In Vivo Anti-Inflammatory Activity of Four Edible Cactaceae Flowers from Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Hydroalcoholic Extracts
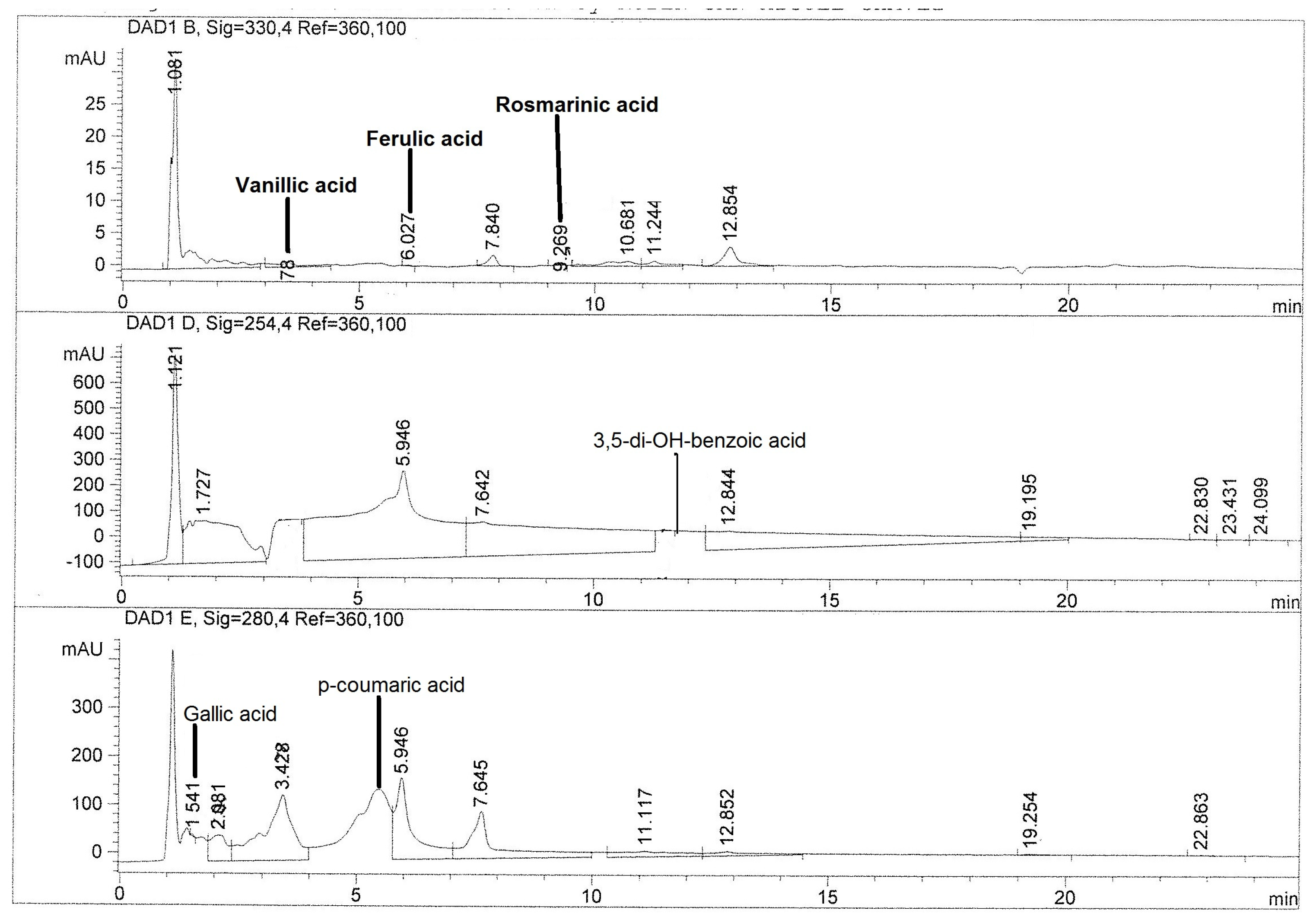
2.3. Quantification of Phenolic Compounds by HPLC
2.4. In Vivo Anti-Inflammatory Activity
2.5. Statistical Analysis
3. Results
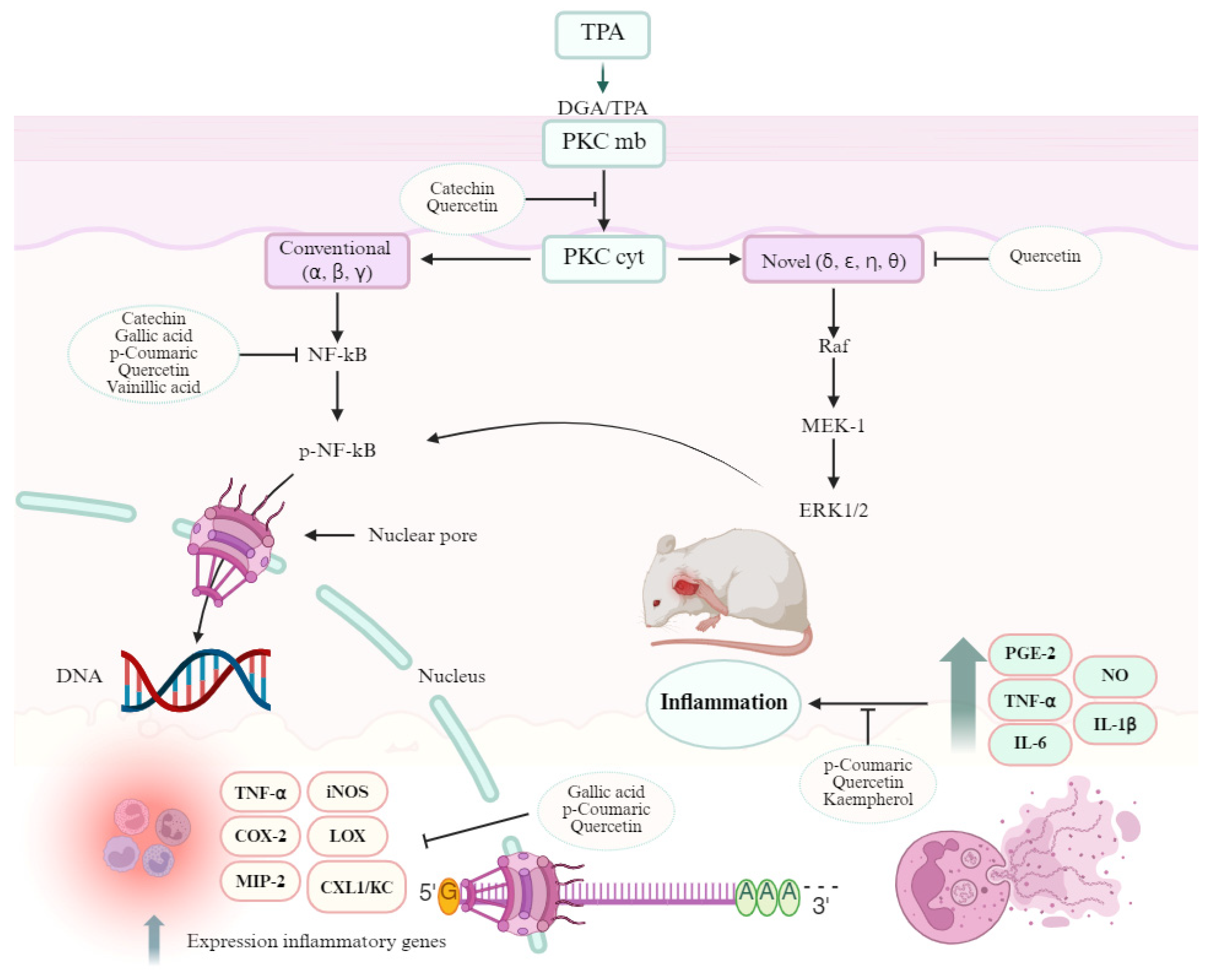
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular Pharmacology of Inflammation: Medicinal Plants as Anti-Inflammatory Agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Natural Products Isolation, 3rd ed.; Humana: Totowa, NJ, USA, 2012; pp. XII, 552. [Google Scholar]
- Benvenuti, S.; Bortolotti, E.; Maggini, R. Antioxidant Power, Anthocyanin Content and Organoleptic Performance of Edible Flowers. Sci. Hortic. 2016, 199, 170–177. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers: Emerging Components in the Diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Wang, T.; Huang, H.; Zhang, Y.; Li, X.; Li, H.; Jiang, Q.; Gao, W. Role of Effective Composition on Antioxidant, Anti-Inflammatory, Sedative-Hypnotic Capacities of 6 Common Edible Lilium Varieties. J. Food Sci. 2015, 80, H857–H868. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Escandón, P.; Ramírez-Hernández, A.; Flores, J.; Juan-Vicedo, J.; Martínez-Falcón, A.P. A Systematic Review on Opuntia (Cactaceae; Opuntioideae) Flower-Visiting Insects in the World with Emphasis on Mexico: Implications for Biodiversity Conservation. Plants 2022, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Cassani, L.; Gomez-Zavaglia, A.; Garcia-Perez, P.; Seyyedi-Mansour, S.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Application of Fermentation for the Valorization of Residues from Cactaceae Family. Food Chem. 2023, 410, 135369. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Underutilized Plants of the Cactaceae Family: Nutritional Aspects and Technological Applications. Food Chem. 2021, 362, 130196. [Google Scholar] [CrossRef]
- Aispuro-Hernández, E.; Vergara-Jiménez, M.J.; Cárdenas-Torres, F.I.; Martínez-Téllez, M.A.; Ontiveros, N. Cactaceae Plants as Sources of Active Bioavailable Phytochemicals. Food Funct. 2022, 13, 9720–9733. [Google Scholar] [CrossRef]
- Pensamiento-Niño, C.A.; Campos-Montiel, R.G.; Añorve-Morga, J.; Ramírez-Moreno, E.; Ascacio-Valdés, J.A.; Hernández-Fuentes, A.D. Nutritional Characterization of the Functional and Antioxidant Activity of Cactus Flowers from Hidalgo, Mexico. Appl. Sci. 2021, 11, 5965. [Google Scholar] [CrossRef]
- Joaquín-Ramos, A.d.J.; López-Palestina, C.U.; Pinedo-Espinoza, J.M.; Altamirano-Romo, S.E.; Santiago-Saenz, Y.O.; Aguirre-Mancilla, C.L.; Gutiérrez-Tlahque, J. Phenolic Compounds, Antioxidant Properties and Antifungal Activity of Jarilla (Barkleyanthus salicifolius ENT#91;KunthENT#93; H. Rob & Brettell). Chil. J. Agric. Res. 2020, 80, 352–360. [Google Scholar]
- Rivero-Pérez, N.; Ayala-Martínez, M.; Zepeda-Bastida, A.; Meneses-Mayo, M.; Ojeda-Ramírez, D. Anti-Inflammatory Effect of Aqueous Extracts of Spent Pleurotus Ostreatus Substrates in Mouse Ears Treated with 12-O-Tetradecanoylphorbol-13-Acetate. Indian J. Pharmacol. 2016, 48, 141–144. [Google Scholar] [CrossRef] [PubMed]
- NOM-062-ZOO-1999; Technical Specifications for the Reproduction, Care, and Use of Laboratory Animals. Ministry of Agriculture, Livestock, Rural Development, Fisheries, and Food: Mexico City, Mexico, 1999.
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological Actions of Opuntia ficus Indica: A Review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) Plant Compounds, Biological Activities and Prospects–A Comprehensive Review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-Inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Skrajda-Brdak, M.; Dąbrowski, G.; Konopka, I. Edible Flowers, a Source of Valuable Phytonutrients and Their pro-Healthy Effects—A Review. Trends Food Sci. Technol. 2020, 103, 179–199. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, B.; Xu, B. An Update on the Health Benefits Promoted by Edible Flowers and Involved Mechanisms. Food Chem. 2021, 340, 127940. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Rivera, D.; Franco, L.A. Topical Anti-Inflammatory Activity in TPA-Induced Mouse Ear Edema Model and in vitro Antibacterial Properties of Cordia Alba Flowers. J. Pharm. Investig. 2019, 49, 331–336. [Google Scholar] [CrossRef]
- Lee, D.Y.; Choi, G.; Yoon, T.; Cheon, M.S.; Choo, B.K.; Kim, H.K. Anti-Inflammatory Activity of Chrysanthemum indicum Extract in Acute and Chronic Cutaneous Inflammation. J. Ethnopharmacol. 2009, 123, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, K.; Matsubara, H.; Sano, Y. Inhibitory Effect of the Flowers of Artichoke (Cynaracardunculus) on TPA-Induced Inflammation and Tumor Promotion in Two-Stage Carcinogenesis in Mouse Skin. J. Nat. Med. 2010, 64, 388–391. [Google Scholar] [CrossRef]
- Li, D.; Tang, X.; Liu, C.; Li, H.; Li, S.; Sun, S.; Zheng, X.; Wu, P.; Xu, X.; Zhang, K.; et al. Jasmine (Jasminum grandiflorum) Flower Extracts Ameliorate Tetradecanoylphorbol Acetate Induced Ear Edema in Mice. Nat. Prod. Commun. 2020, 15, 1934578X20917498. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Leppänen, T.; Tuominen, R.K.; Moilanen, E. Protein Kinase C and Its Inhibitors in the Regulation of Inflammation: Inducible Nitric Oxide Synthase as an Example. Basic Clin. Pharmacol. Toxicol. 2014, 114, 37–43. [Google Scholar] [CrossRef]
- Miao, L.N.; Pan, D.; Shi, J.; Du, J.P.; Chen, P.F.; Gao, J.; Yu, Y.; Shi, D.Z.; Guo, M. Role and Mechanism of PKC-δ for Cardiovascular Disease: Current Status and Perspective. Front. Cardiovasc. Med. 2022, 9, 816369. [Google Scholar] [CrossRef]
- Passos, G.F.; Medeiros, R.; Marcon, R.; Nascimento, A.F.Z.; Calixto, J.B.; Pianowski, L.F. The Role of PKC/ERK1/2 Signaling in the Anti-Inflammatory Effect of Tetracyclic Triterpene Euphol on TPA-Induced Skin Inflammation in Mice. Eur. J. Pharmacol. 2013, 698, 413–420. [Google Scholar] [CrossRef]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol Compounds and PKC Signaling. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2107–2121. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.-B.; Hwang, E.-S.; Kim, E.; Kim, S.-S.; Jeon, T.-D.; Song, M.; Lee, J.-S.; Chung, M.-C.; Maeng, S.; et al. Antidepressant-like Effects of p-coumaric Acid on LPS-Induced Depressive and Inflammatory Changes in Rats. Exp. Neurobiol. 2018, 27, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; You, Y.; Xi, Y.; Ni, B.; Chu, X.; Zhang, R.; You, H. P-Coumaric Acid Attenuates IL-1β-Induced Inflammatory Responses and Cellular Senescence in Rat Chondrocytes. Inflammation 2020, 43, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Murunikkara, V.; Sabina, E.P.; Rasool, M. Ameliorative Effect of p-coumaric Acid, a Common Dietary Phenol, on Adjuvant-Induced Arthritis in Rats. Rheumatol. Int. 2013, 33, 325–334. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Ojeaburu, S.I.; Oriakhi, K. Hepatoprotective, Antioxidant and, Anti-Inflammatory Potentials of Gallic Acid in Carbon Tetrachloride-Induced Hepatic Damage in Wistar Rats. Toxicol. Rep. 2021, 8, 177–185. [Google Scholar] [CrossRef]
- Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes. Biomolecules 2020, 10, 932. [Google Scholar] [CrossRef]
- Gupta, A.; Birhman, K.; Raheja, I.; Sharma, S.K.; Kar, H.K. Quercetin: A Wonder Bioflavonoid with Therapeutic Potential in Disease Management. Asian Pacific J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]


| Group | Left Ear | Right Ear |
|---|---|---|
| Negative control | TPA + ethanol | ethanol |
| Positive control | TPA + ethanol/Indometacin + acetone | ethanol + acetone |
| Cardon flower | TPA + ethanol/CAHE + ethanol | ethanol |
| Xoconostle ulapa flower | TPA + ethanol/XUHE + ethanol | ethanol |
| Xoconostle cuaresmeño flower | TPA + ethanol/XCHE + ethanol | ethanol |
| Pitaya flower | TPA + ethanol/PIHE + ethanol | ethanol |
| Compounds | CAHE | XUHE | XCHE | PIHE |
|---|---|---|---|---|
| Phenolic acids | ||||
| 3,5-di-OH-benzoic acid | 0.96 ± 0.03 b | 0.89 ± 0.00 c | ND | 9.04 ± 0.05 a |
| β-resorcylic acid | ND | 2.08 ± 0.00 b | 2.10 ± 0.06 b | 2.79 ± 0.11 a |
| Chlorogenic acid | ND | ND | ND | 0.08 ± 0.01 |
| Ferulic acid | 0.40 ± 0.00 b | 0.42 ± 0.00 a | ND | ND |
| Gallic acid | 2.85 ± 0.24 c | 4.99 ± 0.01 b | 2.41 ± 0.07 d | 7.84 ± 0.03 a |
| p-coumaric acid | 75.13 ± 0.07 a | 1.60 ± 0.00 c | 1.59 ± 0.00 d | 5.60 ± 0.04 b |
| p-hidroxybenzoic acid | 0.32 ± 0.01 a | ND | 0.33 ± 0.05 a | ND |
| Protocatechuic acid | ND | ND | 3.1 ± 0.02 b | 25.12 ± 0.08 a |
| Rosmarinic acid | 0.91 ± 0.02 b | 5.49 ± 0.12 a | ND | ND |
| Sinapic acid | ND | 0.95 ± 0.00 a | 0.94 ± 0.00 b | ND |
| Vanillic acid | 1.90 ± 0.36 | ND | ND | ND |
| Flavonoids | ||||
| Apigenin | ND | 1.98 ± 0.01 | ND | ND |
| Catechin | 1.44 ± 0.11 b | ND | ND | 8.84 ± 1.53 a |
| Phloretin | 0.09 ± 0.00 a | 0.01 ± 0.00 b | ND | ND |
| Phloridzin | 0.08 ± 0.02 a | ND | ND | 0.07 ± 0.00 a |
| Isorhamnetin | 0.97 ± 0.02 a | 0.78 ± 0.01 b | ND | ND |
| Kaempferol | 1.65 ± 0.01 b | 2.61 ± 0.01 a | ND | ND |
| Myricetin | ND | 1.76 ± 0.00 | ND | ND |
| Naringenin | 0.10 ± 0.03 a | 0.01 ± 0.01 b | ND | ND |
| Quercetin | 1.94 ± 0.03 c | 2.14 ± 0.03 a | 8.94 ± 0.04 d | 2.02 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pensamiento-Niño, C.A.; Hernández-Fuentes, A.D.; Añorve-Morga, J.; Duarte-Sierra, A.; Ramírez-Moreno, E.; Sosa-Gutiérrez, C.G.; Ojeda-Ramírez, D. In Vivo Anti-Inflammatory Activity of Four Edible Cactaceae Flowers from Mexico. Metabolites 2025, 15, 665. https://doi.org/10.3390/metabo15100665
Pensamiento-Niño CA, Hernández-Fuentes AD, Añorve-Morga J, Duarte-Sierra A, Ramírez-Moreno E, Sosa-Gutiérrez CG, Ojeda-Ramírez D. In Vivo Anti-Inflammatory Activity of Four Edible Cactaceae Flowers from Mexico. Metabolites. 2025; 15(10):665. https://doi.org/10.3390/metabo15100665
Chicago/Turabian StylePensamiento-Niño, Christian Alfredo, Alma Delia Hernández-Fuentes, Javier Añorve-Morga, Arturo Duarte-Sierra, Esther Ramírez-Moreno, Carolina Guadalupe Sosa-Gutiérrez, and Deyanira Ojeda-Ramírez. 2025. "In Vivo Anti-Inflammatory Activity of Four Edible Cactaceae Flowers from Mexico" Metabolites 15, no. 10: 665. https://doi.org/10.3390/metabo15100665
APA StylePensamiento-Niño, C. A., Hernández-Fuentes, A. D., Añorve-Morga, J., Duarte-Sierra, A., Ramírez-Moreno, E., Sosa-Gutiérrez, C. G., & Ojeda-Ramírez, D. (2025). In Vivo Anti-Inflammatory Activity of Four Edible Cactaceae Flowers from Mexico. Metabolites, 15(10), 665. https://doi.org/10.3390/metabo15100665








