The Hidden Players of the Fecal Metabolome: Metabolic Dysregulation Beyond SCFAs Under a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Housing
2.2. Metabolomics by Proton (1H) Nuclear Magnetic Resonance (NMR) and Metabolite Interpretation
2.3. Microbiota Analysis
2.4. Statistical Analysis
3. Results
3.1. Group Segregation by Fecal Underexplored Metabolite Profiles After 21 Weeks of HFD
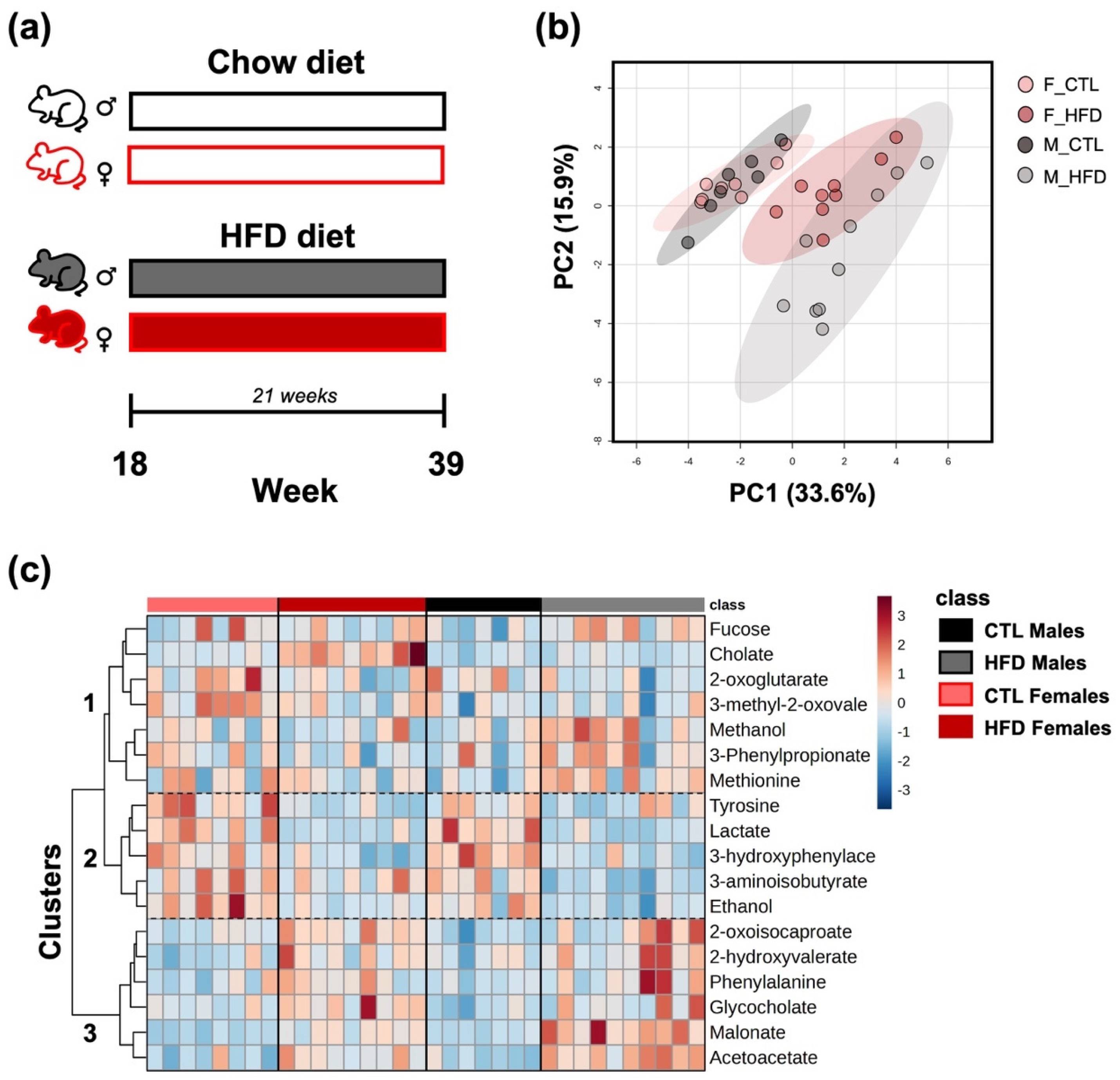
3.2. Endogenous Metabolites Derived from Hepatic Metabolism

3.3. Metabolites Related to Energy Metabolism

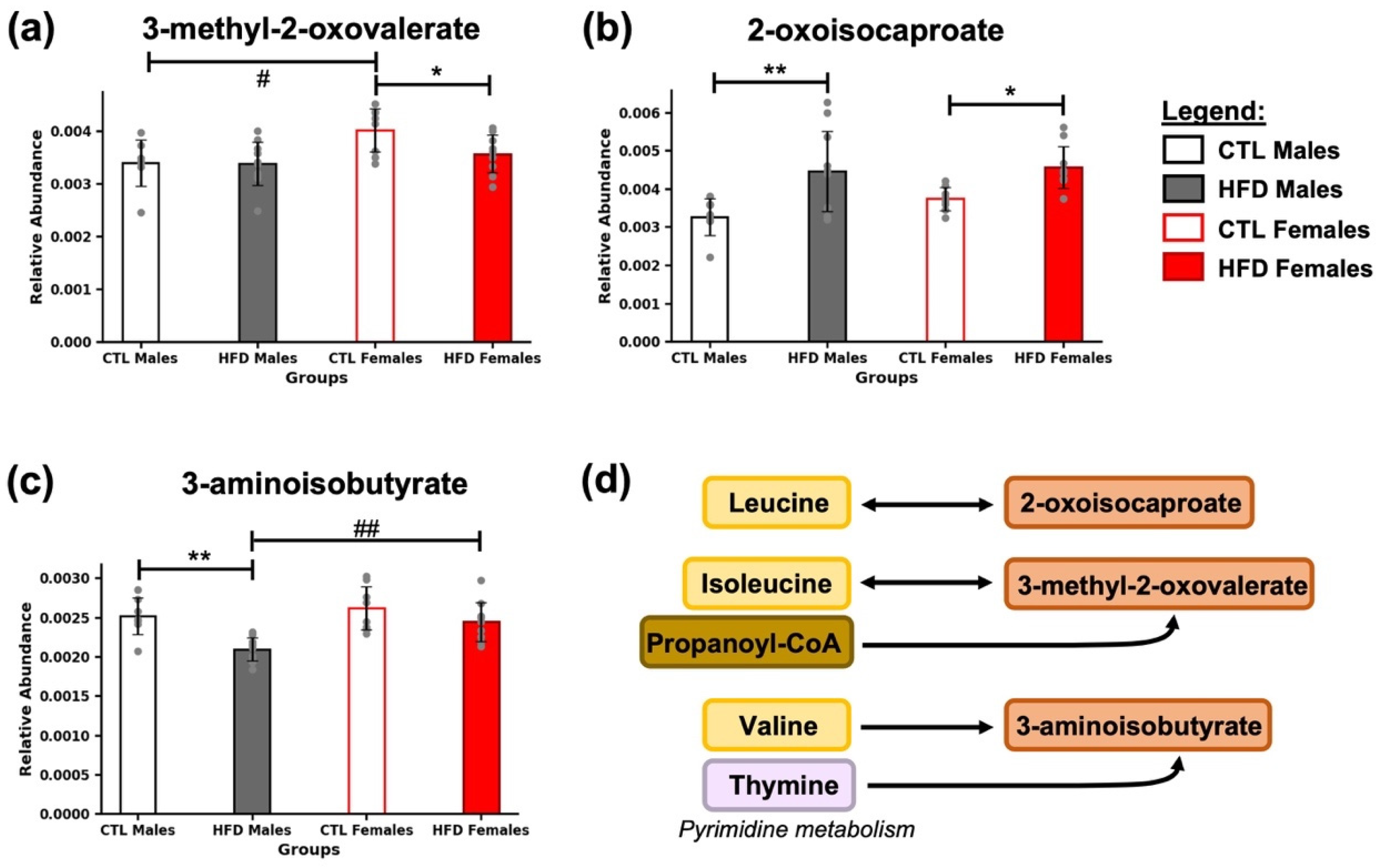
3.4. Intermediates of BCAA Metabolism
3.5. Presence of Amino Acids and Their Derivatives
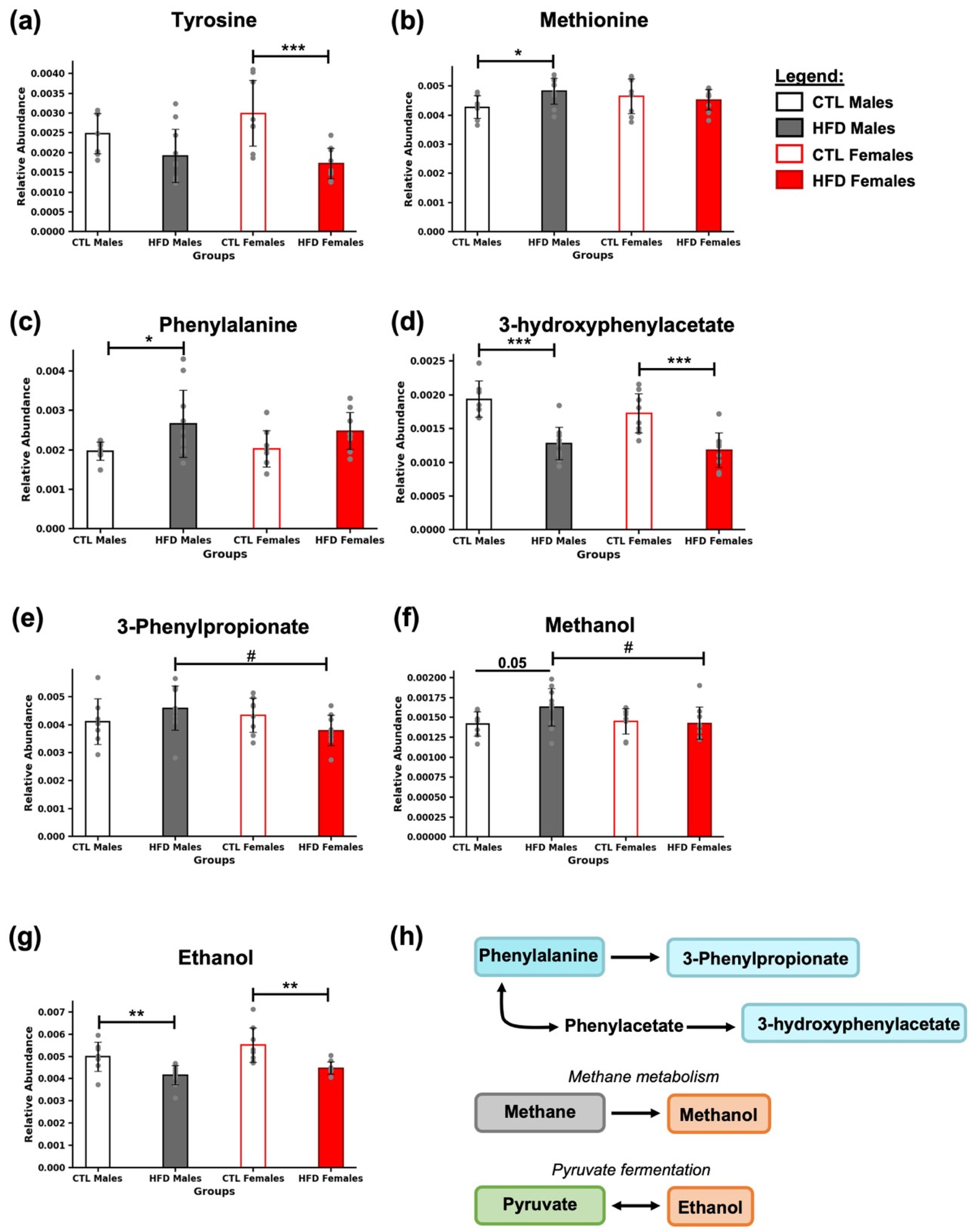
3.6. Presence of Characteristic Microbial Metabolites
3.7. Correlation Analysis Among Newly Identified Metabolites and Microbiota

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | Bile acid |
| BCAA | Branched amino acids |
| CTL | Chow diet |
| HFD | High-fat diet |
| HMDB | Human Metabolome Database |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NMR | Nuclear magnetic resonance |
| PCA | Principal Component Analysis |
| SCFA | Short-chain fatty acids |
| TMAO | Trimethylamine N-oxide |
References
- Iturbe-Rey, S.; Maccali, C.; Arrese, M.; Aspichueta, P.; Oliveira, C.P.; Castro, R.E.; Lapitz, A.; Izquierdo-Sanchez, L.; Bujanda, L.; Perugorria, M.J.; et al. Lipotoxicity-driven metabolic dysfunction-associated steatotic liver disease (MASLD). Atherosclerosis 2025, 400, 119053. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Wong, G.; Anstee, Q.M.; Henry, L. The Global Burden of Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 1978–1991. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Martin-Grau, M.; Monleon, D. Sex dimorphism and metabolic profiles in management of metabolic-associated fatty liver disease. World J. Clin. Cases 2023, 11, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Martin-Grau, M.; Monleón, D. The Role of Microbiota-Related Co-Metabolites in MASLD Progression: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 6377–6389. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Schnabl, B. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Martín-Grau, M.; Casanova, P.; Moreno-Morcillo, L.; Morales, J.M.; Marrachelli, V.G.; Monleón, D. Microbiota Co-Metabolism Alterations Precede Changes in the Host Metabolism in the Early Stages of Diet-Induced MASLD in Wistar Rats. Int. J. Mol. Sci. 2025, 26, 1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jin, Y.S.; Kim, K.H. L-Fucose is involved in human-gut microbiome interactions. Appl. Microbiol. Biotechnol. 2023, 107, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Al-Shareffi, E.; Haltiwanger, R.S. Biological functions of fucose in mammals. Glycobiology 2017, 27, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Kimura, K.; Maezawa, Y.; Ohata, M.; Mizuhara, Y.; Hirakawa, J.; Nakajima, H.; Toda, G. Urinary level of L-fucose as a marker of alcoholic liver disease. Alcohol. Clin. Exp. Res. 1993, 17, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Niu, M.; Tang, W.; Hu, J.; Wei, G.; He, Z.; Chen, Y.; Jiang, Y.; Chen, P. L-Fucose ameliorates high-fat diet-induced obesity and hepatic steatosis in mice. J. Transl. Med. 2018, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Valvano, C.M.; De Nardo, W.; Watt, M.J. Integrative metabolism in MASLD and MASH: Pathophysiology and emerging mechanisms. J. Hepatol. 2025, 83, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.; Felicianna Xu, J.H.; Zhan, Q.; Zeng, Z.; El-Nezami, H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines 2022, 10, 1444. [Google Scholar] [CrossRef] [PubMed]
- Gozdzik, P.; Magkos, F.; Sledzinski, T.; Mika, A. Monomethyl branched-chain fatty acids: Health effects and biological mechanisms. Prog. Lipid Res. 2023, 90, 101226. [Google Scholar] [CrossRef] [PubMed]
| N. | Compound Name | 1H-NMR Chemical Shift (ppm), and Multiplicity | HMDB | KEGG |
|---|---|---|---|---|
| 1 | 2-hydroxyvalerate | 0.90 (t), 1.3 (m), 1.4 (m), 1.6 (m), 1.7 (m), 4.0 (m) | HMDB0001863 | - |
| 2 | 2-oxoglutarate | 2.4 (t), 3.0 (t) | HMDB0000208 | C00026 |
| 3 | 2-oxoisocaproate | 0.9 (d), 2.1 (m), 2.6 (d) | HMDB0000695 | C00233 |
| 4 | 3-aminoisobutyrate | 1.2 (d), 2.6 (m), 3.0 (m), 3.1 (m) | HMDB0003911 | C01205 |
| 5 | 3-hydroxyphenylacetate | 3.5 (s), 6.8 (d), 6.8 (s), 6.9 (d), 7.2 (t) | HMDB0000440 | C05593 |
| 6 | 3-methyl-2-oxovalerate | 0.9 (t), 1.1 (d), 1.4 (m), 1.7 (m), 2.9 (m) | HMDB0000491 | C00671 |
| 7 | 3-phenylpropionate | 2.5 (m), 2.9 (m), 7.3 (t), 7.3 (d), 7.4 (t) | HMDB0000764 | C05629 |
| 8 | Acetoacetate | 2.3 (s), 3.4 (s) | HMDB0000060 | C00164 |
| 9 | Cholate | 0.7 (s), 0.9 (s), 1.0 (d, td), 1.2 (m), 1.3 (m), 1.4 (m), 1.5 (d), 1.6 (m), 1.7 (m), 1.8 (m), 1.9 (m), 2.0 (d, m), 2.1 (m), 2.2 (m), 3.5 (m), 3.9 (d), 4.1 (s) | HMDB0000619 | C00695 |
| 10 | Ethanol | 1.2 (t), 3.6 (q) | HMDB0000108 | C00469 |
| 11 | (L)-Fucose | 1.2 (d), 3.4 (q), 3.6 (dd), 3.7 (d), 3.8 (d, dd, q), 3.9 (dd), 4.0 (t, m), 4.1 (q), 4.2 (q), 4.5 (d), 5.2 (d), 5.3 (d) | HMDB0000174 | C01019 |
| 12 | Glycocholate | 0.7 (s), 0.9 (s), 1.0 (d, m), 1.1 (m), 1.3 (m), 1.4 (m), 1.5 (m), 1.6 (m), 1.7 (m), 1.8 (m), 1.9 (m), 2.0 (q), 2.1 (dt), 2.2 (m), 2.4 (m), 3.5 (m), 3.7 (dd), 3.8 (dd), 3.9 (s), 4.0 (s), 7.9 (t) | HMDB0000138 | C01921 |
| 13 | Lactate | 1.3 (d), 4.1 (q) | HMDB0000190 | C00186 |
| 14 | Malonate | 3.1 (s) | - | C00383 |
| 15 | Methanol | 3.4 (s) | HMDB0001875 | C00132 |
| 16 | L-Methionine | 2.1 (s, m), 2.2 (m), 2.6 (t), 3.9 (q) | HMDB0000696 | C00073 |
| 17 | L-Phenylalanine | 3.1 (q), 3.3 (dd), 4.0 (q), 7.3 (d), 7.4 (t) | HMDB0000159 | C00079 |
| 18 | L-Tyrosine | 3.0 (q), 3.2 (dd), 3.9 (q), 6.9 (d), 7.2 (d) | HMDB0000158 | C00082 |
| Metabolite | Probable Origin | Metabolic Pathway | Biological Significance in Feces |
|---|---|---|---|
| 2-hydroxyvalerate | Host and Microbiota | Branched-chain fatty acid metabolism | From BCAA or intermediate in energy metabolism |
| 2-oxglutarate | Host and Microbiota | TCA cycle | Key intermediate in energy metabolism |
| 2-oxoisocaproate | Host and Microbiota | Leucine metabolism | Intermediate in leucine catabolism |
| 3-aminoisobutyrate | Host and Microbiota | BCAA or thymine metabolism | Microbial metabolism changes |
| 3-hydroxyphenylacetate | Microbiota | Phenylalanine metabolism | Indicator of changes in microbial diversity |
| 3-methyl-2-isovalerate | Host and Microbiota | Isoleucine and propanoyl-CoA metabolism | Microbial metabolism changes |
| 3-Phenylpropionate | Microbiota | Phenylalanine metabolism | Indicator of changes in microbial diversity |
| Acetoacetate | Host and Microbiota | Acetyl-CoA metabolism | Elevated in increased lipid oxidation or ketogenic state |
| Cholate | Host | Primary BA metabolism | Marker of liver function or indicator of changes in enterohepatic activity |
| Ethanol | Microbiota | Pyruvate fermentation | Indicator of changes in microbial diversity |
| (L)-Fucose | Host | Carbohydrate metabolism (fructose and mannose) | Liver metabolism imbalance |
| Glycocholate | Host and Microbiota | BA metabolism; conjugation/deconjugation processes | Marker of liver function or indicator of changes in enterohepatic activity |
| Lactate | Host and Microbiota | Anaerobic glycolysis | Indicator of changes in microbial diversity |
| Malonate | Host and Microbiota | TCA cycle inhibitor/FA metabolism | Regulator of energy metabolism |
| Methanol | Microbiota | Methane metabolism | Indicator of changes in microbial diversity |
| Methionine | Host and Microbiota | Amino acid metabolism | Involved in methylation and antioxidant defense |
| Phenylalanine | Host and Microbiota | Aromatic amino acid metabolism | Precursor of microbial products |
| Tyrosine | Host and Microbiota | Aromatic amino acid metabolism | Precursor of microbial products; also it can be a neurotransmitter precursor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Grau, M.; Casanova, P.; Morales, J.M.; Marrachelli, V.G.; Monleón, D. The Hidden Players of the Fecal Metabolome: Metabolic Dysregulation Beyond SCFAs Under a High-Fat Diet. Metabolites 2025, 15, 660. https://doi.org/10.3390/metabo15100660
Martín-Grau M, Casanova P, Morales JM, Marrachelli VG, Monleón D. The Hidden Players of the Fecal Metabolome: Metabolic Dysregulation Beyond SCFAs Under a High-Fat Diet. Metabolites. 2025; 15(10):660. https://doi.org/10.3390/metabo15100660
Chicago/Turabian StyleMartín-Grau, María, Pilar Casanova, José Manuel Morales, Vannina González Marrachelli, and Daniel Monleón. 2025. "The Hidden Players of the Fecal Metabolome: Metabolic Dysregulation Beyond SCFAs Under a High-Fat Diet" Metabolites 15, no. 10: 660. https://doi.org/10.3390/metabo15100660
APA StyleMartín-Grau, M., Casanova, P., Morales, J. M., Marrachelli, V. G., & Monleón, D. (2025). The Hidden Players of the Fecal Metabolome: Metabolic Dysregulation Beyond SCFAs Under a High-Fat Diet. Metabolites, 15(10), 660. https://doi.org/10.3390/metabo15100660






