Targeting Metabolic Dysregulation in Obesity and Metabolic Syndrome: The Emerging Role of N-Acetylcysteine
Abstract
1. Introduction
2. Methodology
3. MetS and Metabolic Disruption
3.1. Visceral Adipose Tissue
3.2. Lipid Metabolism and Its Deregulation
3.3. Glucose Metabolism and Its Deregulation
3.4. Adipokine Profile of Visceral Adiposity
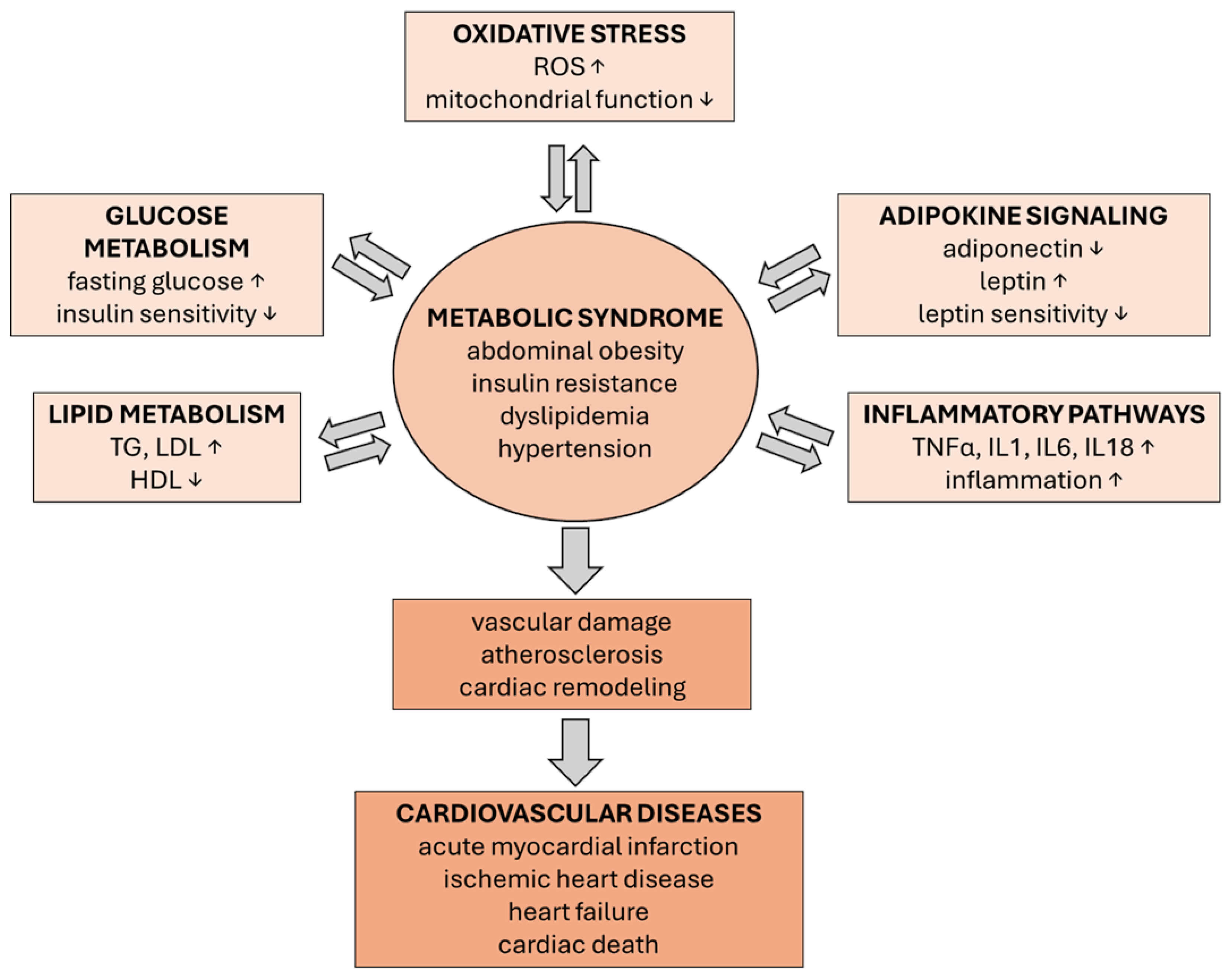
3.5. Integrated Metabolic Disruption in CVD
3.6. Mitochondrial Dysfunction in Obesity, MetS, and CVD
4. N-Acetylcysteine
5. NAC and MetS—Evidence from Cellular, Animal, and Human Studies
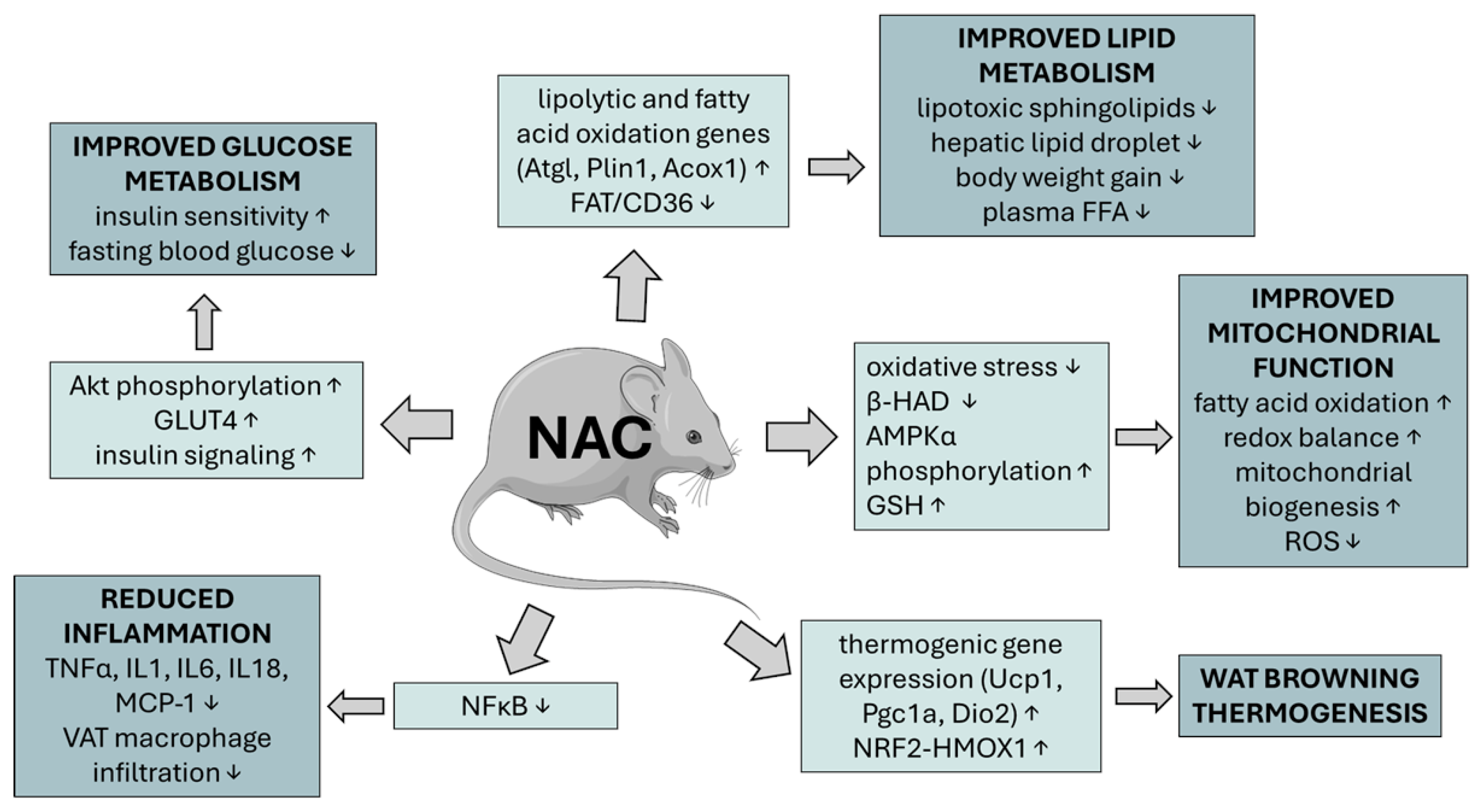
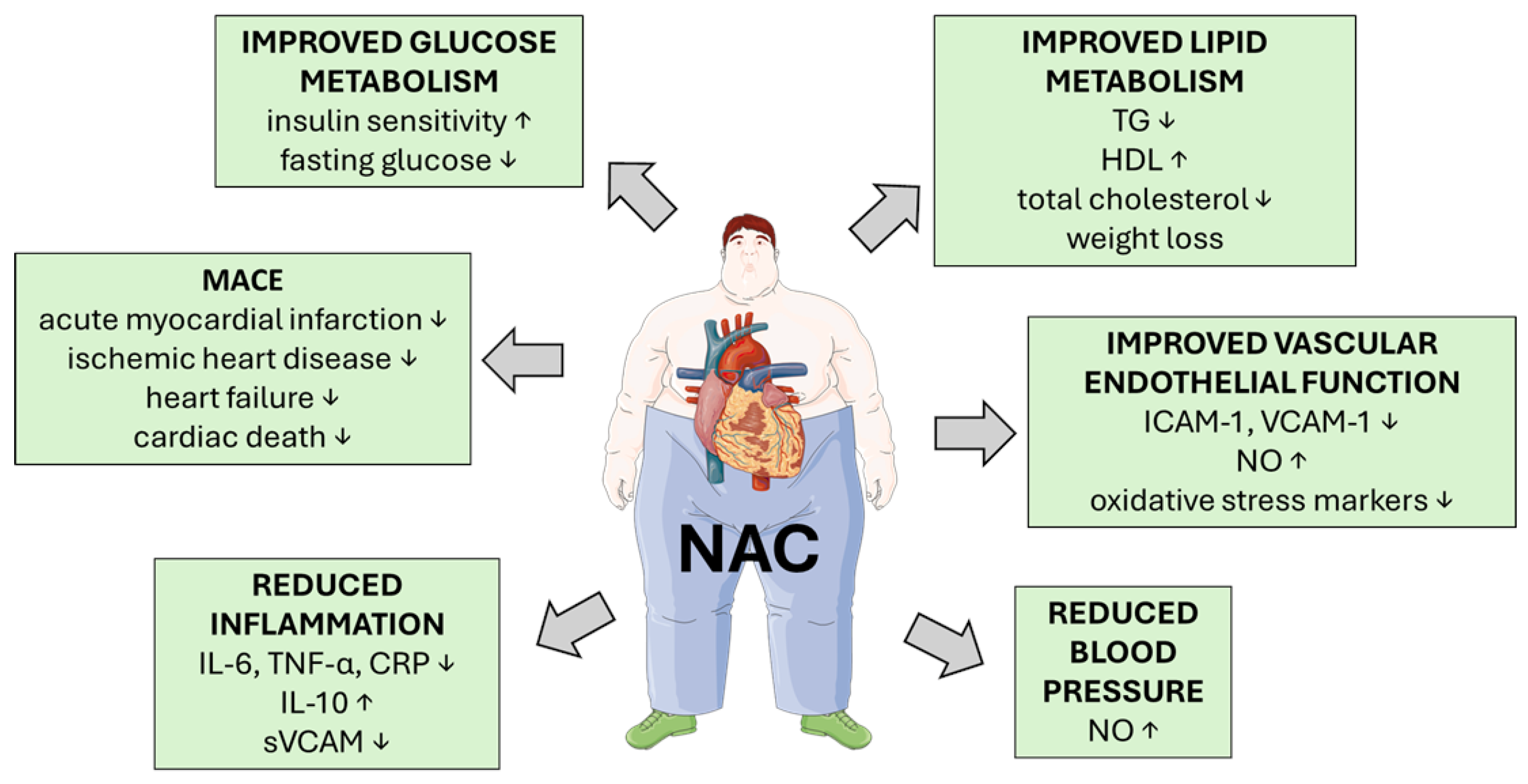
5.1. Effect of NAC on Glucose Metabolism and Its Deregulation
5.2. From Oxidative Stress and Inflammation to Senescence and Aging
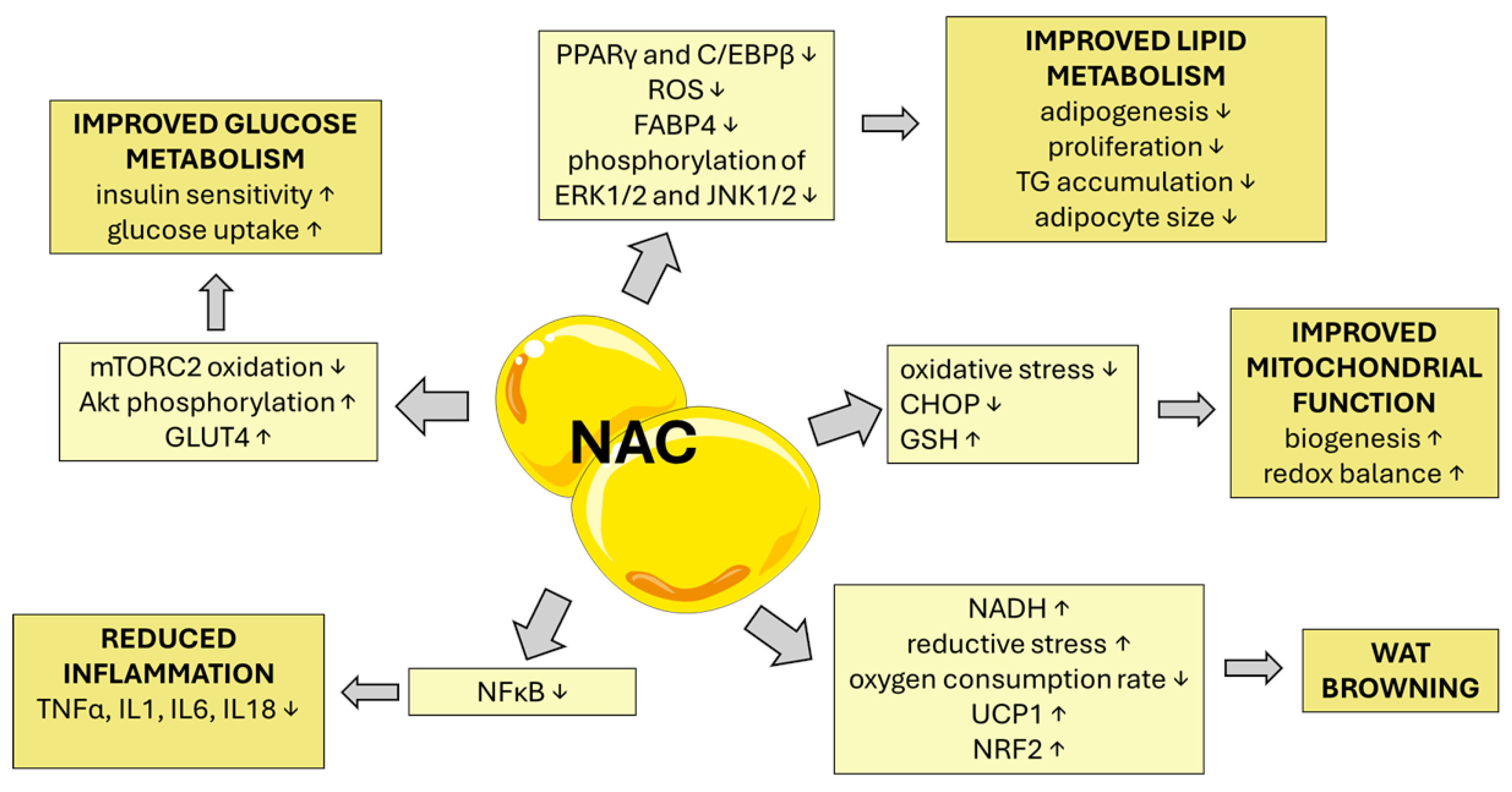
5.3. Effect of NAC on Adipogenesis, Visceral Obesity, Lipid Metabolism, and Its Deregulation
5.4. Effect of NAC on Hepatic Lipid Accumulation
5.5. Effect of NAC on Mitochondrial Dysfunction in Obesity, MetS, and CVD
5.6. Effects of NAC on Chronic Metabolic Inflammation (Metaflammation) and Immune Modulation
5.7. Obesity and the Brain: A Self-Perpetuating Neuro-Metabolic Disorder
5.8. NAC and Its Role in Cardiovascular Protection
| Design | Population | Dose/Route | Duration | Comparators | Effect Sizes | Safety | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled clinical trial [127] | 40 obese adults (BMI ≥ 35 kg/m2), aged 25–50, candidates for bariatric surgery | 600 mg/day oral NAC | 4 weeks before bariatric surgery | Placebo | Adipose tissue sample: SA-β-gal ↓ staining (p = 0.001), IL-6 ↓ (p = 0.014), P16 ↓ (p = 0.047) gene expression Blood: hsCRP, IL-6 FSB, insulin ↓ (p < 0.001) HOMA-IR ↓ (p < 0.001) | No adverse events reported | Low |
| Randomized, double-blind, placebo-controlled clinical trial [128] | 24 older adults (61–80 years, BMI > 27), 12 young adults (21–40 years) | GlyNAC: glycine (100 mg/kg/day) + NAC (100 mg/kg/day), oral | 16 weeks (YA received GlyNAC for 2 weeks) | Placebo | Muscle sample: GSH ↑ (p < 0.001) Blood: RBC total GSH ↑ (p < 0.001), TBARS ↓ (p < 0.001), IL-6 ↓ (p < 0.001), TNFα ↓ (p < 0.001), hsCRP ↓ (p < 0.001), IL-10 ↑ (p < 0.001), sICAM1 ↓ (p < 0.001), sVCAM1 ↓ (p < 0.001), HOMA-IR ↓ (p < 0.001), improved cognition, gait speed | No adverse events reported | Low |
| Double-blind, randomized, placebo-controlled crossover trial [150] | 11 adults (mean age 58), with elevated plasma lipoprotein(a) | 2 g NAC twice daily, oral | 2 weeks NAC, 2 weeks placebo, with 2-week washout between | Placebo | Homocysteine ↓ (p < 0.0001); Cysteinyl glycine ↓ (p = 0.0001); Cysteine ↓ (p = 0.013) | Well tolerated; minor side effects (flatulence, bad taste) | Low |
| Randomized, double-blind, placebo-controlled, multicenter trial (NACIAM) [163] | 112 STEMI patients undergoing PCI | NAC 29 g IV over 48 h + nitroglycerin 7.2 mg IV over 48 h | 48 h treatment; 3-month follow-up | Placebo + nitroglycerin | Infarct size ↓ (p = 0.02); Myocardial salvage ↑ (p < 0.01); Late infarct size ↓ (p = 0.02) | No increase in hypotension, bleeding, or nephropathy; 2 deaths in placebo group, none in NAC group | Low |
| Randomized, double-blind, placebo-controlled, 4-arm trial [153] | 200 patients with unstable angina not requiring emergency revascularization | NAC 600 mg orally, 3× daily; Nitroglycerin 10 mg transdermal patch daily | 4 months | Placebo, Nitroglycerin alone, NAC alone, Nitroglycerin + NAC | Combined therapy reduced outcome events (death, MI, refractory angina): vs. placebo (OR 0.25; 95% CI 0.07–0.7; p = 0.0022); vs. Nitroglycerin (OR 0.31; 95% CI 0.098–0.90; p = 0.018); vs. NAC (OR 0.19; 95% CI 0.06–0.57; p = 0.0008) | High incidence of severe headache in combined group (31% vs. 19% in Nitroglycerin alone); 33% discontinued due to side effects | Low |
| Randomized, single-blind, placebo-controlled trial (LIPSIA-N-ACC) [162] | 251 STEMI patients undergoing PCI | NAC 6 g IV over 48 h (1.2 g bolus + 2 × 1.2 g/day) | 48 h treatment; 6-month follow-up | Placebo + hydration | No significant difference in CIN, oxidative stress markers ↓ (p < 0.05) | No adverse events | Low |
| Open-label clinical trial [88] | 8 OA (71–80 years), compared to 8 YA (21–30 years) | GlyNAC: glycine (1.33 mmol/kg/day) + NAC (0.81 mmol/kg/day), oral | 24 weeks supplementation + 12 weeks withdrawal | Young adults (baseline comparison) | Blood: RBC-reduced GSH ↑ (p = 0.0000) TBARS ↓ (p = 0.0000), IL-6 ↓ (p = 0.0000), TNF-α ↓ (p = 0.0000), hsCRP ↓ (p = 0.0000), IL-10 ↑ (p = 0.0000), sICAM1 ↓ (p = 0.0000), sVCAM1 ↓ (p = 0.0002), glucose ↓ (p = 0.04), HOMA-IR ↓ (p = 0.0000), improved cognition, gait speed | No adverse events reported | Moderate |
| Randomized controlled pilot trial [90] | 27 adults (45–65 years) with MetS and at risk of MASLD | MetioNac®: 3 capsules/day (each capsule: SAMe 200 mg, NAC 100 mg, thioctic acid 75 mg, vitamin B6 0.65 mg), oral | 3 months | Control: semipersonalized MD | Blood: TG ↓ (p = 0.043), VLDL ↓ (p = 0.048) | No adverse events reported | Moderate |
| Randomized, double-blind, placebo-controlled trial [154] | 24 male patients with T2DM and hypertension | NAC 600 mg orally twice daily + L-arginine 1200 mg orally once daily | 6 months | Placebo | Significant reductions in SBP and DBP (p = 0.05), total cholesterol (p = 0.01), LDL (p = 0.005), ox-LDL (p = 0.05), hsCRP (p = 0.05), sICAM (p = 0.05), sVCAM (p = 0.01) | No adverse effects | Moderate |
| Population-based cohort study with propensity score matching [157] | 46,718 patients with T2DM | Oral NAC; average daily dose ≥ 600 mg | Up to 13 years (2008–2021) | Non-NAC users | Overall MACE: average dose aHR 0.84 (95% CI: 0.81–0.86, p < 0.0001); highest dose aHR 0.61 (95% CI: 0.58–0.64, p < 0.0001) | No adverse effects reported | Moderate |
| Open-label, within-patient crossover study [155] | 18 hypertensive smokers (15 males, 3 females; mean age 69 ± 5 years) on ACEi (captopril or enalapril) | NAC 600 mg orally three times daily (1800 mg/day) added to ACEi | 21 days per treatment arm (ACEi alone vs. ACEi + NAC), with 5-day washout | ACEi alone | 24 h SBP ↓ (p < 0.05); 24 h DBP ↓ (p = 0.01); Daytime SBP and DBP ↓ (both p < 0.05) | No adverse effects reported | Moderate |
| Double-blind, randomized, placebo-controlled clinical trial [158] | 98 patients with STEMI | NAC 600 mg twice daily, oral | 3 days (acute phase), with 1-year follow-up for MACE | Placebo | 72 h: MMP-9 ↓ (p = 0.014); MMP-2 ↓ (p = 0.045); MACE: 14% (NAC) vs. 25% (placebo), p = 0.024; Reinfarction: 4% vs. 16.7%, p = 0.007 | No adverse effects reported | Moderate |
| Controlled, non-randomized clinical trial [159] | 30 patients with acute MI, admitted within 6 h of symptom onset | NAC 15 g IV over 24 h, combined with streptokinase and nitroglycerin | 24 h for oxidative stress markers; 3 months for echocardiographic follow-up | Streptokinase + nitroglycerin | MDA ↓ at 4 h and 24 h in NAC group (p < 0.01); LVEF ↑ at day 3 and month 3 (p < 0.05); LVESD ↓ at day 3 and month 3 (p < 0.001); WMSI ↓ at day 3 and month 3 (p < 0.05) | No adverse effects reported | Moderate |
| Randomized, open-label, safety-focused pilot trial [160] | 27 AMI patients treated with streptokinase and nitroglycerin | NAC 15 g IV over 24 h | 24 h treatment; 7-day follow-up | Streptokinase + nitroglycerin | GSH:GSSG ratio ↑ (p < 0.05); MDA ↓ over first 8h (p < 0.001); Cardiac index ↑ (p = 0.009) | No deaths; minor bleeding in 3 NAC patients; headache in 4; no hypotension | Moderate |
| Randomized, controlled, open-label pilot study [161] | 16 AMI patients (mean age 52) | NAC 15 g IV over 24 h + nitroglycerin + streptokinase | 48 h treatment; 6-month follow-up | Nitroglycerin + streptokinase | Plasma hydroperoxides ↓ at 24 h (p = 0.039); PMN count ↓ at 6–24 h (p < 0.01) | No adverse events | Moderate |
| Randomized, double-blind, placebo-controlled pilot study [144] | 42 adults with HCM, LV wall thickness ≥ 15 mm | NAC: 600 mg twice daily for 3 months, then 1200 mg twice daily for 9 months, oral capsules | 12 months | Placebo | LV mass index: Δ = 5.99 g/m2 (95% CI: −19.10, 31.10), wall thickness: Δ = 0.79 mm (95% CI: −2.97, 1.39) | 6 serious adverse events in NAC group (e.g., pneumonia, CVA, seizure), none attributed to NAC | Moderate to high |
6. Concluding Remarks, Clinical Implications, and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organisation |
| Mets | Metabolic Syndrome |
| LAP | Lipid Accumulation Product |
| CMI | Cardiometabolic Index |
| BMI | Body Mass Index |
| Cvd | Cardiovascular Disease |
| Nac | N-Acetylcysteine |
| Masld | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| HF | heart failure |
| Ppar | Peroxisome Proliferator-Activated Receptor |
| Fgf21 | Fibroblast Growth Factor 21 |
| Glp-1 | Glucagon-Like Peptide-1 |
| Sat | Subcutaneous Adipose Tissue |
| Vat | Visceral Adipose Tissue |
| Ffa | Free Fatty Acid |
| Eat | Epicardial Adipose Tissue |
| Tg | Triglycerides |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| Ros | Reactive Oxygen Species |
| Hif-1 | Hypoxia Inducible Factor 1 |
| Mcp-1 | Monocyte Chemoattractant Protein-1 |
| TNF-A | Tumor Necrosis Factor A |
| Prdx3 | Peroxiredoxin-3 |
| Cyslt | Cysteinyl Leukotriene |
| Ltb4 | Leukotriene B4 |
| NF-Κb | Nuclear Factor Kappa-Light-Chain Enhancer Of Activated B Cells |
| Mapk | Mitogen-Activated Protein Kinase |
| Pi3k/Akt | Phosphoinositide 3-Kinase/Protein Kinase B |
| Ampk | Amp-Activated Protein Kinase |
| Sirt3 | Sirtuin 3 |
| Gsh | Glutathione |
| Ace | Angiotensin Converting Enzyme |
| Sod | Superoxide Dismutase |
| Jnk | C-Jun N-Terminal Kinase |
| Erk | Extracellular Signal-Regulated Kinase |
| Ap-1 | Activator Protein 1 |
| Sapk/Ink | Stress-Activated Protein Kinases/Cyclin-Dependent Kinase Inhibitor |
| Stats | Signal Transducers And Activators Of Transcription |
| 8-Oh-G | 8-Hydroxyguanine |
| Hdacs | Histone Deacetylases |
| Hats | Histone Acetyltransferases |
| Pparγ | Peroxisome Proliferator-Activated Receptor Gamma |
| C/Ebpβ | CCAAT/Enhancer Binding Protein Beta |
| Mtorc2 | Mechanistic Target Of Rapamycin Complex 2 |
| Er | Endoplasmic Reticulum |
| Mtupr | Mitochondrial Unfolded Protein Response |
| Hfd | Hight Fat Diet |
| Lc3-Ii | Autophagy-Related Protein Light Chain 3 |
| Gpx | Glutathione Peroxidase |
| Irs1 | Insulin Receptor Substrate 1 |
| Pdx-1 | Pancreatic-Duodenal Homeobox-1 |
| Mda | Malondialdehyde |
| Gsh/Gssg | Glutathione/Oxidised Glutathione |
| Sasp | Senescence Secretory Phenotype |
| CMR | Cardiac magnetic resonance |
| Tbars | Thiobarbituric Acid-Reactive Substances |
| Ph2ax | Phospho-H2A Histone Family Member X |
| Fabp4 | Fatty Acid Binding Protein 4 |
| Hsp70 | Heat Shock Protein 70 |
| Maoa | Monoamine Oxidase A |
| Acy-1 | Aminoacylase-1 |
| Tkt | Transketolase |
| Mt3 | Metallothionein 3 |
| Mc | Metabolic Cofactor |
| Homa-Ir | Homeostasis Model Assessment Of Insulin Resistance |
| Atgl | Adipose Triglyceride Lipase |
| Plin1 | Perilipin 1 |
| Acox1 | Acyl-Coa Oxidase 1 |
| Hscrp | High-Sensitivity C-Reactive Protein |
| Bmat | Bone Marrow Adipose Tissue |
| Ho-1 | Heme Oxygenase-1 |
| Dgat1 | Diacylglycerol Acyltransferase |
| S1p | Sphingosine-1-Phosphate |
| Sa1p | Sphinganine-1-Phosphate |
| GSK3α/Β | Glycogen Synthase Kinase-3α/Β |
| S6rp | S6 Ribosomal Protein |
| Chop | C/Ebp Homologous Protein |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| Fat/Cd36 | Fatty Acid Translocase |
| Fabppm | Fatty Acid Binding Protein |
| Fatp1 | Long-Chain Fatty Acid Transport Protein 1 |
| Β-HAD | Β-Hydroxyacyl-Coa Dehydrogenase |
| Ucp1 | Uncoupling Protein 1 |
| PGC1α/Β | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| Bha | Butylated Hydroxyanisole |
| Bat | Brown Adipose Tissue |
| Nox4 | Nicotinamide Adenine Dinucleotide Phosphate Oxidase 4 |
| Pa | Palmitate |
| Grp78 | Er Stress Markers 78 Kda Glucose-Regulated Protein |
| Ire1 | Inositol-Requiring Protein 1 |
| VLDL | Very Low-Density Lipoprotein |
| MitoTEMPO | Mitochondria-Targeted Superoxide Dismutase Mimetic |
| Pai-1 | Plasminogen Activator Inhibitor-1 |
| Inos | Inducible Nitric Oxide Synthase |
| GSK3β | glycogen synthase kinase 3 beta |
| Γ-H2AX | H2A Histone Family Member X |
| Atm | Ataxia-Telangiectasia Mutated |
| AGE-Albumin | Advanced Glycated Albumin |
| Aa | Arachidonic Acid |
| Cox-2 | Cyclooxygenase-2 |
| 5-Lox | 5-Lipoxygenase |
| Pge2 | Prostaglandin E2 |
| Ltc4 | Leukotriene C4 |
| Lxa4 | Lipoxin A4 |
| 4-Hne | 4-Hydroxynonenal |
| Ripk3 | Receptor-Interacting Protein Kinase-3 |
| Nlrp3 | Nod Like Receptor Protein 3 |
| Mlkl | Mixed Lineage Kinase Domain-Like Protein |
| Asc | Apoptosis-Associated Speck-Like Proteins |
| Gfap | Glial Fibrillary Acidic Protein |
| Mmp-9 | Matrix Metalloproteinase 9 |
| Wd | Western Diet |
| Hfhs | High-Fat, High-Sugar |
| DIO | diet-induced obesity |
| Ec | Endothelial Cell |
| Ace | Angiotensin-Converting Enzyme |
| No | Nitric Oxide |
| Nox | Nadph Oxidase |
| Sptlc2 | Serine Palmitoyltransferase Long Chain Base Subunit 2 |
| Lass5 | Ceramide Synthase 5 |
| Asah2 | Neutral Ceramidase |
| Alk-Smase | Alkaline Sphingomyelinase |
| N-Smase | Neutral Sphingomyelinase |
| Drp1 | Dynamin-Related Protein 1 |
| Puma | P53-Upregulated Modulator Of Apoptosis |
| Mfn-2 | Mitofusin-2 |
| Alp | Allopurinol |
| Mg | Methylglyoxal |
| Htert | Telomerase |
| Pml | Promyelocytic Leukemia Protein |
| Hcy | Homocysteine |
| Svcam | Soluble Vascular Cell Adhesion Molecule |
| Arg | L-Arginine |
| Mace | Major Adverse Cardiac Events |
| AMI | Acute Myocardial Infarction |
| Stemi | St-Elevation Myocardial Infarction |
| Rooh | Lipid Hydroperoxide |
| Pmn | Polymorphonuclear Neutrophil |
| Cin | Contrast-Induced Nephropathy |
| Pci | Percutaneous Coronary Intervention |
| Msi | Myocardial Salvage Index |
| T2dm | Type 2 Diabetes Mellitus |
| Pkc | Phosphokinase C |
| Il | Interleukin |
| Prdx2 | Peroxiredoxin 2 |
| Mtor | Mammalian Target Of Rapamycin |
| SA-Β-Gal | Senescence Activated Β-Galactosidase |
References
- Collaborators, G.B.D.A.B. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Pigeot, I.; Ahrens, W. Epidemiology of metabolic syndrome. Pflug. Arch. 2025, 477, 669–680. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Sun, J.; Jiang, Y.; Tang, Y. Identification of metabolic syndrome using lipid accumulation product and cardiometabolic index based on NHANES data from 2005 to 2018. Nutr. Metab. 2024, 21, 96. [Google Scholar] [CrossRef]
- Kwon, J.H.; Jang, H.Y.; Oh, M.J.; Rho, J.S.; Jung, J.H.; Yum, K.S.; Han, J.W. Association of visceral fat and risk factors for metabolic syndrome in children and adolescents. Yonsei Med. J. 2011, 52, 39–44. [Google Scholar] [CrossRef]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, J. The pathophysiology of visceral adipose tissues in cardiometabolic diseases. Biochem. Pharmacol. 2024, 222, 116116. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; De Michele, G.; Fimiani, F.; Acerbo, V.; Scherillo, G.; Signore, G.; Rotolo, F.P.; Scialla, F.; Raucci, G.; Panico, D.; et al. Visceral adipose tissue and residual cardiovascular risk: A pathological link and new therapeutic options. Front. Cardiovasc. Med. 2023, 10, 1187735. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Metabolic Modulators in Heart Disease: Past, Present, and Future. Can. J. Cardiol. 2017, 33, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Rehberger-Likozar, A.; Sebestjen, M. Influence of trimetazidine and ranolazine on endothelial function in patients with ischemic heart disease. Coron. Artery Dis. 2015, 26, 651–656. [Google Scholar] [CrossRef]
- Minano, S.; Gonzalez-Correa, C.; Moleon, J.; Duarte, J. Metabolic Modulators in Cardiovascular Complications of Systemic Lupus Erythematosus. Biomedicines 2023, 11, 3142. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G.; Tilg, H. Thyroid hormone receptor-beta agonists: New MASLD therapies on the horizon. Gut 2024, 73, 573–581. [Google Scholar] [CrossRef]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Tenorio, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Sharma, K.; Yanek, L.R.; Vaidya, D.; Schar, M.; Markl, M.; Subramanya, V.; Soleimani, S.; Ouyang, P.; Michos, E.D.; et al. Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 2535–2545. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Khawaja, T.; Nied, M.; Wilgor, A.; Neeland, I.J. Impact of Visceral and Hepatic Fat on Cardiometabolic Health. Curr. Cardiol. Rep. 2024, 26, 1297–1307. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef]
- Bhat, N.; Mani, A. Dysregulation of Lipid and Glucose Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients 2023, 15, 2323. [Google Scholar] [CrossRef]
- Ko, C.W.; Qu, J.; Black, D.D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- Kersten, S. The impact of fasting on adipose tissue metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159262. [Google Scholar] [CrossRef]
- Deng, L.; Vrieling, F.; Stienstra, R.; Hooiveld, G.J.; Feitsma, A.L.; Kersten, S. Macrophages take up VLDL-sized emulsion particles through caveolae-mediated endocytosis and excrete part of the internalized triglycerides as fatty acids. PLoS Biol. 2022, 20, e3001516. [Google Scholar] [CrossRef]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr. Diab Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, J.; van Veldhuisen, D.J.; van der Meer, P.; Meems, L.M.G. Heart Failure and Obesity: Unraveling Molecular Mechanisms of Excess Adipose Tissue. J. Am. Coll. Cardiol. 2024, 84, 1666–1677. [Google Scholar] [CrossRef]
- Trandafir, L.M.; Dodi, G.; Frasinariu, O.; Luca, A.C.; Butnariu, L.I.; Tarca, E.; Moisa, S.M. Tackling Dyslipidemia in Obesity from a Nanotechnology Perspective. Nutrients 2022, 14, 3774. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Satoh, T. Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Int. J. Mol. Sci. 2014, 15, 18677–18692. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Halse, R.; Bonavaud, S.M.; Armstrong, J.L.; McCormack, J.G.; Yeaman, S.J. Control of glycogen synthesis by glucose, glycogen, and insulin in cultured human muscle cells. Diabetes 2001, 50, 720–726. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Carpentier, A.C.; Poirier, P.; Despres, J.P. Adiposity, type 2 diabetes and atherosclerotic cardiovascular disease risk: Use and abuse of the body mass index. Atherosclerosis 2024, 394, 117546. [Google Scholar] [CrossRef] [PubMed]
- Feijoo-Bandin, S.; Aragon-Herrera, A.; Morana-Fernandez, S.; Anido-Varela, L.; Tarazon, E.; Rosello-Lleti, E.; Portoles, M.; Moscoso, I.; Gualillo, O.; Gonzalez-Juanatey, J.R.; et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Clemente-Suarez, V.J.; Redondo-Florez, L.; Beltran-Velasco, A.I.; Martin-Rodriguez, A.; Martinez-Guardado, I.; Navarro-Jimenez, E.; Laborde-Cardenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Hemat Jouy, S.; Mohan, S.; Scichilone, G.; Mostafa, A.; Mahmoud, A.M. Adipokines in the Crosstalk between Adipose Tissues and Other Organs: Implications in Cardiometabolic Diseases. Biomedicines 2024, 12, 2129. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Q.; Wu, C. The role of adiponectin in cardiovascular disease. Cardiovasc. Pathol. 2023, 64, 107514. [Google Scholar] [CrossRef]
- Mellott, E.; Faulkner, J.L. Mechanisms of leptin-induced endothelial dysfunction. Curr. Opin. Nephrol. Hypertens. 2023, 32, 118–123. [Google Scholar] [CrossRef]
- Bays, H.E.; Kirkpatrick, C.; Maki, K.C.; Toth, P.P.; Morgan, R.T.; Tondt, J.; Christensen, S.M.; Dixon, D.; Jacobson, T.A. Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. Obes. Pillars 2024, 10, 100108. [Google Scholar] [CrossRef]
- Tian, X.; Chen, S.; Wang, P.; Xu, Q.; Zhang, Y.; Luo, Y.; Wu, S.; Wang, A. Insulin resistance mediates obesity-related risk of cardiovascular disease: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Luciani, L.; Pedrelli, M.; Parini, P. Modification of lipoprotein metabolism and function driving atherogenesis in diabetes. Atherosclerosis 2024, 394, 117545. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhu, Q.; Hao, H.; Flaker, G.C.; Liu, Z. N-Acetylcysteine and Atherosclerosis: Promises and Challenges. Antioxidants 2023, 12, 2073. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Panday, S.; Talreja, R.; Kavdia, M. The role of glutathione and glutathione peroxidase in regulating cellular level of reactive oxygen and nitrogen species. Microvasc. Res. 2020, 131, 104010. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Mirabelli, M.; Misiti, R.; Sicilia, L.; Brunetti, F.S.; Chiefari, E.; Brunetti, A.; Foti, D.P. Hypoxia in Human Obesity: New Insights from Inflammation towards Insulin Resistance-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9802. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.A.; Reaven, P.D. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim. Biophys. Acta 2012, 1821, 858–866. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Mancuso, P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.F. Subcellular aspects of the response to trauma. Br. Med. Bull. 1985, 41, 240–245. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Garcia-Banuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef]
- Zhang, W.; Elimban, V.; Nijjar, M.S.; Gupta, S.K.; Dhalla, N.S. Role of mitogen-activated protein kinase in cardiac hypertrophy and heart failure. Exp. Clin. Cardiol. 2003, 8, 173–183. [Google Scholar] [PubMed]
- Li, J.; Sun, M.; Tang, M.; Song, X.; Zheng, K.; Meng, T.; Li, C.; Du, L. Mechanism of PI3K/Akt-mediated mitochondrial pathway in obesity-induced apoptosis (Review). Biomed. Rep. 2025, 22, 40. [Google Scholar] [CrossRef]
- Wu, S.; Zou, M.H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Chen, Q.; Liu, N.; Yan, Y.; Liu, B. SIRT3: A New Regulator of Cardiovascular Diseases. Oxid. Med. Cell Longev. 2018, 2018, 7293861. [Google Scholar] [CrossRef]
- Zhang, Q.; Siyuan, Z.; Xing, C.; Ruxiu, L. SIRT3 regulates mitochondrial function: A promising star target for cardiovascular disease therapy. Biomed. Pharmacother. 2024, 170, 116004. [Google Scholar] [CrossRef]
- Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya, U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-Acetylcysteine and Its Immunomodulatory Properties in Humans and Domesticated Animals. Antioxidants 2023, 12, 1867. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Yi, D.; Wu, G. N-acetylcysteine and intestinal health: A focus on its mechanism of action. Front. Biosci. 2015, 20, 872–891. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, K.Q.; Moura, F.A.; dos Santos, J.M.; de Araujo, O.R.; de Farias Santos, J.C.; Goulart, M.O. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef]
- Mlejnek, P.; Dolezel, P.; Kriegova, E.; Pastvova, N. N-acetylcysteine Can Induce Massive Oxidative Stress, Resulting in Cell Death with Apoptotic Features in Human Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 12635. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Monroy-Ramirez, H.C.; Caloca-Camarena, F.; Arceo-Orozco, S.; Muriel, P.; Sandoval-Rodriguez, A.; Garcia-Banuelos, J.; Garcia-Gonzalez, A.; Navarro-Partida, J.; Armendariz-Borunda, J. A new opportunity for N-acetylcysteine. An outline of its classic antioxidant effects and its pharmacological potential as an epigenetic modulator in liver diseases treatment. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 2365–2386. [Google Scholar] [CrossRef]
- Ezerina, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H(2)S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459.e444. [Google Scholar] [CrossRef]
- Lee, S.J.; Noh, H.J.; Sung, E.G.; Song, I.H.; Kim, J.Y.; Kwon, T.K.; Lee, T.J. Berberine sensitizes TRAIL-induced apoptosis through proteasome-mediated downregulation of c-FLIP and Mcl-1 proteins. Int. J. Oncol. 2011, 38, 485–492. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Mumby, S.; Quinlan, G.J.; Chung, K.F.; Evans, T.W. Pro-oxidant iron is present in human pulmonary epithelial lining fluid: Implications for oxidative stress in the lung. Biochem. Biophys. Res. Commun. 1996, 220, 1024–1027. [Google Scholar] [CrossRef]
- Yu, N.; Wu, X.; Zhang, C.; Qin, Q.; Gu, Y.; Ke, W.; Liu, X.; Zhang, Q.; Liu, Z.; Chen, M.; et al. NADPH and NAC synergistically inhibits chronic ocular hypertension-induced neurodegeneration and neuroinflammation through regulating p38/MAPK pathway and peroxidation. Biomed. Pharmacother. 2024, 175, 116711. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.P.; Cheng, W.L.; Yi, W.; Cai, H.H.; Deng, K.; Cao, J.L.; Zeng, Z.; Wang, H.; Wu, X. N-Acetylcysteine Alleviates Phenylephrine-Induced Cardiomyocyte Dysfunction via Engaging PI3K/AKT Signaling Pathway. Am. J. Hypertens. 2024, 37, 230–238. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Leng, Y.; Xiong, Y.; Xue, R.; Chen, R.; Xia, Z. Mechanism of N-acetylcysteine in alleviating diabetic myocardial ischemia reperfusion injury by regulating PTEN/Akt pathway through promoting DJ-1. Biosci. Rep. 2020, 40, BSR20192118. [Google Scholar] [CrossRef]
- Radomska-Lesniewska, D.M.; Sadowska, A.M.; Van Overveld, F.J.; Demkow, U.; Zielinski, J.; De Backer, W.A. Influence of N-acetylcysteine on ICAM-1 expression and IL-8 release from endothelial and epithelial cells. J. Physiol. Pharmacol. 2006, 57 (Suppl. 4), 325–334. [Google Scholar] [PubMed]
- Sadowska, A.M.; Manuel-y-Keenoy, B.; Vertongen, T.; Schippers, G.; Radomska-Lesniewska, D.; Heytens, E.; De Backer, W.A. Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: In vivo and in vitro study. Pharmacol. Res. 2006, 53, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Lesniewska, D.M.; Skopinska-Rozewska, E.; Jankowska-Steifer, E.; Sobiecka, M.; Sadowska, A.M.; Hevelke, A.; Malejczyk, J. N-acetylcysteine inhibits IL-8 and MMP-9 release and ICAM-1 expression by bronchoalveolar cells from interstitial lung disease patients. Pharmacol. Rep. 2010, 62, 131–138. [Google Scholar] [CrossRef]
- Fan, H.; Le, J.W.; Zhu, J.H. Protective Effect of N-Acetylcysteine Pretreatment on Acute Kidney Injury in Septic Rats. J. Surg. Res. 2020, 254, 125–134. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Cai, J.; Liu, K.; Liu, M.; Wang, H.; Zhang, H. N-acetylcysteine alleviates H2O2-induced damage via regulating the redox status of intracellular antioxidants in H9c2 cells. Int. J. Mol. Med. 2019, 43, 199–208. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, L.; Zhu, L.; Ran, B.; Xue, X.; Wang, Z. N-acetylcysteine alleviated bisphenol A-induced testicular DNA hypermethylation of rare minnow (Gobiocypris rarus) by increasing cysteine contents. Ecotoxicol. Environ. Saf. 2019, 173, 243–250. [Google Scholar] [CrossRef]
- Aksit, H.; Bildik, A. Determination of DNA damage in experimental liver intoxication and role of N-acetyl cysteine. Cell Biochem. Biophys. 2014, 70, 1119–1125. [Google Scholar] [CrossRef]
- Wang, L.L.; Huang, Y.H.; Yan, C.Y.; Wei, X.D.; Hou, J.Q.; Pu, J.X.; Lv, J.X. N-acetylcysteine Ameliorates Prostatitis via miR-141 Regulating Keap1/Nrf2 Signaling. Inflammation 2016, 39, 938–947. [Google Scholar] [CrossRef]
- Yang, W.; Guo, R.; Pi, A.; Ding, Q.; Hao, L.; Song, Q.; Chen, L.; Dou, X.; Na, L.; Li, S. Long non-coding RNA-EN_181 potentially contributes to the protective effects of N-acetylcysteine against non-alcoholic fatty liver disease in mice. Br. J. Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Kolanu, N.D.; Syeda, Z.R.; Joshi, N.; Singh, P.; Erukulla, M. The Differential Impact of Medical Therapy and Lifestyle Modification on Cardiovascular Health and Risk of Adverse Cardiovascular Events: A Narrative Review. Cureus 2024, 16, e57742. [Google Scholar] [CrossRef] [PubMed]
- Straub, L.G.; Efthymiou, V.; Grandl, G.; Balaz, M.; Challa, T.D.; Truscello, L.; Horvath, C.; Moser, C.; Rachamin, Y.; Arnold, M.; et al. Antioxidants protect against diabetes by improving glucose homeostasis in mouse models of inducible insulin resistance and obesity. Diabetologia 2019, 62, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef]
- Quesada-Vazquez, S.; Antolin, A.; Colom-Pellicer, M.; Aragones, G.; Herrero, L.; Del Bas, J.M.; Caimari, A.; Escote, X. Reduction of Obesity and Insulin Resistance through Dual Targeting of VAT and BAT by a Novel Combination of Metabolic Cofactors. Int. J. Mol. Sci. 2022, 23, 14923. [Google Scholar] [CrossRef] [PubMed]
- Garicano Vilar, E.; Sanz Rojo, S.; Lopez Oliva, S.; Martinez, S.; Terren Lora, A.; San Mauro Martin, I. Effect of MetioNac(R) in patients with metabolic syndrome who are at risk of metabolic dysfunction associated fatty liver disease: A randomized controlled trial. Nutr. Hosp. 2023, 40, 755–762. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Tiano, L.; Marcheggiani, F.; Cirilli, I.; Louw, J.; Nkambule, B.B. The beneficial effects of N-acetyl cysteine (NAC) against obesity associated complications: A systematic review of pre-clinical studies. Pharmacol. Res. 2019, 146, 104332. [Google Scholar] [CrossRef]
- Calzadilla, P.; Gomez-Serrano, M.; Garcia-Santos, E.; Schiappacasse, A.; Abalde, Y.; Calvo, J.C.; Peral, B.; Guerra, L.N. N-Acetylcysteine affects obesity-related protein expression in 3T3-L1 adipocytes. Redox Rep. 2013, 18, 210–218. [Google Scholar] [CrossRef]
- Raffaele, M.; Barbagallo, I.; Licari, M.; Carota, G.; Sferrazzo, G.; Spampinato, M.; Sorrenti, V.; Vanella, L. N-Acetylcysteine (NAC) Ameliorates Lipid-Related Metabolic Dysfunction in Bone Marrow Stromal Cells-Derived Adipocytes. Evid. Based Complement. Altern. Med. 2018, 2018, 5310961. [Google Scholar] [CrossRef]
- da Silva, K.S.; Pinto, P.R.; Fabre, N.T.; Gomes, D.J.; Thieme, K.; Okuda, L.S.; Iborra, R.T.; Freitas, V.G.; Shimizu, M.H.M.; Teodoro, W.R.; et al. N-acetylcysteine Counteracts Adipose Tissue Macrophage Infiltration and Insulin Resistance Elicited by Advanced Glycated Albumin in Healthy Rats. Front. Physiol. 2017, 8, 723. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Dajnowicz-Brzezik, P.; Lukaszuk, B.; Zebrowska, E.; Maciejczyk, M.; Zalewska, A.; Kasacka, I.; Chabowski, A. Diverse impact of N-acetylcysteine or alpha-lipoic acid supplementation during high-fat diet regime on fatty acid transporters in visceral and subcutaneous adipose tissue. Adv. Med. Sci. 2022, 67, 216–228. [Google Scholar] [CrossRef]
- Manuel, A.M.; Walla, M.D.; Dorn, M.T.; Tanis, R.M.; Piroli, G.G.; Frizzell, N. Fumarate and oxidative stress synergize to promote stability of C/EBP homologous protein in the adipocyte. Free Radic. Biol. Med. 2020, 148, 70–82. [Google Scholar] [CrossRef]
- Pieri, B.; Rodrigues, M.S.; Farias, H.R.; Silveira, G.B.; Ribeiro, V.; Silveira, P.C.L.; De Souza, C.T. Role of Oxidative Stress on Insulin Resistance in Diet-Induced Obesity Mice. Int. J. Mol. Sci. 2023, 24, 12088. [Google Scholar] [CrossRef]
- Olson, D.H.; Burrill, J.S.; Kuzmicic, J.; Hahn, W.S.; Park, J.M.; Kim, D.H.; Bernlohr, D.A. Down regulation of Peroxiredoxin-3 in 3T3-L1 adipocytes leads to oxidation of Rictor in the mammalian-target of rapamycin complex 2 (mTORC2). Biochem. Biophys. Res. Commun. 2017, 493, 1311–1317. [Google Scholar] [CrossRef]
- Hodun, K.; Sztolsztener, K.; Chabowski, A. Antioxidants Supplementation Reduces Ceramide Synthesis Improving the Cardiac Insulin Transduction Pathway in a Rodent Model of Obesity. Nutrients 2021, 13, 3413. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Pieri, B.; Silveira, G.B.; Zaccaron, R.P.; Venturini, L.M.; Comin, V.H.; Luiz, K.D.; Silveira, P.C.L. Reduction of oxidative stress improves insulin signaling in cardiac tissue of obese mice. Einstein 2020, 18, eAO5022. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Dai, Z.; Sun, Y. Intermittent high glucose stimulate MCP-l, IL-18, and PAI-1, but inhibit adiponectin expression and secretion in adipocytes dependent of ROS. Cell Biochem. Biophys. 2009, 55, 173–180. [Google Scholar] [CrossRef]
- Fang, X.; Liu, L.; Zhou, S.; Zhu, M.; Wang, B. N-acetylcysteine inhibits atherosclerosis by correcting glutathione-dependent methylglyoxal elimination and dicarbonyl/oxidative stress in the aorta of diabetic mice. Mol. Med. Rep. 2021, 23, 201. [Google Scholar] [CrossRef]
- Keshk, W.A.; Ibrahim, M.A.; Shalaby, S.M.; Zalat, Z.A.; Elseady, W.S. Redox status, inflammation, necroptosis and inflammasome as indispensable contributors to high fat diet (HFD)-induced neurodegeneration; Effect of N-acetylcysteine (NAC). Arch. Biochem. Biophys. 2020, 680, 108227. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Boss, M.; Zezina, E.; Weigert, A.; Dehne, N.; Fleming, I.; Brune, B.; Namgaladze, D. Hypoxia Potentiates Palmitate-induced Pro-inflammatory Activation of Primary Human Macrophages. J. Biol. Chem. 2016, 291, 413–424. [Google Scholar] [CrossRef]
- Diaz-Saez, F.; Balcells, C.; Rossello, L.; Lopez-Soldado, I.; Romero, M.; Sebastian, D.; Lopez-Soriano, F.J.; Busquets, S.; Cascante, M.; Ricart, W.; et al. Neuregulin 4 Downregulation Alters Mitochondrial Morphology and Induces Oxidative Stress in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2024, 25, 11718. [Google Scholar] [CrossRef]
- Sztolsztener, K.; Bzdega, W.; Hodun, K.; Chabowski, A. N-Acetylcysteine Decreases Myocardial Content of Inflammatory Mediators Preventing the Development of Inflammation State and Oxidative Stress in Rats Subjected to a High-Fat Diet. Int. J. Inflam. 2023, 2023, 5480199. [Google Scholar] [CrossRef]
- Alnahdi, A.; John, A.; Raza, H. Mitigation of Glucolipotoxicity-Induced Apoptosis, Mitochondrial Dysfunction, and Metabolic Stress by N-Acetyl Cysteine in Pancreatic beta-Cells. Biomolecules 2020, 10, 239. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Im, S.S.; Bae, J.H.; Song, D.K. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biol. 2020, 37, 101749. [Google Scholar] [CrossRef]
- Chen, Y.W.; Harris, R.A.; Hatahet, Z.; Chou, K.M. Ablation of XP-V gene causes adipose tissue senescence and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2015, 112, E4556–E4564. [Google Scholar] [CrossRef]
- Sztolsztener, K.; Michalak, D.; Chabowski, A. N-acetylcysteine influence on PI3K/Akt/mTOR and sphingolipid pathways in rats with MASLD induced by HFD: A promising new therapeutic purpose. Mol. Cell Endocrinol. 2025, 603, 112545. [Google Scholar] [CrossRef]
- Bauza-Thorbrugge, M.; Peris, E.; Zamani, S.; Micallef, P.; Paul, A.; Bartesaghi, S.; Benrick, A.; Wernstedt Asterholm, I. NRF2 is essential for adaptative browning of white adipocytes. Redox Biol. 2023, 68, 102951. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, X.; Wang, Y.; Chen, H.; Chai, Y. ROS-activated NLRP3 inflammasome initiates inflammation in delayed wound healing in diabetic rats. Int. J. Clin. Exp. Pathol. 2017, 10, 9902–9909. [Google Scholar] [PubMed]
- Soto, D.; Gomez-Serrano, M.; Pieralisi, A.; Calvo, J.C.; Peral, B.; Guerra, L.N. N-acetylcysteine inhibits kinase phosphorylation during 3T3-L1 adipocyte differentiation. Redox Rep. 2017, 22, 265–271. [Google Scholar] [CrossRef]
- Wang, T.; Mao, X.; Li, H.; Qiao, S.; Xu, A.; Wang, J.; Lei, S.; Liu, Z.; Ng, K.F.; Wong, G.T.; et al. N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic. Biol. Med. 2013, 63, 291–303. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.; Li, D.; Zhang, X.; Shi, B.; Liu, Q.; Fang, C.; Xu, S.; Zhang, Z. Hippo-YAP/TAZ-ROS signaling axis regulates metaflammation induced by SelenoM deficiency in high-fat diet-derived obesity. J. Adv. Res. 2025, 71, 603–620. [Google Scholar] [CrossRef]
- Russell-Guzman, J.; Americo-Da Silva, L.; Cadagan, C.; Maturana, M.; Palomero, J.; Estrada, M.; Barrientos, G.; Buvinic, S.; Hidalgo, C.; Llanos, P. Activation of the ROS/TXNIP/NLRP3 pathway disrupts insulin-dependent glucose uptake in skeletal muscle of insulin-resistant obese mice. Free Radic. Biol. Med. 2024, 222, 187–198. [Google Scholar] [CrossRef]
- Argaev-Frenkel, L.; Rosenzweig, T. Complexity of NAC Action as an Antidiabetic Agent: Opposing Effects of Oxidative and Reductive Stress on Insulin Secretion and Insulin Signaling. Int. J. Mol. Sci. 2022, 23, 2965. [Google Scholar] [CrossRef]
- Schuurman, M.; Wallace, M.; Sahi, G.; Barillaro, M.; Zhang, S.; Rahman, M.; Sawyez, C.; Borradaile, N.; Wang, R. N-acetyl-L-cysteine treatment reduces beta-cell oxidative stress and pancreatic stellate cell activity in a high fat diet-induced diabetic mouse model. Front. Endocrinol. 2022, 13, 938680. [Google Scholar] [CrossRef]
- Kaneto, H.; Kajimoto, Y.; Miyagawa, J.; Matsuoka, T.; Fujitani, Y.; Umayahara, Y.; Hanafusa, T.; Matsuzawa, Y.; Yamasaki, Y.; Hori, M. Beneficial effects of antioxidants in diabetes: Possible protection of pancreatic beta-cells against glucose toxicity. Diabetes 1999, 48, 2398–2406. [Google Scholar] [CrossRef]
- Pereira, S.; Shah, A.; George Fantus, I.; Joseph, J.W.; Giacca, A. Effect of N-acetyl-l-cysteine on insulin resistance caused by prolonged free fatty acid elevation. J. Endocrinol. 2015, 225, 1–7. [Google Scholar] [CrossRef]
- Grzych, G.; Pekar, J.D.; Chevalier-Curt, M.J.; Decoin, R.; Vergriete, P.; Henry, H.; Odou, P.; Maboudou, P.; Brousseau, T.; Vamecq, J. Antioxidants other than vitamin C may be detected by glucose meters: Immediate relevance for patients with disorders targeted by antioxidant therapies. Clin. Biochem. 2021, 92, 71–76. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Bahijri, S.; Eldakhakhny, B.; Tuomilehto, J.; Wu, F.; Ren, J. Hallmarks and mechanisms of cellular senescence in aging and disease. Cell Death Discov. 2025, 11, 364. [Google Scholar] [CrossRef]
- Owesny, P.; Grune, T. The link between obesity and aging—Insights into cardiac energy metabolism. Mech. Ageing Dev. 2023, 216, 111870. [Google Scholar] [CrossRef]
- Salvestrini, V.; Sell, C.; Lorenzini, A. Obesity May Accelerate the Aging Process. Front. Endocrinol. 2019, 10, 266. [Google Scholar] [CrossRef]
- Ou, M.Y.; Zhang, H.; Tan, P.C.; Zhou, S.B.; Li, Q.F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 300. [Google Scholar] [CrossRef]
- Behtaj, D.; Ghorbani, A.; Eslamian, G.; Malekpour Alamdari, N.; Abbasi, M.; Zand, H.; Shakery, A.; Shimi, G.; Sohouli, M.H.; Fazeli Taherian, S. Ex vivo Anti-Senescence Activity of N-Acetylcysteine in Visceral Adipose Tissue of Obese Volunteers. Obes. Facts 2024, 17, 355–363. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Eslamian, G.; Malekpour Alamdari, N.; Abbasi, M.; Fazeli Taherian, S.; Behtaj, D.; Zand, H. Effects of N-acetylcysteine on aging cell and obesity complications in obese adults: A randomized, double-blind clinical trial. Front. Nutr. 2023, 10, 1237869. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R.V. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 75–89. [Google Scholar] [CrossRef]
- Calzadilla, P.; Sapochnik, D.; Cosentino, S.; Diz, V.; Dicelio, L.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011, 12, 6936–6951. [Google Scholar] [CrossRef]
- Pieralisi, A.; Martini, C.; Soto, D.; Vila, M.C.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine inhibits lipid accumulation in mouse embryonic adipocytes. Redox Biol. 2016, 9, 39–44. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.H.; Piao, M.; Kim, H.S.; Lee, K.Y. Metallothionein 3 Inhibits 3T3-L1 Adipocyte Differentiation via Reduction of Reactive Oxygen Species. Antioxidants 2023, 12, 640. [Google Scholar] [CrossRef]
- Devlin, M.J.; Rosen, C.J. The bone-fat interface: Basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015, 3, 141–147. [Google Scholar] [CrossRef]
- Ferraro, F.; Lymperi, S.; Mendez-Ferrer, S.; Saez, B.; Spencer, J.A.; Yeap, B.Y.; Masselli, E.; Graiani, G.; Prezioso, L.; Rizzini, E.L.; et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci. Transl. Med. 2011, 3, 104ra101. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Li, H.; Wang, C.; Chen, C.; Tang, J.; Zhou, M.; Hong, X.; Cheng, Y.; Wu, Q.; Zhang, X.; et al. Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: A meta-analysis. Front. Endocrinol. 2022, 13, 934225. [Google Scholar] [CrossRef]
- Ro, S.H.; Nam, M.; Jang, I.; Park, H.W.; Park, H.; Semple, I.A.; Kim, M.; Kim, J.S.; Park, H.; Einat, P.; et al. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc. Natl. Acad. Sci. USA 2014, 111, 7849–7854. [Google Scholar] [CrossRef]
- Peris, E.; Micallef, P.; Paul, A.; Palsdottir, V.; Enejder, A.; Bauza-Thorbrugge, M.; Olofsson, C.S.; Wernstedt Asterholm, I. Antioxidant treatment induces reductive stress associated with mitochondrial dysfunction in adipocytes. J. Biol. Chem. 2019, 294, 2340–2352. [Google Scholar] [CrossRef]
- Garcia-Serrano, A.M.; Vieira, J.P.P.; Fleischhart, V.; Duarte, J.M.N. Taurine and N-acetylcysteine treatments prevent memory impairment and metabolite profile alterations in the hippocampus of high-fat diet-fed female mice. Nutr. Neurosci. 2023, 26, 1090–1102. [Google Scholar] [CrossRef]
- Hurley, M.M.; Resch, J.M.; Maunze, B.; Frenkel, M.M.; Baker, D.A.; Choi, S. N-acetylcysteine decreases binge eating in a rodent model. Int. J. Obes. 2016, 40, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Sketriene, D.; Battista, D.; Perry, C.J.; Sumithran, P.; Lawrence, A.J.; Brown, R.M. N-acetylcysteine reduces addiction-like behaviour towards high-fat high-sugar food in diet-induced obese rats. Eur. J. Neurosci. 2021, 54, 4877–4887. [Google Scholar] [CrossRef] [PubMed]
- John, P.; Marenco, R.E.F. The Clinical Use of N-Acetylcysteine in Cardiology, 1st ed.; Adis Singapore: Singapore, 2018; p. 394. [Google Scholar]
- Bartkowiak, K.; Bartkowiak, M.; Jankowska-Steifer, E.; Ratajska, A.; Kujawa, M.; Aniolek, O.; Niderla-Bielinska, J. Metabolic Syndrome and Cardiac Vessel Remodeling Associated with Vessel Rarefaction: A Possible Underlying Mechanism May Result from a Poor Angiogenic Response to Altered VEGF Signaling Pathways. J. Vasc. Res. 2024, 61, 151–159. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, I.; Bashir, Z.; Sarwar, M.; Arshad, M.; Ishtiaq, A.; Khan, W.; Khan, U.; Tabassum, S.; Ali, T.; Fatima, T.; et al. N-Acetyl Cysteine, Selenium, and Ascorbic Acid Rescue Diabetic Cardiac Hypertrophy via Mitochondrial-Associated Redox Regulators. Molecules 2021, 26, 7285. [Google Scholar] [CrossRef]
- Marian, A.J.; Tan, Y.; Li, L.; Chang, J.; Syrris, P.; Hessabi, M.; Rahbar, M.H.; Willerson, J.T.; Cheong, B.Y.; Liu, C.Y.; et al. Hypertrophy Regression With N-Acetylcysteine in Hypertrophic Cardiomyopathy (HALT-HCM): A Randomized, Placebo-Controlled, Double-Blind Pilot Study. Circ. Res. 2018, 122, 1109–1118. [Google Scholar] [CrossRef]
- Kelley, R.C.; Lapierre, S.S.; Muscato, D.R.; Hahn, D.; Christou, D.D.; Ferreira, L.F. Cardiac and respiratory muscle responses to dietary N-acetylcysteine in rats consuming a high-saturated fat, high-sucrose diet. Exp. Physiol. 2022, 107, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Sivasinprasasn, S.; Chattipakorn, K.; Pratchayasakul, W.; Chattipakorn, S.C.; Chattipakorn, N. N-Acetylcysteine enhances low-dose estrogen efficacy against ischemia-reperfusion injury in estrogen-deprived obese insulin-resistant rats. Menopause 2025, 32, 81–90. [Google Scholar] [CrossRef]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Mamarbachi, A.M.; Villeneuve, L.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech. Ageing Dev. 2008, 129, 261–270. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Al-Kassab-Cordova, A.; Cabrera-Guzman, J.C.; Herrera-Anazco, P.; Benites-Zapata, V.A. Vitamin B12, folate, and homocysteine in metabolic syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1221259. [Google Scholar] [CrossRef]
- Ventura, P.; Panini, R.; Pasini, M.C.; Scarpetta, G.; Salvioli, G. N -Acetyl-cysteine reduces homocysteine plasma levels after single intravenous administration by increasing thiols urinary excretion. Pharmacol. Res. 1999, 40, 345–350. [Google Scholar] [CrossRef]
- Wiklund, O.; Fager, G.; Andersson, A.; Lundstam, U.; Masson, P.; Hultberg, B. N-acetylcysteine treatment lowers plasma homocysteine but not serum lipoprotein(a) levels. Atherosclerosis 1996, 119, 99–106. [Google Scholar] [CrossRef]
- Hildebrandt, W.; Sauer, R.; Bonaterra, G.; Dugi, K.A.; Edler, L.; Kinscherf, R. Oral N-acetylcysteine reduces plasma homocysteine concentrations regardless of lipid or smoking status. Am. J. Clin. Nutr. 2015, 102, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Faghfouri, A.H.; Zarezadeh, M.; Tavakoli-Rouzbehani, O.M.; Radkhah, N.; Faghfuri, E.; Kord-Varkaneh, H.; Tan, S.C.; Ostadrahimi, A. The effects of N-acetylcysteine on inflammatory and oxidative stress biomarkers: A systematic review and meta-analysis of controlled clinical trials. Eur. J. Pharmacol. 2020, 884, 173368. [Google Scholar] [CrossRef]
- Ardissino, D.; Merlini, P.A.; Savonitto, S.; Demicheli, G.; Zanini, P.; Bertocchi, F.; Falcone, C.; Ghio, S.; Marinoni, G.; Montemartini, C.; et al. Effect of transdermal nitroglycerin or N-acetylcysteine, or both, in the long-term treatment of unstable angina pectoris. J. Am. Coll. Cardiol. 1997, 29, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Martina, V.; Masha, A.; Gigliardi, V.R.; Brocato, L.; Manzato, E.; Berchio, A.; Massarenti, P.; Settanni, F.; Della Casa, L.; Bergamini, S.; et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care 2008, 31, 940–944. [Google Scholar] [CrossRef]
- Barrios, V.; Calderon, A.; Navarro-Cid, J.; Lahera, V.; Ruilope, L.M. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002, 11, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Lodola, E.; Licciardello, L.; Colombo, A. Use of N-acetylcysteine in the management of coronary artery diseases. Cardiologia 1999, 44, 633–637. [Google Scholar] [PubMed]
- Sun, M.; Lu, Z.; Chen, W.M.; Lv, S.; Fu, N.; Yang, Y.; Wang, Y.; Miao, M.; Wu, S.Y.; Zhang, J. N-acetylcysteine therapy reduces major adverse cardiovascular events in patients with type 2 diabetes mellitus. Atherosclerosis 2025, 402, 119117. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Khalili, H.; Fahimi, F.; Jenab, Y.; Broumand, M.A.; Salarifar, M.; Darabi, F. Effects of N-acetylcysteine on the cardiac remodeling biomarkers and major adverse events following acute myocardial infarction: A randomized clinical trial. Am. J. Cardiovasc. Drugs 2014, 14, 51–61. [Google Scholar] [CrossRef]
- Yesilbursa, D.; Serdar, A.; Senturk, T.; Serdar, Z.; Sag, S.; Cordan, J. Effect of N-acetylcysteine on oxidative stress and ventricular function in patients with myocardial infarction. Heart Vessel. 2006, 21, 33–37. [Google Scholar] [CrossRef]
- Arstall, M.A.; Yang, J.; Stafford, I.; Betts, W.H.; Horowitz, J.D. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects. Circulation 1995, 92, 2855–2862. [Google Scholar] [CrossRef]
- Sajkowska, A.; Wykretowicz, A.; Szczepanik, A.; Kempa, M.; Minczykowski, A.; Wysocki, H. Fibrinolytic therapy and n-acetylocysteine in the treatment of patients with acute myocardial infarction: Its influence on authentic plasma hydroperoxide levels and polymorphonuclear neutrophil oxygen metabolism. Cardiology 1999, 91, 60–65. [Google Scholar] [CrossRef]
- Thiele, H.; Hildebrand, L.; Schirdewahn, C.; Eitel, I.; Adams, V.; Fuernau, G.; Erbs, S.; Linke, A.; Diederich, K.W.; Nowak, M.; et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J. Am. Coll. Cardiol. 2010, 55, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early Use of N-acetylcysteine With Nitrate Therapy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction Reduces Myocardial Infarct Size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [CrossRef] [PubMed]
| Metabolic Pathways | Physiological Function | Effect of Dysregulation | Impact of NAC | Model/Dosage |
|---|---|---|---|---|
| Lipidmetabolism | Fatty acid oxidation, energy balance | Dyslipidemia, ectopic fat accumulation | Increases fatty acid oxidation, reduces lipogenesis, normalizes lipid profiles | cultured 3T3-L1 preadipocytes, 10 μM NAC [92] BMAT in vitro, 10 mM NAC [93] Wistar rats injected AGE-albumin, NAC in drinking water (600 mg/L) [94] HFD rat, 500 mg/kg NAC [95] |
| Glucose metabolism | Maintaining energy homeostasis via ATP production, glucose synthesis, redox balance, and storage | Dysregulated glucose flux, lactate accumulation, oxidative stress, insulin resistance | Improves insulin signaling, reduces oxidative stress, enhances glycogen synthesis, modulates glycolysis, improves glucose tolerance and uptake | 3T3-L1 murine fibroblasts, 2.5 or 5 mM NAC [96] iFIRKO mice, 15 mmol/L NAC in drinking water [87] HFD mice, 50 mg/kg NAC [97] |
| Insulin signaling | Glucose uptake, lipid metabolism | Insulin resistance, hyperglycemia | Improves insulin sensitivity, reduces inflammation in adipose tissue | 3T3-L1 fibroblasts, 50 μM NAC [98] HFD rat, 500 mg/kg NAC [99] HFD mice, 50 mg/kg NAC [100] |
| Oxidative stress response | ROS detoxification, redox balance | Increased accumulation of ROS—endothelial dysfunction, mitochondrial damage | Increases GSH, scavenges ROS, protects mitochondria and vascular endothelium | 3T3-L1 adipocytes, 1.0 mmol/L NAC [101] ApoE-/- mice, 2 mmol/L NAC in drinking water [102] HFD rat, 150 mg/kg NAC [103] |
| Metaflammation | Immune regulation, tissue repair | Chronic low-grade Inflammation, elevated proinflammatory cytokines such tnf-α, il-6 | Reduces pro-inflammatory cytokines, attenuates chronic low-grade inflammation, improves adiponectin levels | human ATM, 5 mM NAC [104] 3T3-L1 adipocytes, 1 mM NAC [105] HFD rat, 500 mg/kg NAC [106] |
| Mitochondrial metabolism | Energy production, apoptosis regulation | Mitochondrial dysfunction, reduced membrane potential | Improves energy metabolism and prevents mitochondrial dysfunction in adipose tissue and cardiomyocytes, restores mitochondrial membrane potential | pancreatic-βcells, 10 mM NAC [107] HFD mice, 60 mg/kg NAC [108] |
| Molecular hallmarks of aging | Increase in activity with age | Increase in sa–β-gal and p16, p21 expression | Reduces SA–β-gal activity and expression of p16, p21 | pol η−/− mice, 1 mg/mL NAC in drinking water [109] |
| Apoptosis | Cell growth, metabolism, and survival | Hypertrophy and apoptosis of cardiomyocytes | Reduces oxidative stress-induced hypertrophy and cardiomyocyte apoptosis | pancreatic-βcells, 10 mM NAC [107] NRCM cardiomyocytes, 100 µM NAC [75] |
| Cellular Signaling | Physiological Role | Effect of Dysregulation | Impact of NAC | Model/Dosage |
|---|---|---|---|---|
| AMPK | Energy sensor, catabolic pathways activation at low ATP | Impaired energy balance, lipid accumulation | Indirectly supports AMPK activation, restores redox balance, | pancreatic-βcells, 10 mM NAC [107] HFD mice, 60 mg/kg NAC [108] |
| m-TOR | Cell growth, protein synthesis regulation, and nutrient sensing | Lipogenesis promotion, insulin resistance | Reduces oxidative stress, may normalize mTOR signaling | pancreatic-βcells, 10 mM NAC [107] HFD rat, 500 mg/kg NAC [110] |
| ROS/NRF2 | cellular resistance to oxidation regulation | Inflammation, cellular damage | Boosts glutathione, activates NRF2, reduces ROS | 3T3-L1 murine fibro-blasts, 2.5 or 5 mM NAC [96] HFD mice, 0.5, 2 or 10 g/L NAC in drinking water [111] |
| ROS–NLRP3 | Inflammatory response triggered by mitochondrial DAMPs | Chronic inflammation and tissue injury, insulin resistance in skeletal muscle | Inhibits NLRP3 activation by lowering ROS restored insulin-dependent glucose uptake | HFD rat, 150 mg/kg NAC [103] HFD diabetic rat, 20 mg/kg NAC [112] |
| PI3K/AKT | Insulin signaling mediation, glucose uptake regulation | Insulin resistance, hyperglycemia, metabolic imbalance, oxidative stress and inflammation increase, mitochondrial dysfunction and apoptosis of cardiomyocytes, pathological cardiac remodeling and progression to HF | Improves insulin sensitivity by reducing oxidative and ER stress, enhances AKT expression, elevates phosphorylated AKT levels | NRCM cardiomyocytes, 100 µM NAC [75] 3T3-L1 fibroblasts, 50 μM NAC [98] HFD mice, 50 mg/kg NAC [97] HFD mice, 50 mg/kg NAC [100] |
| SIRT1/ SIRT3 | Mitochondrial function oxidative stress, and metabolism regulation | Mitochondrial dysfunction and ROS accumulation | Increases the NAD+/NADH ratio, supports SIRT1/3 activity, reduces inflammation | BMAT in vitro, 10 mM NAC [93] |
| NFκB/ AP-1/ JNK | Inflammatory response regulation | Chronic inflammation; insulin resistance, endothelial dysfunction | Inhibits NFκB-mediated inflammation | 3T3-L1 adipocytes, 1 mM NAC [105] HFD rat, 500 mg/kg NAC [110] |
| MAPK (ERK/JNK) | Cellular proliferation, differentiation, and stress response regulation | Adipogenesis and inflammation activation | Inhibits ERK1/2 and JNK1/2 phosphorylation; suppresses adipocyte differentiation and inflammatory signaling | 3T3-L1 preadipocytes, 0.01 or 1 mM NAC [113] |
| STAT3 | Cytokine signaling and immune response regulation | Chronic inflammation and metabolic dysfunction activation | Inhibits STAT3 activation via antioxidant and anti-inflammatory mechanisms | HFD diabetic rat, 1.5 g/kgNAC [114] |
| GSK3β | Glycogen synthesis and insulin signaling regulation | Altered glucose metabolism and defective insulin signaling | Restores GSK3β phosphorylation; improves insulin signaling | HFD rat, 500 mg/kg NAC [110] T3-L1 preadipocytes, 100 µM NAC [115] |
| IRS1/PI3K | Insulin signaling cascade initiation | Glucose uptake dysfunction, insulin resistance | Enhances IRS1/PI3K association, improves insulin signalling | HFD mice, 50 mg/kg NAC [97] |
| CHOP | ER stress marker, apoptosis promotion | ER stress, mitochondrial dysfunction, inflammatory activation | Reduces CHOP accumulation; restores anti-inflammatory cytokine secretion | 3T3-L1 murine fibroblasts, 2.5 or 5 mM NAC [96] |
| UCP1/ PGC-1α | Thermogenic response and mitochondrial biogenesis induction | Lowered energy output and adiposity development | Induces browning of WAT via NRF2/HO-1 axis; upregulates UCP1 and PGC-1α | HFD mice, 400 mg/kg NAC (with other cofactors) [89] HFD mice, 0.5, 2, or 10 g/L NAC in drinking water 111 [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radomska-Leśniewska, D.M.; Niderla-Bielińska, J.; Kujawa, M.; Jankowska-Steifer, E. Targeting Metabolic Dysregulation in Obesity and Metabolic Syndrome: The Emerging Role of N-Acetylcysteine. Metabolites 2025, 15, 645. https://doi.org/10.3390/metabo15100645
Radomska-Leśniewska DM, Niderla-Bielińska J, Kujawa M, Jankowska-Steifer E. Targeting Metabolic Dysregulation in Obesity and Metabolic Syndrome: The Emerging Role of N-Acetylcysteine. Metabolites. 2025; 15(10):645. https://doi.org/10.3390/metabo15100645
Chicago/Turabian StyleRadomska-Leśniewska, Dorota Magdalena, Justyna Niderla-Bielińska, Marek Kujawa, and Ewa Jankowska-Steifer. 2025. "Targeting Metabolic Dysregulation in Obesity and Metabolic Syndrome: The Emerging Role of N-Acetylcysteine" Metabolites 15, no. 10: 645. https://doi.org/10.3390/metabo15100645
APA StyleRadomska-Leśniewska, D. M., Niderla-Bielińska, J., Kujawa, M., & Jankowska-Steifer, E. (2025). Targeting Metabolic Dysregulation in Obesity and Metabolic Syndrome: The Emerging Role of N-Acetylcysteine. Metabolites, 15(10), 645. https://doi.org/10.3390/metabo15100645




