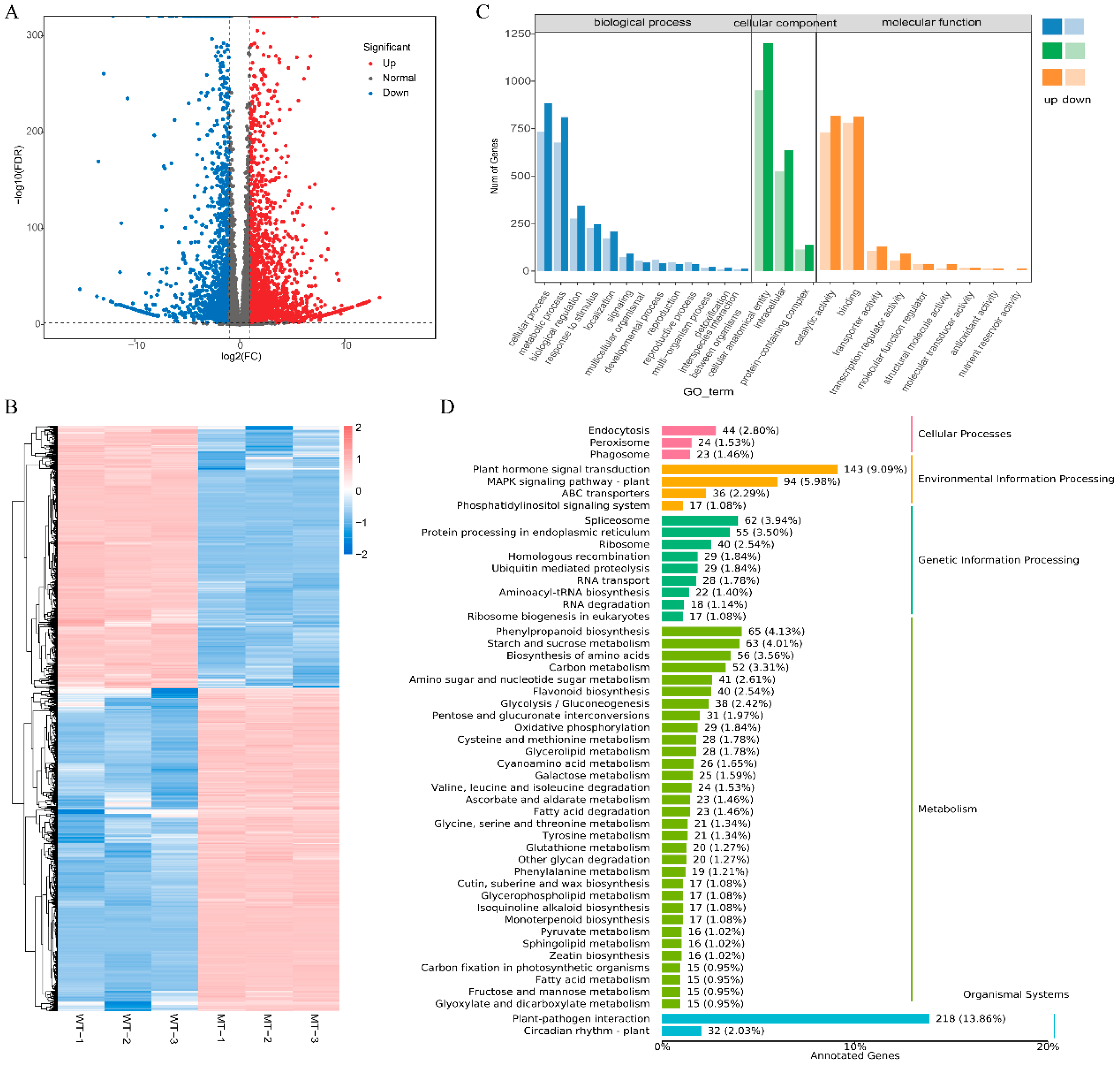
3.4. Transcriptomic Analysis of DEGs
3.5. Integrated Transcriptome and Metabolome Analysis
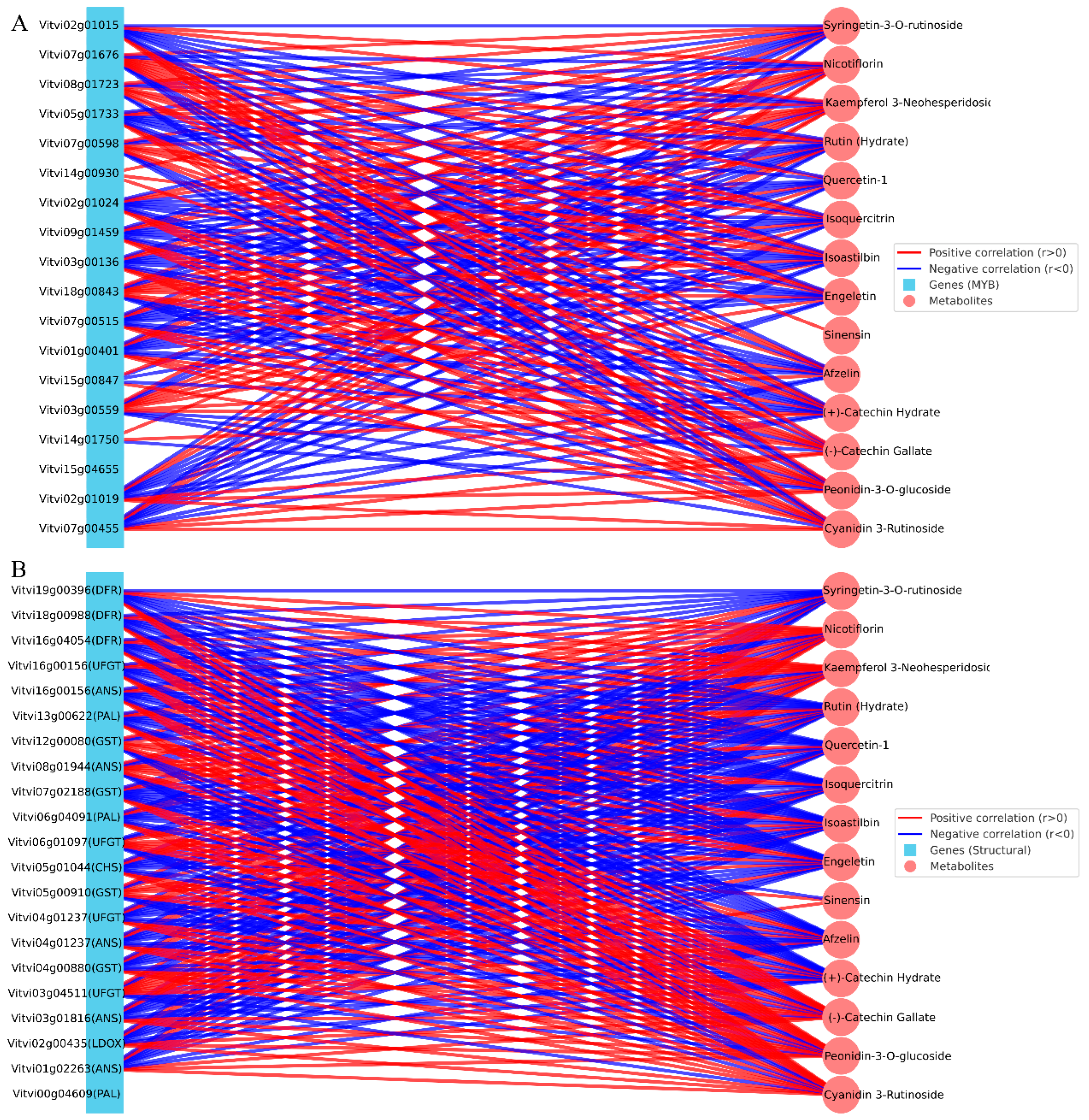
3.6. Integrated Correlation Network Between DEGs and DAMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS/LDOX | Anthocyanidin synthase/Leucoanthocyanidin dioxygenase |
| bHLH | Basic helix–loop–helix (transcription factor) |
| CHI | Chalcone isomerase |
| CHS | Chalcone synthase |
| C4H | Cinnamate-4-hydroxylase |
| DAMs | Differentially accumulated metabolites |
| DEGs | Differentially expressed genes |
| DFR | Dihydroflavonol reductase |
| F3H | Flavanone 3-hydroxylase |
| F3′H | Flavonoid 3′-hydroxylase |
| FPKM | Fragments per kilobase of transcript per million mapped reads |
| GST | Glutathione S-transferase |
| HMDB | Human Metabolome Database |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC–MS | Liquid chromatography–mass spectrometry |
| LMSD | LIPID MAPS Structure Database |
| MBW | MYB–bHLH–WD40 transcriptional complex |
| MRM | Multiple reaction monitoring |
| MT | Mutant (bud sport of Vitis vinifera ‘Zaoheibao’) |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PAL | Phenylalanine ammonia-lyase |
| PCA | Principal component analysis |
| PC | Principal component |
| QC | Quality control |
| RNA-seq | RNA sequencing |
| UFGT | UDP-glucose: flavonoid 3-O-glucosyltransferase |
| VIP | Variable importance in projection |
| WT | Wild type (Vitis vinifera ‘Zaoheibao’) |
| WGCNA | Weighted gene co-expression network analysis |
References
- Ranganath, K.G. Pigments that colour our fruits: An overview. Erwerbs-Obstbau 2022, 64, 535–547. [Google Scholar] [CrossRef]
- Muhammad, N.; Luo, Z.; Yang, M.; Li, X.; Liu, Z.; Liu, M. The joint role of the late anthocyanin biosynthetic UFGT-encoding genes in the flowers and fruits coloration of horticultural plants. Sci. Hortic. 2022, 301, 111110. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jia, Y.; Wu, Y.; Ren, F. Anthocyanin and its bioavailability, health benefits, and applications: A comprehensive review. Food Rev. Int. 2024, 40, 3666–3689. [Google Scholar] [CrossRef]
- Giacosa, S.; Ferrero, L.; Paissoni, M.A.; Segade, S.R.; Gerbi, V.; Rolle, L. Grape skin anthocyanin extraction from red varieties during simulated maceration: Influence of grape seeds and pigments adsorption on their surface. Food Chem. 2023, 424, 136463. [Google Scholar] [CrossRef]
- Foster, T.M.; Aranzana, M.J. Attention sports fans! The far-reaching contributions of bud sport mutants to horticulture and plant biology. Hortic. Res. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, D.P.; Dogra, R.K.; Singh, G.; Sharma, R.; Kumar, P. Identification and characterization of Apple (Malus× domestica Borkh.) bud sports mutations in the apple growing Northwestern Himalayan region. Sci. Hortic. 2022, 304, 111308. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, R.; Feng, W.; Zhang, S.; Wang, Z.; Qiu, Z.; Zhang, J.; Ma, F.; Xu, L. Proteomic analysis of ‘Zaosu’pear (Pyrus bretschneideri Rehd.) and its early-maturing bud sport. Plant Sci. 2014, 224, 120–135. [Google Scholar] [CrossRef]
- Leng, F.; Ye, Y.; Zhu, X.; Zhang, Y.; Zhang, Z.; Shi, J.; Shen, N.; Jia, H.; Wang, L. Comparative transcriptomic analysis between ‘Summer Black’ and its bud sport ‘Nantaihutezao’ during developmental stages. Planta 2021, 253, 23. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.H.; Fan, X.C.; Zhang, Y.; Sun, L.; Jiang, J.F.; Guo, D.L. Advances in research on bud mutantation mechanism in grape. J. Fruit Sci. 2022, 39, 474–482. [Google Scholar]
- Jun, C.; Xiaoping, T.; Dengke, L.; Xiaohe, M.; Zhigang, D. ‘Zaoheibao’—An Early-maturing, Good Quality and Large Berry Grape Variety. Acta Hortic. Sin. 2001, 28, 277. [Google Scholar]
- Lu, R.; Song, M.; Wang, Z.; Zhai, Y.; Hu, C.; Perl, A.; Ma, H. Independent flavonoid and anthocyanin biosynthesis in the flesh of a red-fleshed table grape revealed by metabolome and transcriptome co-analysis. BMC Plant Biol. 2023, 23, 361. [Google Scholar] [CrossRef]
- Xie, S.; Lei, Y.; Chen, H.; Li, J.; Chen, H.; Zhang, Z. R2R3-MYB transcription factors regulate anthocyanin biosynthesis in grapevine vegetative tissues. Front. Plant Sci. 2020, 11, 527. [Google Scholar] [CrossRef]
- Francisco, R.M.; Regalado, A.; Ageorges, A.; Burla, B.J.; Bassin, B.; Eisenach, C.; Zarrouk, O.; Vialet, S.; Marlin, T.; Chaves, M.M. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-glucosides. Plant Cell 2013, 25, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Transcriptional control of flavonoid biosynthesis: Fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal. Behav. 2014, 9, e27522. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Oglesby, L.; Ananga, A.; Obuya, J.; Ochieng, J.; Cebert, E.; Tsolova, V. Anthocyanin accumulation in muscadine berry skins is influenced by the expression of the MYB transcription factors, MybA1, and MYBCS1. Antioxidants 2016, 5, 35. [Google Scholar] [CrossRef]
- Jiu, S.; Guan, L.; Leng, X.; Zhang, K.; Haider, M.S.; Yu, X.; Zhu, X.; Zheng, T.; Ge, M.; Wang, C. The role of VvMYBA2r and VvMYBA2w alleles of the MYBA2 locus in the regulation of anthocyanin biosynthesis for molecular breeding of grape (Vitis spp.) skin coloration. Plant Biotechnol. J. 2021, 19, 1216–1239. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, G.; Liu, L.; Zhang, Q.; Han, Z.; Chen, X.; Li, B. A R2R3-MYB transcription factor, VvMYBC2L2, functions as a transcriptional repressor of anthocyanin biosynthesis in grapevine (Vitis vinifera L.). Molecules 2018, 24, 92. [Google Scholar] [CrossRef]
- Cavallini, E.; Zenoni, S.; Finezzo, L.; Guzzo, F.; Zamboni, A.; Avesani, L.; Tornielli, G.B. Functional diversification of grapevine MYB5a and MYB5b in the control of flavonoid biosynthesis in a petunia anthocyanin regulatory mutant. Plant Cell Physiol. 2014, 55, 517–534. [Google Scholar] [CrossRef]
- Wang, N.; Qu, C.; Jiang, S.; Chen, Z.; Xu, H.; Fang, H.; Su, M.; Zhang, J.; Wang, Y.; Liu, W. The proanthocyanidin-specific transcription factor Md MYBPA 1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018, 96, 39–55. [Google Scholar] [CrossRef]
- Yao, G.; Ming, M.; Allan, A.C.; Gu, C.; Li, L.; Wu, X.; Wang, R.; Chang, Y.; Qi, K.; Zhang, S. Map-based cloning of the pear gene MYB 114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. Plant J. 2017, 92, 437–451. [Google Scholar] [CrossRef]
- Ge, M.; Sadeghnezhad, E.; Hakeem, A.; Zhong, R.; Wang, P.; Shangguan, L.; Fang, J. Integrated transcriptomic and metabolic analyses unveil anthocyanins biosynthesis metabolism in three different color cultivars of grape (Vitis vinifera L.). Sci. Hortic. 2022, 305, 111418. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, Q.; Wu, H.; Ma, X.; Xu, W.; Li, L.; Liang, Q.; Wang, S. Metabolomics and transcriptomics analyses reveal the potential molecular mechanisms of flavonoids and carotenoids in guava pulp with different colors. Sci. Hortic. 2022, 305, 111384. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Cui, L.; Li, Y.; Liang, Y.; Wang, S.; Chen, Y.; Zhou, L.; Zhang, Y.; Li, F. Transcriptome and metabolome analyses reveal anthocyanins pathways associated with fruit color changes in plum (Prunus salicina Lindl.). PeerJ 2022, 10, e14413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, C.; Yan, P.; An, S.; Ma, L.; Zheng, Q.; Deng, Y.; Chen, Q. Metabolomic and Transcriptomic Analyses Reveal the Factors Underlying Mature Fruit Pericarp Color Variations in the ‘Xinli No. 7’ Pear (Pyrus sinkiangensis). Metabolites 2025, 15, 81. [Google Scholar] [CrossRef]
- Ding, R.; Che, X.; Shen, Z.; Zhang, Y. Metabolome and transcriptome profiling provide insights into green apple peel reveals light-and UV-B-responsive pathway in anthocyanins accumulation. BMC Plant Biol. 2021, 21, 351. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, H.; Kaur, R.; Garg, R.; Prasad, R.; Assouguem, A.; Kara, M.; Bahhou, J. A Review on the Nutritional Value and Health Benefits of Different Parts of Grape (Vitis vinifera L.). Trop. J. Nat. Prod. Res. 2023, 7, 3874–3880. [Google Scholar]
- Furiya, T.; Suzuki, S.; Sueta, T.; Takayanagi, T. Molecular characterization of a bud sport of Pinot gris bearing white berries. Am. J. Enol. Vitic. 2009, 60, 66–73. [Google Scholar] [CrossRef]
- Bao, X.; Dong, J.; Niu, M.; Wang, Z.; Xu, G. Transcriptome and Metabolome Analyses of Aroma Differences between Chardonnay and a Chardonnay Bud Sport. Molecules 2024, 29, 3671. [Google Scholar] [CrossRef]
- Kumar, A.P.; Bhasker, K.; Nikhil, B.; Srinivas, P. Role of phenylpropanoids and flavonoids in plant defense mechanism. Int. J. Env. Clim. Change 2023, 13, 2951–2960. [Google Scholar] [CrossRef]
- Sun, L.; Fan, X.; Zhang, Y.; Jiang, J.; Sun, H.; Liu, C. Transcriptome analysis of genes involved in anthocyanins biosynthesis and transport in berries of black and white spine grapes (Vitis davidii). Hereditas 2016, 153, 17. [Google Scholar] [CrossRef]
- Ferreira, V.; Matus, J.T.; Pinto-Carnide, O.; Carrasco, D.; Arroyo-García, R.; Castro, I. Genetic analysis of a white-to-red berry skin color reversion and its transcriptomic and metabolic consequences in grapevine (Vitis vinifera cv. ‘Moscatel Galego’). BMC Genom. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ruan, J.; Mumm, R.; de Vos, R.C.; Liu, M.-Y. Dynamic changes in the antioxidative defense system in the tea plant reveal the photoprotection-mediated temporal accumulation of flavonoids under full sunlight exposure. Plant Cell Physiol. 2022, 63, 1695–1708. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Tang, R.; Wang, R.; Ahmad, S.; Liu, Z.; Peng, D. Research Progress on Anthocyanin-Mediated Regulation of ‘Black’ Phenotypes of Plant Organs. Curr. Issues Mol. Biol. 2023, 45, 7242–7256. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, C.; Li, Y.; Zeng, Y.; Jiang, J.; Wu, L.; Yang, S.; Yuan, D.; Chen, L.; Pei, Z.; et al. Transcriptomic Insights into Higher Anthocyanin Accumulation in ‘Summer Black’ Table Grapes in Winter Crop Under Double-Cropping Viticulture System. Plants 2025, 14, 26. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.-X.; Zhang, Z.; Fang, Y.; Li, D.; Chen, X.-S.; Feng, S.-Q. Ethylene precisely regulates anthocyanin synthesis in apple via a module comprising MdEIL1, MdMYB1, and MdMYB17. Hortic. Res. 2022, 9, uhac034. [Google Scholar] [CrossRef]
- Meng, J.; Sun, S.; Li, A.; Pan, L.; Duan, W.; Cui, G.; Xu, J.; Niu, L.; Wang, Z.; Zeng, W. A NAC transcription factor, PpNAC1, regulates the expression of PpMYB10. 1 to promote anthocyanin biosynthesis in the leaves of peach trees in autumn. Hortic. Adv. 2023, 1, 8. [Google Scholar] [CrossRef]
- Li, S.; Qin, Y.; Jing, S.; Wang, D.; Zhang, Z.; Qin, Y.; Hu, G.; Zhao, J. Metabolome and transcriptome analyses reveal the molecular mechanisms of LcMYB1 regulating anthocyanin accumulation in litchi hairy roots. Plant Physiol. Biochem. 2023, 200, 107749. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Zhuge, Y.; Zhang, M.; Liu, C.; Jia, H.; Fang, J. Integrative Analyses of Metabolomes and Transcriptomes Provide Insights into Flavonoid Variation in Grape Berries. J. Agric. Food Chem. 2021, 69, 12354–12367. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, W.; Xu, J.; Lu, X.; Liu, Y. Anthocyanin biosynthesis induced by MYB transcription factors in plants. Int. J. Mol. Sci. 2022, 23, 11701. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Dai, X.; Zhang, L.; Zhu, Y.; Wang, M.; Xun, Z.; Zhao, Q.; Zhang, J. Multi-Omics Insights into Altered Flavonoid Metabolism Underlying Skin Color Variation in a Bud Mutant of Vitis vinifera Zaoheibao. Metabolites 2025, 15, 675. https://doi.org/10.3390/metabo15100675
Huang L, Dai X, Zhang L, Zhu Y, Wang M, Xun Z, Zhao Q, Zhang J. Multi-Omics Insights into Altered Flavonoid Metabolism Underlying Skin Color Variation in a Bud Mutant of Vitis vinifera Zaoheibao. Metabolites. 2025; 15(10):675. https://doi.org/10.3390/metabo15100675
Chicago/Turabian StyleHuang, Liping, Xi Dai, Linan Zhang, Yue Zhu, Min Wang, Zhili Xun, Qifeng Zhao, and Jiancheng Zhang. 2025. "Multi-Omics Insights into Altered Flavonoid Metabolism Underlying Skin Color Variation in a Bud Mutant of Vitis vinifera Zaoheibao" Metabolites 15, no. 10: 675. https://doi.org/10.3390/metabo15100675
APA StyleHuang, L., Dai, X., Zhang, L., Zhu, Y., Wang, M., Xun, Z., Zhao, Q., & Zhang, J. (2025). Multi-Omics Insights into Altered Flavonoid Metabolism Underlying Skin Color Variation in a Bud Mutant of Vitis vinifera Zaoheibao. Metabolites, 15(10), 675. https://doi.org/10.3390/metabo15100675




