Longitudinal Plasma Lipidomics Reveals Distinct Signatures Following Surgery in Patients with Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Sample Collection
2.2. Sample Preparation and Lipidomics Analysis
2.3. Statistical Analysis
2.4. Machine Learning
3. Results
3.1. Plasma Lipidomic Signatures Differ by Treatment Stage
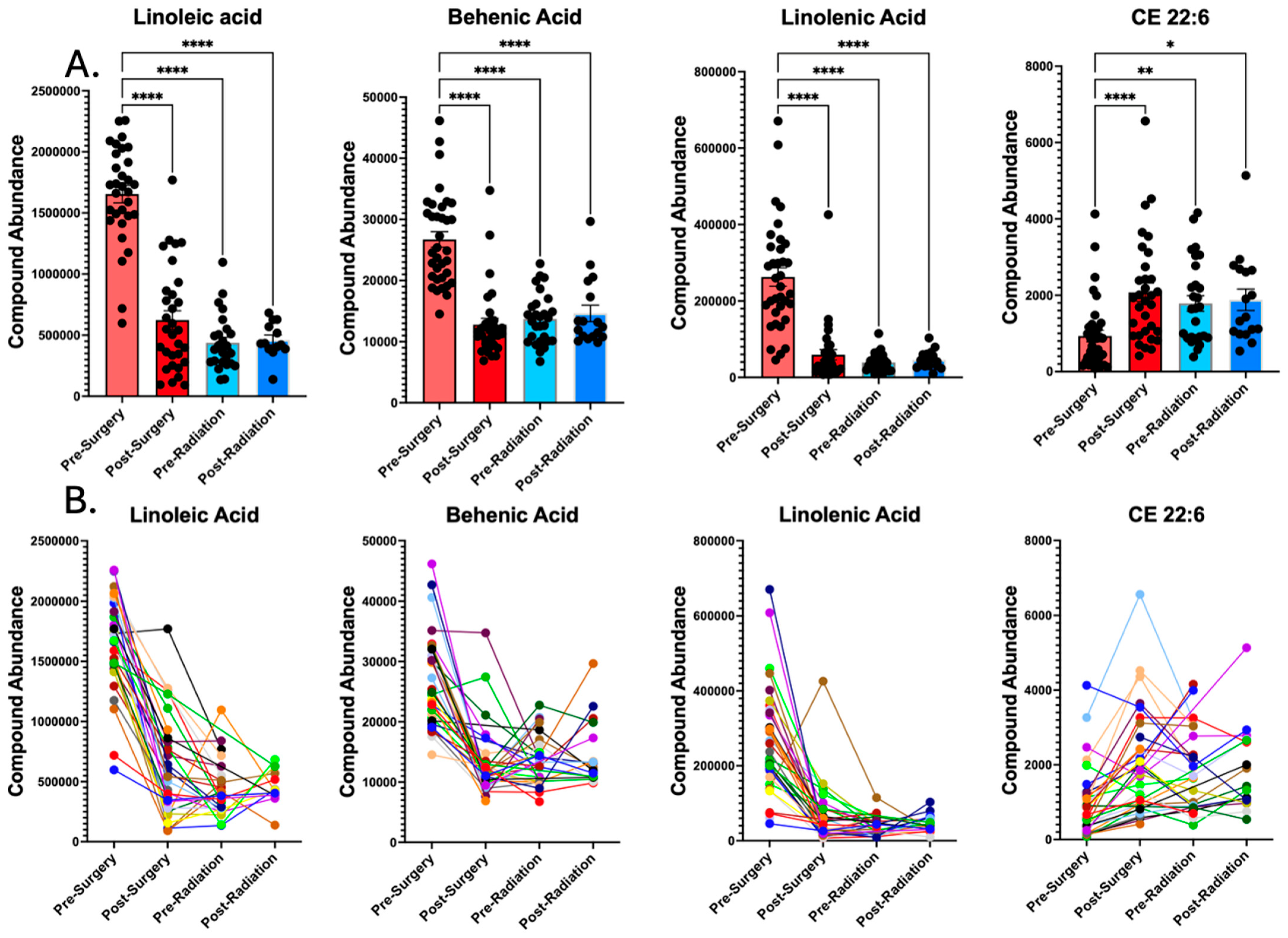
3.2. Surgery Alters the Plasma Lipidome
3.3. Chemoradiation Is Not Associated with Significant Plasma Lipidomic Changes
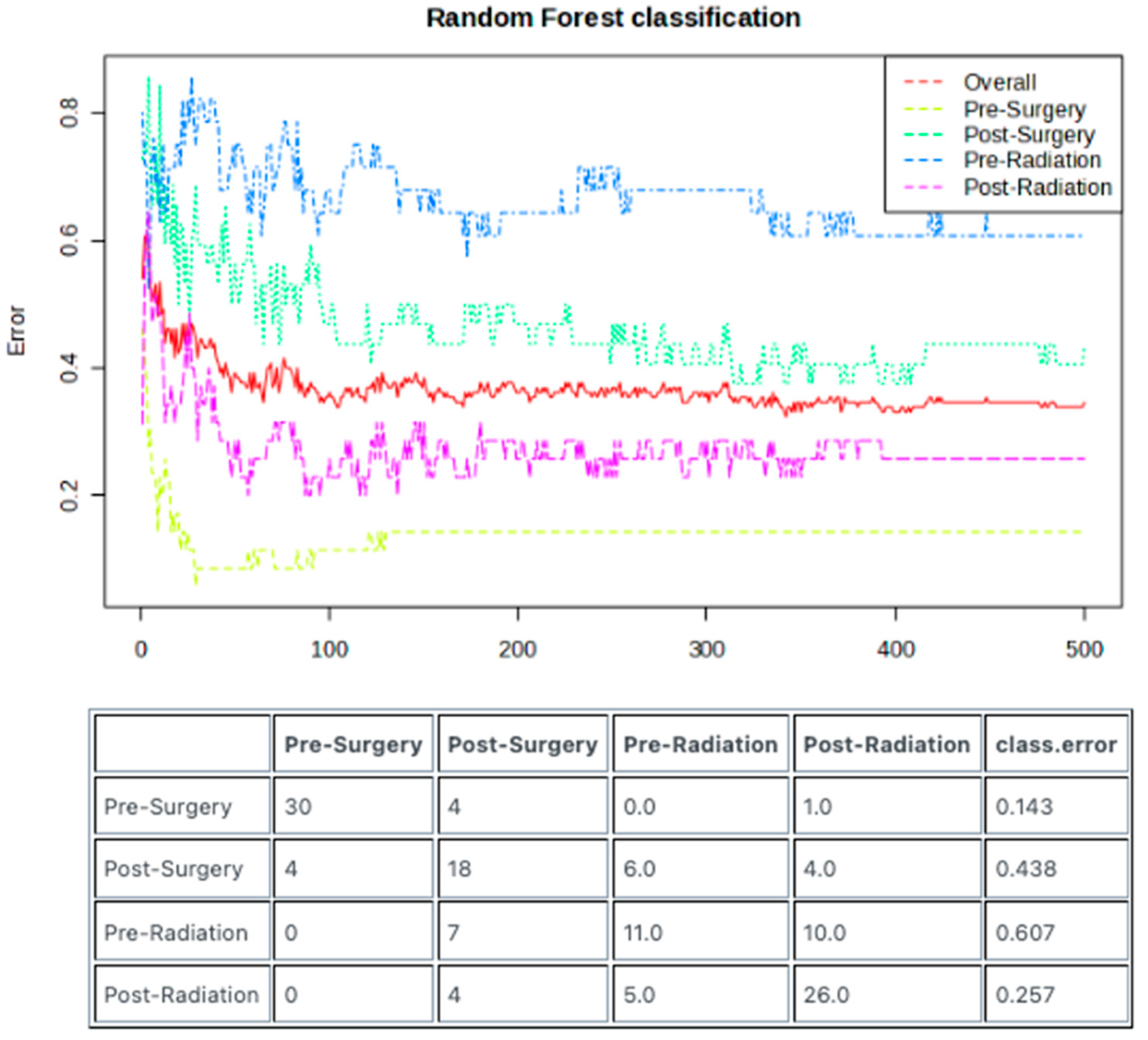
3.4. Random Forest Model Enables Predictive Sample Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Garcia, J.H.; Jain, S.; Aghi, M.K. Metabolic Drivers of Invasion in Glioblastoma. Front. Cell Dev. Biol. 2021, 9, 683276. [Google Scholar] [CrossRef]
- Aboubechara, J.P.; Aboud, O. Metabolic Risk Factors and Survival in Patients with Glioblastoma. Cancers 2024, 16, 3666. [Google Scholar] [CrossRef]
- Lucas, D.; Carvalho, B.; Tuna, R.; Linhares, P. Metabolic Syndrome and Survival in Glioblastoma Patients: Retrospective Cohort Study and Review of the Literature. Cureus 2024, 16, e53641. [Google Scholar] [CrossRef] [PubMed]
- McManus, E.J.; Frampton, C.; Tan, A.; Phillips, M.C.L. Metabolics risk factors in a New Zealand glioblastoma cohort. Neurooncol. Pract. 2022, 9, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.R.; Ostrom, Q.T.; Schroer, J.; Vengoechea, J.; Li, L.; Gerson, S.; Nock, C.J.; Machtay, M.; Selman, W.; Lo, S.; et al. Association of metabolic syndrome with glioblastoma: A retrospective cohort study and review. Neurooncol. Pract. 2020, 7, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Bettegowda, C.; Mellinghoff, I.K.; Warren, K.E.; Ahluwalia, M.S.; De Groot, J.F.; Galanis, E.; Gilbert, M.R.; Jaeckle, K.A.; Le Rhun, E.; et al. Liquid biopsy in gliomas: A RANO review and proposals for clinical applications. Neuro Oncol. 2022, 24, 855–871. [Google Scholar] [CrossRef]
- Mair, R.; Mouliere, F. Cell-free DNA technologies for the analysis of brain cancer. Br. J. Cancer 2022, 126, 371–378. [Google Scholar] [CrossRef]
- Ferrasi, A.C.; Puttini, R.; Galvani, A.F.; Hamamoto Filho, P.T.; Delafiori, J.; Argente, V.D.; de Oliveira, A.N.; Dias-Audibert, F.L.; Catharino, R.R.; Silva, O.C.; et al. Metabolomics Approach Reveals Important Glioblastoma Plasma Biomarkers for Tumor Biology. Int. J. Mol. Sci. 2023, 24, 8813. [Google Scholar] [CrossRef]
- Fontanilles, M.; Heisbourg, J.D.; Daban, A.; Di Fiore, F.; Pépin, L.F.; Marguet, F.; Langlois, O.; Alexandru, C.; Tennevet, I.; Ducatez, F.; et al. Metabolic remodeling in glioblastoma: A longitudinal multi-omics study. Acta Neuropathol. Commun. 2024, 12, 162. [Google Scholar] [CrossRef]
- Godlewski, A.; Mojsak, P.; Pienkowski, T.; Lyson, T.; Mariak, Z.; Reszec, J.; Kaminski, K.; Moniuszko, M.; Kretowski, A.; Ciborowski, M. Metabolomic profiling of plasma from glioma and meningioma patients based on two complementary mass spectrometry techniques. Metabolomics 2025, 21, 33. [Google Scholar] [CrossRef]
- Zhao, H.; Heimberger, A.B.; Lu, Z.; Wu, X.; Hodges, T.R.; Song, R.; Shen, J. Metabolomics profiling in plasma samples from glioma patients correlates with tumor phenotypes. Oncotarget 2016, 7, 20486–20495. [Google Scholar] [CrossRef]
- Divé, I.; Hahnefeld, L.; Wenger, K.J.; Kögel, D.; Steinbach, J.; Geisslinger, G.; Ronellenfitsch, M.W.; Tegeder, I. Plasma lipidomic and metabolomic profiles in high-grade glioma patients before and after 72-h presurgery water-only fasting. Mol. Oncol. 2025, 19, 2249–2269. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, N.; Wang, G.; Zhang, Y.; Song, H.; Yuan, Y.; Yang, C.; Jin, Y.; Zhang, Z.; Zhang, L.; et al. Metabolic detection of malignant brain gliomas through plasma lipidomic analysis and support vector machine-based machine learning. EBioMedicine 2022, 81, 104097. [Google Scholar] [CrossRef] [PubMed]
- Muller Bark, J.; Karpe, A.V.; Doecke, J.D.; Leo, P.; Jeffree, R.L.; Chua, B.; Day, B.W.; Beale, D.J.; Punyadeera, C. A pilot study: Metabolic profiling of plasma and saliva samples from newly diagnosed glioblastoma patients. Cancer Med. 2023, 12, 11427–11437. [Google Scholar] [CrossRef] [PubMed]
- Aboud, O.; Liu, Y.A.; Fiehn, O.; Brydges, C.; Fragoso, R.; Lee, H.S.; Riess, J.; Hodeify, R.; Bloch, O. Application of Machine Learning to Metabolomic Profile Characterization in Glioblastoma Patients Undergoing Concurrent Chemoradiation. Metabolites 2023, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Maraqah, H.H.; Aboubechara, J.P.; Abu-Asab, M.S.; Lee, H.S.; Aboud, O. Excessive lipid production shapes glioma tumor microenvironment. Ultrastruct. Pathol. 2024, 48, 367–377. [Google Scholar] [CrossRef]
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef]
- Fan, S.; Kind, T.; Cajka, T.; Hazen, S.L.; Tang, W.H.W.; Kaddurah-Daouk, R.; Irvin, M.R.; Arnett, D.K.; Barupal, D.K.; Fiehn, O. Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data. Anal. Chem. 2019, 91, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.; Barnett, G.H.; Golubic, M. The eicosanoid cascade: Possible role in gliomas and meningiomas. J. Clin. Pathol. 2004, 57, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Novel metabolism of docosahexaenoic acid in neural cells. J. Biol. Chem. 2007, 282, 18661–18665. [Google Scholar] [CrossRef] [PubMed]
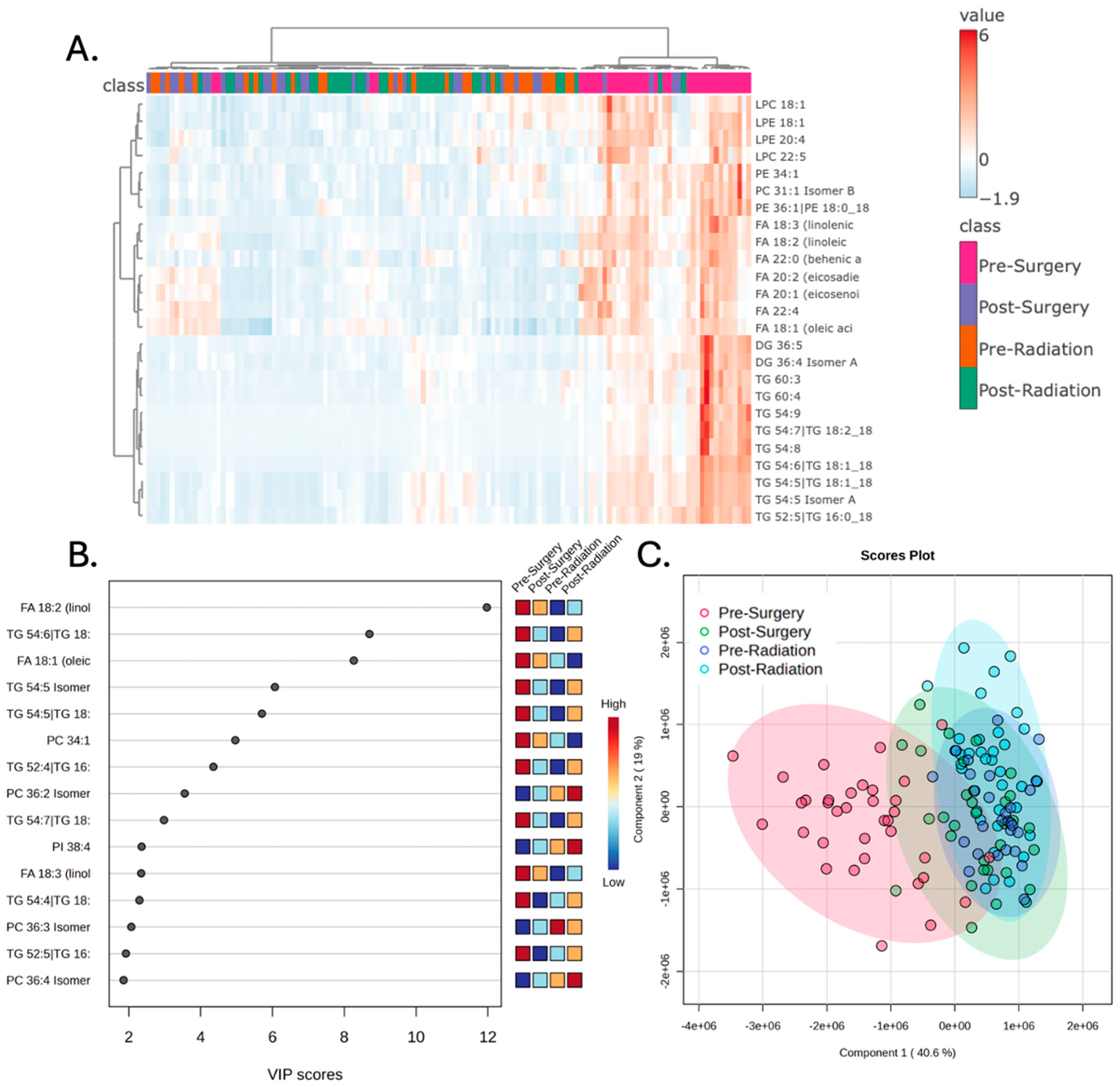
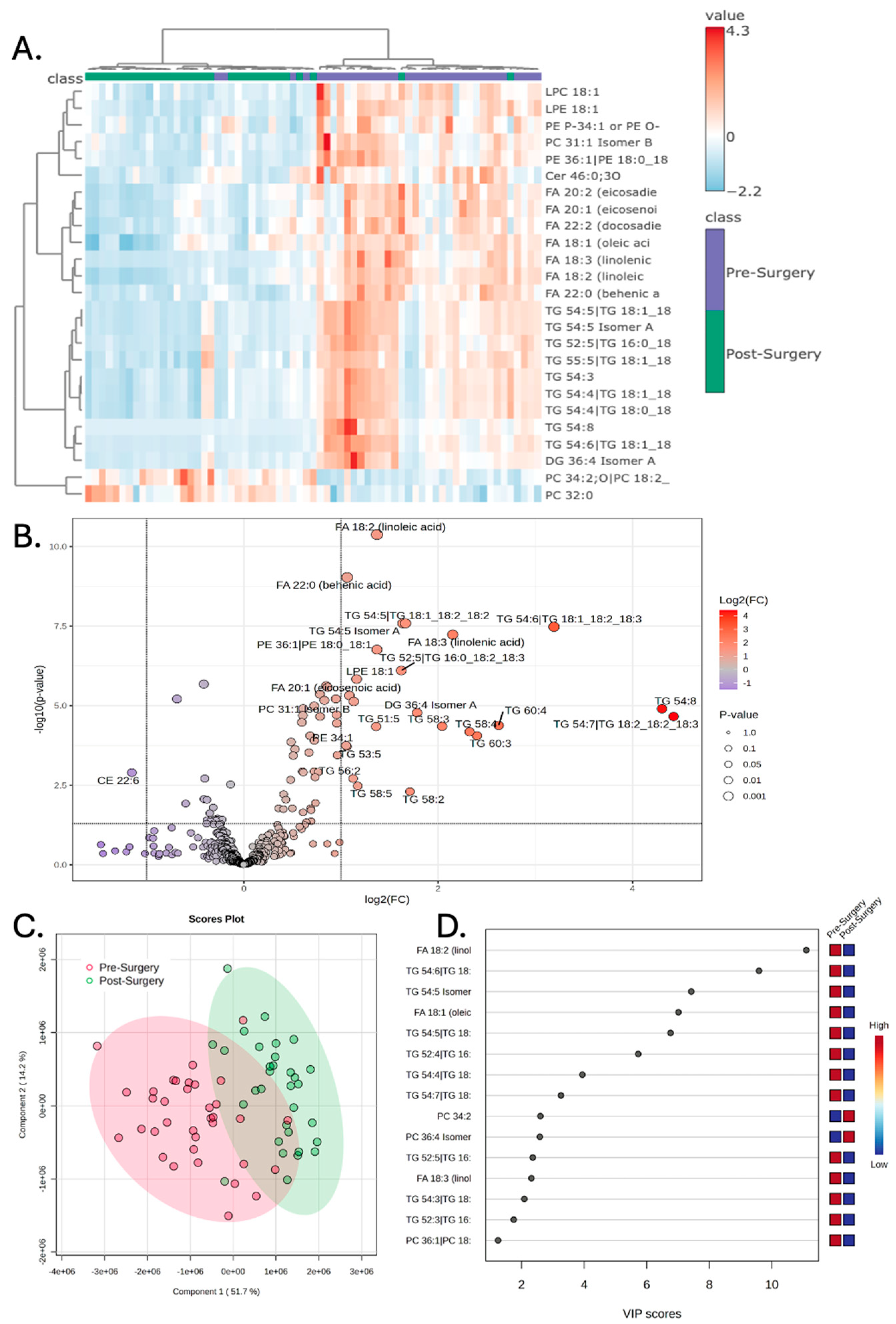
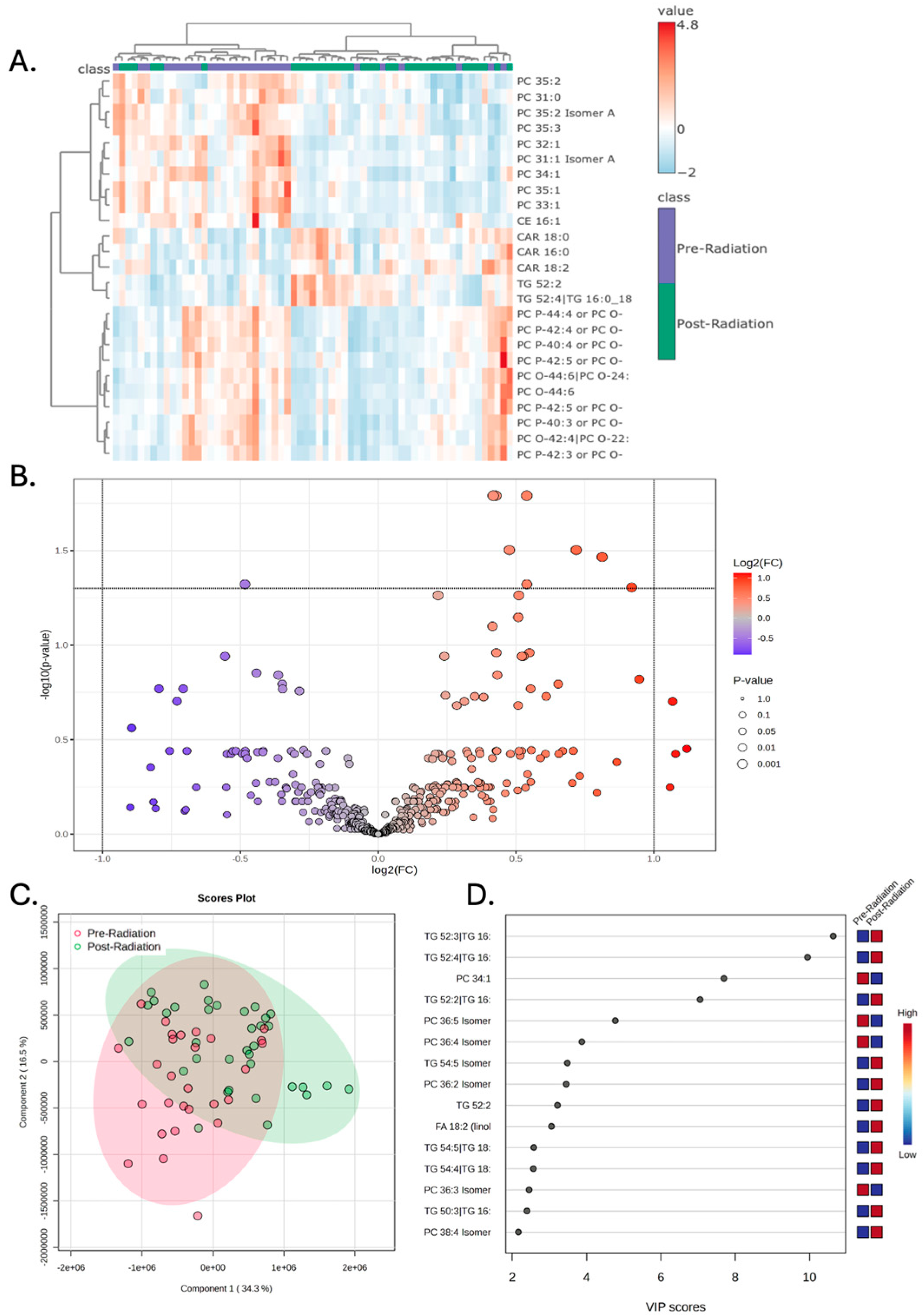
| Patient ID | Sex | Ethnicity | Diagnosis Age (years) | BMI at Diagnosis | Pre-Surgery | Post-Surgery | Pre-Radiation | Post-Radiation |
|---|---|---|---|---|---|---|---|---|
| 1 | M | White | 60 | 40 | X | X | X | X |
| 2 | M | White | 72 | 30 | X | X | X | |
| 3 | M | Hispanic | 43 | 28 | X | X | X | |
| 4 | M | Asian | 49 | 57 | X | X | X | |
| 5 | F | White | 78 | 23 | X | X | ||
| 6 | M | Hispanic | 65 | 22 | X | X | X | |
| 7 | M | White | 72 | 41 | X | X | X | |
| 8 | M | White | 80 | 24 | X | X | X | X |
| 9 | F | White | 61 | 27 | X | X | X | |
| 10 | F | White | 69 | 25 | X | X | X | |
| 11 | M | Indian | 60 | 27 | X | X | X | |
| 12 | F | White | 61 | 25 | X | X | X | |
| 13 | F | White | 52 | 27 | X | X | ||
| 14 | M | White | 62 | 30 | X | X | X | |
| 15 | M | White | 69 | 31 | X | X | X | X |
| 16 | M | White | 67 | 44 | X | X | ||
| 17 | F | White | 82 | 28 | X | X | X | |
| 18 | F | White | 55 | 29 | X | X | ||
| 19 | M | African American | 47 | 37 | X | X | X | X |
| 20 | M | White | 63 | 30 | X | X | X | X |
| 21 | F | White | 86 | 27 | X | X | X | |
| 22 | F | White | 64 | 31 | X | X | X | X |
| 23 | M | White | 56 | 22 | X | X | X | X |
| 24 | F | White | 69 | 26 | X | X | X | X |
| 25 | F | NA | 69 | 27 | X | X | X | |
| 26 | M | White | 64 | 36 | X | X | X | X |
| 27 | M | White | 68 | 28 | X | X | X | |
| 28 | M | White | 69 | 28 | X | X | X | X |
| 29 | F | White | 58 | 27 | X | X | X | |
| 30 | F | white | 66 | 27 | X | X | X | |
| 31 | M | White | 55 | 28 | X | X | X | X |
| 32 | F | White | 60 | 20 | X | X | X | |
| 33 | M | White | 58 | 28 | X | X | X | |
| 34 | M | White | 53 | 30 | X | X | X | |
| 35 | M | White | 58 | 26 | X | X | X | |
| 36 | M | White | 76 | 35 | X |
| Lipid Name | Fold-Change | p-Value |
|---|---|---|
| Linoleic acid | 2.58 | 4.21 × 10−11 |
| Behenic acid | 2.09 | 9.3 × 10−10 |
| TG 54:5 Isomer A | 3.11 | 2.58 × 10−8 |
| TG 54:5 | 3.17 | 2.58 × 10−8 |
| TG 54:6 | 9.14 | 3.33 × 10−8 |
| Linolenic acid | 4.44 | 5.83 × 10−8 |
| PE 36:1 | 2.58 | 1.73 × 10−7 |
| TG 52:5 | 3.07 | 7.84 × 10−7 |
| LPE 18:1 | 2.24 | 1.46 × 10−6 |
| Eicosenoic acid | 2.12 | 4.79 × 10−6 |
| PC 31:1 Isomer B | 2.19 | 7.38 × 10−6 |
| TG 54:8 | 19.8 | 1.25 × 10−5 |
| DG 36:4 Isomer A | 3.44 | 1.66 × 10−5 |
| TG 54:7|TG 18:2_18:2_18:3 | 21.5 | 2.19 × 10−5 |
| TG 60:4 | 6.16 | 4.17 × 10−5 |
| TG 58:3 | 4.11 | 4.45 × 10−5 |
| TG 51:5 | 2.57 | 4.51 × 10−5 |
| TG 58:4 | 5.01 | 6.60 × 10−5 |
| TG 60:3 | 5.28 | 8.77 × 10−5 |
| PE 34:1 | 2.07 | 1.78 × 10−4 |
| TG 53:5 | 2.08 | 1.90 × 10−4 |
| CE 22:6 | 0.45 | 1.26 × 10−3 |
| TG 56:2 | 2.18 | 1.94 × 10−3 |
| TG 58:5 | 2.25 | 3.31 × 10−3 |
| TG 58:2 | 3.27 | 4.99 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboubechara, J.P.; Liu, Y.; Fiehn, O.; Dahabiyeh, L.A.; Fragoso, R.; Lee, H.S.; Riess, J.W.; Hodeify, R.; Bloch, O.; Aboud, O. Longitudinal Plasma Lipidomics Reveals Distinct Signatures Following Surgery in Patients with Glioblastoma. Metabolites 2025, 15, 673. https://doi.org/10.3390/metabo15100673
Aboubechara JP, Liu Y, Fiehn O, Dahabiyeh LA, Fragoso R, Lee HS, Riess JW, Hodeify R, Bloch O, Aboud O. Longitudinal Plasma Lipidomics Reveals Distinct Signatures Following Surgery in Patients with Glioblastoma. Metabolites. 2025; 15(10):673. https://doi.org/10.3390/metabo15100673
Chicago/Turabian StyleAboubechara, John Paul, Yin Liu, Oliver Fiehn, Lina A. Dahabiyeh, Ruben Fragoso, Han Sung Lee, Jonathan W. Riess, Rawad Hodeify, Orin Bloch, and Orwa Aboud. 2025. "Longitudinal Plasma Lipidomics Reveals Distinct Signatures Following Surgery in Patients with Glioblastoma" Metabolites 15, no. 10: 673. https://doi.org/10.3390/metabo15100673
APA StyleAboubechara, J. P., Liu, Y., Fiehn, O., Dahabiyeh, L. A., Fragoso, R., Lee, H. S., Riess, J. W., Hodeify, R., Bloch, O., & Aboud, O. (2025). Longitudinal Plasma Lipidomics Reveals Distinct Signatures Following Surgery in Patients with Glioblastoma. Metabolites, 15(10), 673. https://doi.org/10.3390/metabo15100673









