Serum Uric Acid and Bone Health in Middle-Aged and Elderly Hypertensive Patients: A Potential U-Shaped Association and Implications for Future Fracture Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection and Definitions of Variable Information
2.3. BMD Assessment
2.4. FRAX Scores
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Effect of SUA on BMD
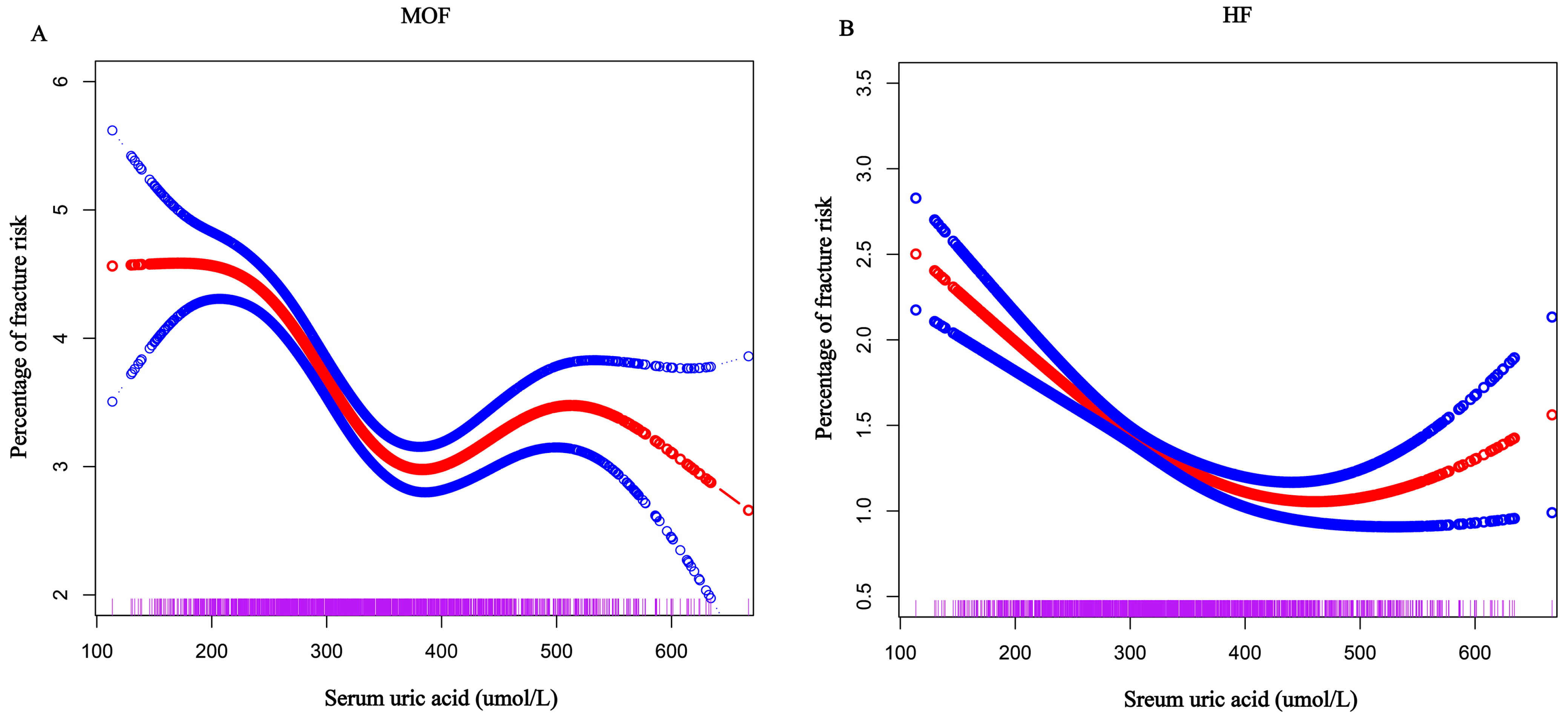
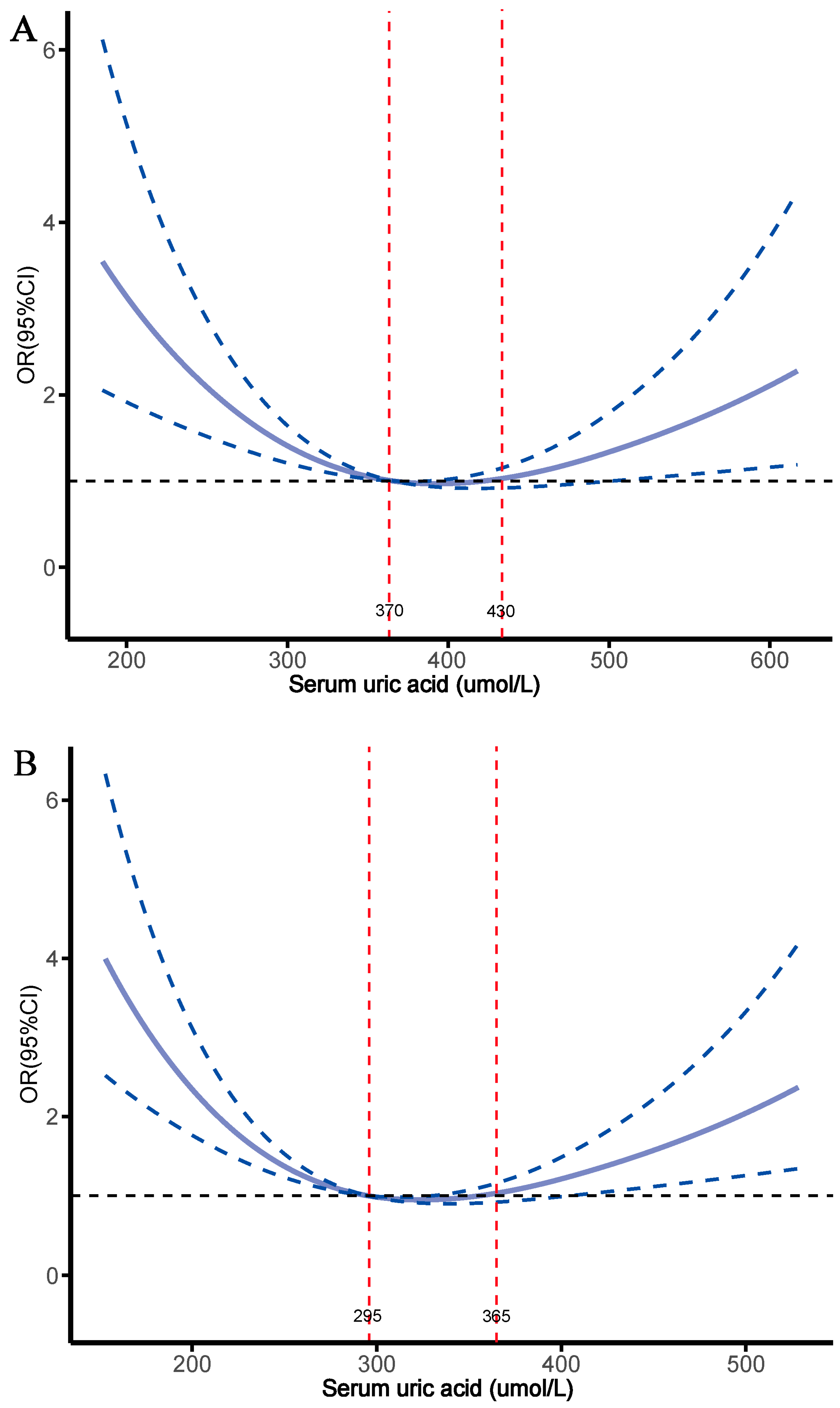
3.3. Relationship Between SUA Levels and FRAX Score
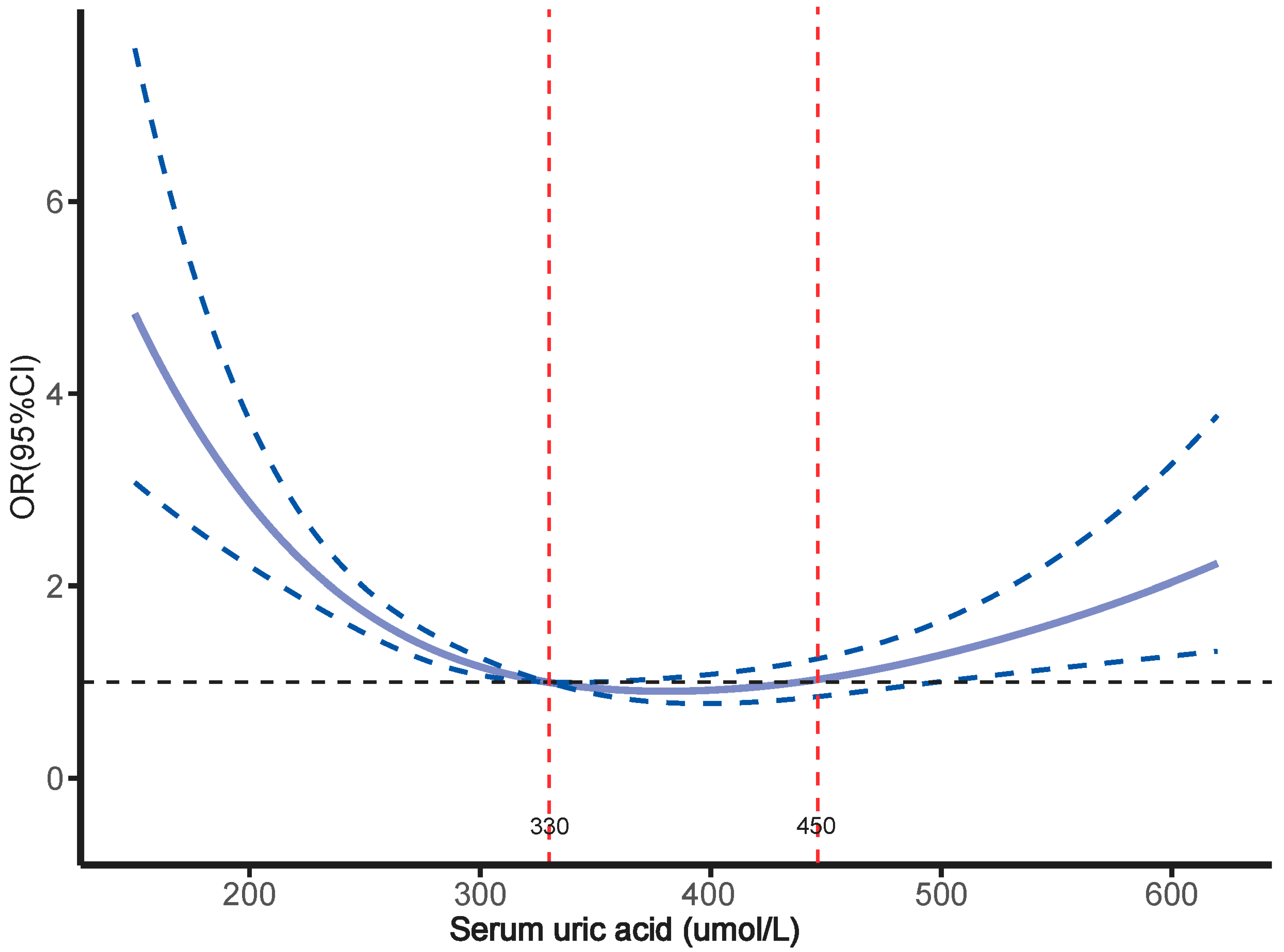
3.4. Relationship Between SUA Levels and Osteoporosis
3.5. Subgroup and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Ilić, K.; Obradović, N.; Vujasinović-Stupar, N. The relationship among hypertension, antihypertensive medications, and osteoporosis: A narrative review. Calcif. Tissue Int. 2013, 92, 217–227. [Google Scholar] [CrossRef]
- Liu, S.; Wu, S.; Bao, X.; Ji, J.; Ye, Y.; Guo, J.; Liu, J.; Wang, X.; Zhang, Y.; Hao, D.; et al. Changes in Blood Pressure is Associated with Bone Loss in US Adults: A Cross-Sectional Study from NHANES 2005-2018. Calcif. Tissue Int. 2024, 114, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Shin, J.; Min, S.Y.; Lim, Y.H.; Kim, K.S.; Kim, S.G.; Kim, J.H.; Lim, H.K. The relationship between bone mineral density and blood pressure in the Korean elderly population: The Korea National Health and Nutrition Examination Survey, 2008–2011. Clin. Exp. Hypertens. 2015, 37, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Sànchez-Riera, L.; Carnahan, E.; Vos, T.; Veerman, L.; Norman, R.; Lim, S.S.; Hoy, D.; Smith, E.; Wilson, N.; Nolla, J.M.; et al. The global burden attributable to low bone mineral density. Ann. Rheum. Dis. 2014, 73, 1635–1645. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, itc17–itc32. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Ann. Intern. Med. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Jt. Surg. 2021, 103, 1451–1461. [Google Scholar] [CrossRef]
- Dalbeth, N.; Choi, H.K.; Joosten, L.A.B.; Khanna, P.P.; Matsuo, H.; Perez-Ruiz, F.; Stamp, L.K. Gout. Nat. Rev. Dis. Primers 2019, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Curhan, G.C. Serum Uric Acid and Risk of Kidney Stones. Am. J. Kidney Dis. 2017, 70, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cai, X.; Hu, J.; Zhu, Q.; Shen, D.; Ma, H.; Zhang, Y.; Ma, R.; Zhou, P.; Yang, W.; et al. Plasma aldosterone concentrations elevation in hypertensive patients: The dual impact on hyperuricemia and gout. Front. Endocrinol. 2024, 15, 1424207. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, J.; Wong, M.C.S. Association between serum uric acid and prostate cancer risk in East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2023, 62, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Tolan, D.R.; Bredesen, D.; Nagel, M.; Sánchez-Lozada, L.G.; Fini, M.; Burtis, S.; Lanaspa, M.A.; Perlmutter, D. Could Alzheimer’s disease be a maladaptation of an evolutionary survival pathway mediated by intracerebral fructose and uric acid metabolism? Am. J. Clin. Nutr. 2023, 117, 455–466. [Google Scholar] [CrossRef]
- Fang, P.; Li, X.; Luo, J.J.; Wang, H.; Yang, X.F. A Double-edged Sword: Uric Acid and Neurological Disorders. Brain Disord. Ther. 2013, 2, 109. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.D.; Wang, J.; Hou, X.H.; Bao, Y.Q.; Zhang, Z.L.; Hu, C.; Jia, W.P. Association of serum uric acid levels with osteoporosis and bone turnover markers in a Chinese population. Acta Pharmacol. Sin. 2018, 39, 626–632. [Google Scholar] [CrossRef]
- Lin, K.M.; Lu, C.L.; Hung, K.C.; Wu, P.C.; Pan, C.F.; Wu, C.J.; Syu, R.S.; Chen, J.S.; Hsiao, P.J.; Lu, K.C. The Paradoxical Role of Uric Acid in Osteoporosis. Nutrients 2019, 11, 2111. [Google Scholar] [CrossRef] [PubMed]
- Chhana, A.; Pool, B.; Callon, K.E.; Tay, M.L.; Musson, D.; Naot, D.; McCarthy, G.; McGlashan, S.; Cornish, J.; Dalbeth, N. Monosodium urate crystals reduce osteocyte viability and indirectly promote a shift in osteocyte function towards a proinflammatory and proresorptive state. Arthritis Res. Ther. 2018, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Song, S.; Hu, J.; Wang, L.; Shen, D.; Zhu, Q.; Yang, W.; Luo, Q.; Hong, J.; Li, N. Systemic Inflammation Response Index as a Predictor of Stroke Risk in Elderly Patients with Hypertension: A Cohort Study. J. Inflamm. Res. 2023, 16, 4821–4832. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Song, S.; Hu, J.; Zhu, Q.; Shen, D.; Yang, W.; Ma, H.; Luo, Q.; Hong, J.; Zhang, D.; et al. Association of the trajectory of plasma aldosterone concentration with the risk of cardiovascular disease in patients with hypertension: A cohort study. Sci. Rep. 2024, 14, 4906. [Google Scholar] [CrossRef]
- Ma, H.; Cai, X.; Hu, J.; Song, S.; Zhu, Q.; Zhang, Y.; Ma, R.; Shen, D.; Yang, W.; Zhou, P.; et al. Association of systemic inflammatory response index with bone mineral density, osteoporosis, and future fracture risk in elderly hypertensive patients. Postgrad Med. 2024, 136, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, Y.; Xu, L.; Yue, C.; Wang, Q.; Chen, R.; Ding, N.; Qu, X.; Ou, Y.; Yang, Y.; et al. Triglyceride Glucose-Body Mass Index Is a Reliable Indicator of Bone Mineral Density and Risk of Osteoporotic Fracture in Middle-Aged and Elderly Nondiabetic Chinese Individuals. J. Clin. Med. 2022, 11, 5694. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Schousboe, J.T.; Broy, S.B.; Engelke, K.; Leslie, W.D. Executive Summary of the 2015 ISCD Position Development Conference on Advanced Measures From DXA and QCT: Fracture Prediction Beyond BMD. J. Clin. Densitom. 2015, 18, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Song, S.; Cai, X.; Hu, J.; Zhu, Q.; Shen, D.; Heizhati, M.; Yang, W.; Hong, J.; Li, N. Correlation between plasma aldosterone concentration and bone mineral density in middle-aged and elderly hypertensive patients: Potential impact on osteoporosis and future fracture risk. Front. Endocrinol. 2024, 15, 1373862. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Yu, J.J.; Wang, X.J.; Jing, Y.X.; Sun, L.H.; Tao, B.; Wang, W.Q.; Ning, G.; Liu, J.M.; Zhao, H.Y. The associations between Hypovitaminosis D, higher Pth levels with bone mineral densities, and risk of the 10-year probability of major osteoporotic fractures in Chinese patients with T2Dm. Endocr. Pract. 2018, 24, 334–341. [Google Scholar] [CrossRef]
- Song, S.; Cai, X.; Hu, J.; Zhu, Q.; Shen, D.; Ma, H.; Zhang, Y.; Ma, R.; Yang, W.; Hong, J.; et al. Effectiveness of Spironolactone in Reducing Osteoporosis and Future Fracture Risk in Middle-Aged and Elderly Hypertensive Patients. Drug Des. Dev. Ther. 2024, 18, 2215–2225. [Google Scholar] [CrossRef]
- Borghi, C.; Agabiti-Rosei, E.; Johnson, R.J.; Kielstein, J.T.; Lurbe, E.; Mancia, G.; Redon, J.; Stack, A.G.; Tsioufis, K.P. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern. Med. 2020, 80, 1–11. [Google Scholar] [CrossRef]
- Jeong, J.; Suh, Y.J. Association between Serum Uric Acid and Metabolic Syndrome in Koreans. J. Korean Med. Sci. 2019, 34, e307. [Google Scholar] [CrossRef]
- İnanir, M. Serum uric acid (SUA) in morbidly obese patients and its relationship with metabolic syndrome. Aging Male 2020, 23, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Karmacharya, U.; Liu, F.; Chen, B.D.; Ma, X.; Ma, Y.T. Uric acid and its correlation with hypertension in postmenopausal women: A multi-ethnic study (Observational study). Clin. Exp. Hypertens. 2020, 42, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Meng, X.F.; He, F.F.; Chen, S.; Su, H.; Xiong, J.; Gao, P.; Tian, X.J.; Liu, J.S.; Zhu, Z.H.; et al. High serum uric acid and increased risk of type 2 diabetes: A systemic review and meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e56864. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Robles-Rivera, K.; Argoty-Pantoja, A.D.; Hidalgo-Bravo, A.; Quezada-Sánchez, A.D.; León-Reyes, G.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R.; Rivera-Paredez, B. Uric Acid Levels Are Associated with Bone Mineral Density in Mexican Populations: A Longitudinal Study. Nutrients 2022, 14, 4245. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Yao, X.; Liu, M.; Zhu, Z. Association of Serum Uric Acid Status With Bone Mineral Density in Adolescents Aged 12–19 Years. Front. Med. 2020, 7, 255. [Google Scholar] [CrossRef]
- Lin, Z.C.; Wu, J.F.; Chang, C.Y.; Lai, K.M.; Yang, H.Y. Association between serum uric acid level and bone mineral density at multiple skeletal sites in middle-aged and elderly men: A cross-sectional study of a healthy population in Taiwan. Arch. Osteoporos. 2022, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yu, R.; Li, J.; Wang, X.; Guo, J.; Xiao, E.; Liu, P. Association of serum uric acid levels with bone mineral density and the presence of osteoporosis in Chinese patients with Parkinson’s disease: A cross-sectional study. J. Bone Miner. Metab. 2023, 41, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, G.; Trentini, A.; Greco, P.; Tisato, V.; Gemmati, D.; Bianchi, N.; Giganti, M.; Rossini, M.; Guglielmi, G.; Cervellati, C. Changes in Adipose Tissue Distribution and Association between Uric Acid and Bone Health during Menopause Transition. Int. J. Mol. Sci. 2019, 20, 6321. [Google Scholar] [CrossRef]
- Wei, J.; Choi, H.K.; Dalbeth, N.; Lane, N.E.; Wu, J.; Lyu, H.; Zeng, C.; Lei, G.; Zhang, Y. Lowering Serum Urate With Urate-Lowering Therapy to Target and Incident Fracture Among People With Gout. Arthritis Rheumatol. 2023, 75, 1456–1465. [Google Scholar] [CrossRef]
- Mehta, T.; Bůžková, P.; Sarnak, M.J.; Chonchol, M.; Cauley, J.A.; Wallace, E.; Fink, H.A.; Robbins, J.; Jalal, D. Serum urate levels and the risk of hip fractures: Data from the Cardiovascular Health Study. Metab. Clin. Exp. 2015, 64, 438–446. [Google Scholar] [CrossRef]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Fellström, B.; Danielson, B.G.; Karlström, B.; Lithell, H.; Ljunghall, S.; Vessby, B. The influence of a high dietary intake of purine-rich animal protein on urinary urate excretion and supersaturation in renal stone disease. Clin. Sci. 1983, 64, 399–405. [Google Scholar] [CrossRef]
- Huang, S.; Liang, H.; Chen, Y.; Liu, C.; Luo, P.; Wang, H.; Du, Q. Hypoxanthine ameliorates diet-induced insulin resistance by improving hepatic lipid metabolism and gluconeogenesis via AMPK/mTOR/PPARα pathway. Life Sci. 2024, 357, 123096. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Kang, K.Y.; Hong, Y.S.; Park, S.H.; Ju, J.H. Low levels of serum uric Acid increase the risk of low bone mineral density in young male patients with ankylosing spondylitis. J. Rheumatol. 2015, 42, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Lee, S.H.; Kim, B.J.; Lim, K.H.; Bae, S.J.; Kim, E.H.; Kim, H.K.; Choe, J.W.; Koh, J.M.; Kim, G.S. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos. Int. 2013, 24, 2961–2970. [Google Scholar] [CrossRef]
- Makovey, J.; Macara, M.; Chen, J.S.; Hayward, C.S.; March, L.; Seibel, M.J.; Sambrook, P.N. Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: A longitudinal study. Bone 2013, 52, 400–406. [Google Scholar] [CrossRef]
- Li, H.Z.; Chen, Z.; Hou, C.L.; Tang, Y.X.; Wang, F.; Fu, Q.G. Uric Acid Promotes Osteogenic Differentiation and Inhibits Adipogenic Differentiation of Human Bone Mesenchymal Stem Cells. J. Biochem. Mol. Toxicol. 2015, 29, 382–387. [Google Scholar] [CrossRef]
- Zhao, D.D.; Jiao, P.L.; Yu, J.J.; Wang, X.J.; Zhao, L.; Xuan, Y.; Sun, L.H.; Tao, B.; Wang, W.Q.; Ning, G.; et al. Higher Serum Uric Acid Is Associated with Higher Bone Mineral Density in Chinese Men with Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2016, 2016, 2528956. [Google Scholar] [CrossRef]
- Adomako, E.; Moe, O.W. Uric Acid and Urate in Urolithiasis: The Innocent Bystander, Instigator, and Perpetrator. Semin. Nephrol. 2020, 40, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Shannon, J.; Frei, B.; Kaye, J.A.; Quinn, J.F. Uric acid as a CNS antioxidant. J. Alzheimer’s Dis. JAD 2010, 19, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, J.; Jung, J.H.; Kim, S.K.; Kim, R.B.; Hahm, J.R. Risk Factors of Bone Mass Loss at the Lumbar Spine: A Longitudinal Study in Healthy Korean Pre- and Perimenopausal Women Older than 40 Years. PLoS ONE 2015, 10, e0136283. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Miyao, M.; Mizuno, Y.; Tanaka-Ishikawa, M.; Akishita, M.; Ouchi, Y. Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos. Int. 2014, 25, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Hwang, J.S. Impact of Type 2 Diabetes Mellitus and Antidiabetic Medications on Bone Metabolism. Curr. Diabetes Rep. 2020, 20, 78. [Google Scholar] [CrossRef]
- Orriss, I.R.; Arnett, T.R.; George, J.; Witham, M.D. Allopurinol and oxypurinol promote osteoblast differentiation and increase bone formation. Exp. Cell Res. 2016, 342, 166–174. [Google Scholar] [CrossRef]
- Chin, K.Y.; Nirwana, S.I.; Ngah, W.Z. Significant association between parathyroid hormone and uric acid level in men. Clin. Interv. Aging 2015, 10, 1377–1380. [Google Scholar] [CrossRef]
- Sumino, H.; Ichikawa, S.; Kanda, T.; Nakamura, T.; Sakamaki, T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet 1999, 354, 650. [Google Scholar] [CrossRef]
- El-Eshmawy, M.M.; Ibrahim, A.; Bahriz, R.; Shams-Eldin, N.; Mahsoub, N. Serum uric acid/creatinine ratio and free androgen index are synergistically associated with increased risk of polycystic ovary syndrome in obese women. BMC Endocr. Disord. 2022, 22, 315. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, H. The estrogen receptor and metabolism. Women’s Health 2024, 20, 17455057241227362. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, J.G.; Drake, M.T.; Khosla, S.; Farr, J.N. Skeletal Aging. Mayo Clin. Proc. 2022, 97, 1194–1208. [Google Scholar] [CrossRef]
- Nie, T.; Venkatesh, V.S.; Golub, S.; Stok, K.S.; Hemmatian, H.; Desai, R.; Handelsman, D.J.; Zajac, J.D.; Grossmann, M.; Davey, R.A. Estradiol increases cortical and trabecular bone accrual and bone strength in an adolescent male-to-female mouse model of gender-affirming hormone therapy. Bone Res. 2024, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Hoebertz, A.; Arnett, T.R.; Burnstock, G. Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol. Sci. 2003, 24, 290–297. [Google Scholar] [CrossRef]
- Solgaard, M.; Jørgensen, N.R. P2 purinergic receptors: Regulation of bone metabolism and therapeutic potential? Ugeskr. Laeger 2005, 167, 3152–3156. [Google Scholar]
- Zhang, S.; Fan, Z.; Ouyang, Z.; Sun, H.; Song, Y.; Yu, H.; Xu, Q.; Liu, S.; Sun, Y.; Shao, F. Purine metabolites promote ectopic new bone formation in ankylosing spondylitis. Int. Immunopharmacol. 2023, 116, 109810. [Google Scholar] [CrossRef]


| Characteristic | T1 | T2 | T3 | p Value |
|---|---|---|---|---|
| <289 (µmol/L) | 289–372 (µmol/L) | >372 (µmol/L) | ||
| N | 762 | 762 | 760 | |
| Age (years) | 58.36 ± 10.32 | 56.96 ± 11.01 | 54.02 ± 11.44 | <0.001 |
| Sex | <0.001 | |||
| Female | 544 (71.39%) | 395 (51.84%) | 230 (30.26%) | |
| Male | 218 (28.61%) | 368 (48.29%) | 529 (69.61%) | |
| BMI (kg/m2) | 26.35 ± 3.89 | 27.41 ± 3.74 | 27.48 ± 3.56 | <0.001 |
| SBP (mmHg) | 144.67 ± 17.66 | 145.06 ± 17.09 | 145.14 ± 18.32 | 0.858 |
| DBP (mmHg) | 85.37 ± 12.48 | 86.38 ± 11.98 | 88.24 ± 13.54 | <0.001 |
| Current smoking (%) | 96 (12.60%) | 186 (24.41%) | 288 (37.89%) | <0.001 |
| Current drinking (%) | 69 (9.06%) | 133 (17.45%) | 235 (30.92%) | <0.001 |
| Menopausal (%) | 401 (52.62%) | 304 (39.90%) | 181 (23.82%) | <0.001 |
| Medical history | ||||
| PA | 136 (17.85%) | 90 (11.81%) | 80 (10.53%) | <0.001 |
| DM | 244 (32.02%) | 248 (32.55%) | 204 (26.84%) | 0.031 |
| CHD | 66 (8.66%) | 53 (6.96%) | 52 (6.84%) | 0.319 |
| Cancer | 41 (5.38%) | 41 (5.38%) | 23 (3.03%) | 0.028 |
| Laboratory tests | ||||
| Serum potassium (mmol/L) | 3.80 ± 0.35 | 3.90 ± 0.33 | 3.96 ± 0.33 | <0.001 |
| PTH (pg/mL) | 50.30 (36.20–68.60) | 53.30 (37.75–71.60) | 50.00 (36.05–65.55) | <0.001 |
| Serum calcium (mmol/L) | 2.32 ± 0.55 | 2.35 ± 0.61 | 2.35 ± 0.54 | 0.564 |
| 25-hydroxyvitamin D (nmol/L) | 19.31 (12.18–29.27) | 18.32 (10.61–28.49) | 19.11 (12.63–29.36) | 0.161 |
| Serum phosphorus (mmol/L) | 1.15 ± 0.17 | 1.15 ± 0.17 | 1.18 ± 0.18 | 0.002 |
| ALT (U/L) | 23.00 (16.00–35.77) | 20.50 (15.00–29.00) | 24.00 (17.00–35.55) | <0.001 |
| AST (U/L) | 20.02 (16.60–26.00) | 19.10 (16.00–24.78) | 20.00 (16.70–25.77) | <0.001 |
| TC (mmol/L) | 4.53 ± 1.06 | 4.46 ± 1.09 | 4.58 ± 1.12 | 0.127 |
| TG (mmol/L) | 1.60 (1.15–2.39) | 1.40 (1.00–2.16) | 1.56 (1.15–2.38) | <0.001 |
| Cr (µmol/L) | 58.84 ± 15.37 | 63.94 ± 14.87 | 70.39 ± 16.25 | <0.001 |
| eGFR (mL/min/1.73 m2) | 125.97 ± 63.61 | 121.50 ± 48.83 | 119.68 ± 57.98 | 0.087 |
| ALP (U/L) | 84.62 ± 29.81 | 82.28 ± 28.84 | 80.90 ± 28.84 | 0.042 |
| TSH (uIU/mL) | 2.17 (1.45–3.33) | 2.31 (1.49–3.45) | 2.09 (1.46–3.36) | 0.008 |
| FPG (mmol/L) | 5.92 ± 2.06 | 5.88 ± 1.98 | 5.65 ± 1.83 | 0.014 |
| SUA (µmol/L) | 237.96 ± 35.98 | 328.75 ± 23.34 | 445.30 ± 64.89 | <0.001 |
| Medications | ||||
| Statins (%) | 174 (22.83%) | 145 (19.03%) | 152 (20.00%) | 0.16 |
| Diuretics (%) | 74 (9.71%) | 81 (10.63%) | 103 (13.55%) | 0.045 |
| Beta-blockers (%) | 138 (18.11%) | 140 (18.37%) | 139 (18.29%) | 0.992 |
| Calcium channel blockers (%) | 417 (54.72%) | 413 (54.20%) | 405 (53.29%) | 0.866 |
| ACEIs/ARBs (%) | 298 (39.11%) | 318 (41.73%) | 356 (46.84%) | 0.007 |
| Oral hypoglycemic agents (%) | 200 (26.25%) | 185 (24.28%) | 152 (20.00%) | 0.014 |
| Insulin (%) | 60 (7.87%) | 48 (6.30%) | 42 (5.53%) | 0.171 |
| DXA BMD T-scores | ||||
| Lumbar 1-4 | −1.20 ± 1.65 | −0.51 ± 1.58 | −0.44 ± 1.68 | <0.001 |
| Neck | −1.05 ± 1.10 | −0.74 ± 1.00 | −0.69 ± 1.01 | <0.001 |
| Total | −0.44 ± 1.12 | −0.04 ± 1.01 | −0.05 ± 1.03 | <0.001 |
| FRAX score (%) | ||||
| MOF | 4.34 ± 3.12 | 3.31 ± 2.46 | 3.17 ± 2.37 | <0.001 |
| HF | 1.81 ± 2.46 | 1.19 ± 1.93 | 1.21 ± 2.00 | <0.001 |
| Osteoporosis (%) | 268 (35.17%) | 115 (15.09%) | 168 (22.11%) | <0.001 |
| Exposure | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| β (95% CI) p Value | β (95% CI) p Value | β (95% CI) p Value | β (95% CI) p Value | |
| Lumbar 1-4 | ||||
| SUA (per 10-µmol/L increase) | 0.032 (0.025, 0.039) <0.001 | 0.017 (0.010, 0.024) 0.010 | 0.016 (0.008, 0.023) <0.001 | 0.010 (0.003, 0.018) 0.006 |
| Tertiles of SUA | ||||
| Tertile 1 | Reference | Reference | Reference | Reference |
| Tertile 2 | 0.687 (0.523, 0.852) <0.001 | 0.514 (0.351, 0.677) <0.001 | 0.504 (0.341, 0.667) <0.001 | 0.409 (0.248, 0.529) <0.001 |
| Tertile 3 | 0.757 (0.592, 0.922) <0.001 | 0.435 (0.520, 0.959) <0.001 | 0.414 (0.242, 0.585) <0.001 | 0.271 (0.099, 0.443) 0.002 |
| Neck | ||||
| SUA (per 10-µmol/L increase) | 0.014 (0.009, 0.018) <0.001 | 0.008 (0.004, 0.013) <0.001 | 0.008 (0.004, 0.013) <0.001 | 0.004 (0.001, 0.008) 0.037 |
| Tertiles of SUA | ||||
| Tertile 1 | Reference | Reference | Reference | Reference |
| Tertile 2 | 0.309 (0.205, 0.413) <0.001 | 0.255 (0.155, 0.356) <0.001 | 0.253 (0.153, 0.354) <0.001 | 0.178 (0.080, 0.276) <0.001 |
| Tertile 3 | 0.367 (0.262, 0.471) <0.001 | 0.258 (0.152, 0.364) <0.001 | 0.259 (0.153, 0.365) <0.001 | 0.139 (0.034, 0.244) 0.009 |
| Total | ||||
| SUA (per 10-µmol/L increase) | 0.013 (0.009, 0.018) <0.001 | 0.008 (0.003, 0.012) 0.002 | 0.008 (0.003, 0.013) 0.001 | 0.003 (−0.002, 0.007) 0.232 |
| Tertiles of SUA | ||||
| Tertile 1 | Reference | Reference | Reference | Reference |
| Tertile 2 | 0.396 (0.290, 0.501) <0.001 | 0.331 (0.228, 0.434) <0.001 | 0.330 (0.227, 0.433) <0.001 | 0.246 (0.147, 0.346) <0.001 |
| Tertile 3 | 0.385 (0.279, 0.491) <0.001 | 0.283 (0.174, 0.392) <0.001 | 0.293 (0.185, 0.402) <0.001 | 0.158 (0.051, 0.264) 0.004 |
| Exposure | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| β (95% CI) p Value | β (95% CI) p Value | β (95% CI) p Value | β (95% CI) p Value | |
| MOF | ||||
| SUA (per 10-µmol/L increase) | −0.045 (−0.056, −0.033) <0.001 | −0.026 (−0.037, −0.014) <0.001 | −0.025 (−0.037, −0.014) <0.001 | −0.013 (−0.024, −0.001) 0.031 |
| Tertiles of SUA | ||||
| Tertile 1 | Reference | Reference | Reference | Reference |
| Tertile 2 | −1.033 (−1.301, −0.765) <0.001 | −0.834 (−1.089, −0.579) <0.001 | −0.826 (−1.081, −0.570) <0.001 | −0.608 (−0.856, −0.361) <0.001 |
| Tertile 3 | −1.168 (−1.437, −0.900) <0.001 | −0.758 (−1.027, −0.490) <0.001 | −0.753 (−1.022, −0.484) <0.001 | −0.413 (−0.678, −0.148) 0.002 |
| HF | ||||
| SUA (per 10-µmol/L increase) | −0.020 (−0.030, −0.011) <0.001 | −0.022 (−0.032, −0.012) <0.001 | −0.022 (−0.032, −0.012) <0.001 | −0.013 (−0.022, −0.003) 0.010 |
| Tertiles of SUA | ||||
| Tertile 1 | Reference | Reference | Reference | Reference |
| Tertile 2 | −0.628 (−0.843, −0.412) <0.001 | −0.632 (−0.844, 0.420) <0.001 | −0.628 (−0.840, −0.416) <0.001 | −0.467 (−674, −0.260) <0.001 |
| Tertile 3 | −0.600 (−0.815, −0.384) <0.001 | −0.644 (−0.867, −0.421) <0.001 | −0.645 (−0.868, −0.421) <0.001 | −0.392 (−0614, −0.170) 0.001 |
| Exposure | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| OR (95%CI) p Value | OR (95%CI) p Value | OR (95%CI) p Value | OR (95%CI) p Value | |
| Tertiles of SUA | ||||
| Tertile 1 | 3.057 (2.386, 3.917) <0.001 | 2.705 (2.095, 3.493) <0.001 | 2.680 (2.074, 3.462) <0.001 | 2.427 (1.869, 3.153) <0.001 |
| Tertile 2 | Reference | Reference | Reference | Reference |
| Tertile 3 | 1.602 (1.232, 2.082) <0.001 | 1.911 (1.455, 2.510) <0.001 | 1.939 (1.475, 2.549) <0.001 | 2.146 (1.622, 2.841) <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Cai, X.; Hu, J.; Zhu, Q.; Shen, D.; Ma, H.; Zhang, Y.; Ma, R.; Zhou, P.; Yang, W.; et al. Serum Uric Acid and Bone Health in Middle-Aged and Elderly Hypertensive Patients: A Potential U-Shaped Association and Implications for Future Fracture Risk. Metabolites 2025, 15, 15. https://doi.org/10.3390/metabo15010015
Song S, Cai X, Hu J, Zhu Q, Shen D, Ma H, Zhang Y, Ma R, Zhou P, Yang W, et al. Serum Uric Acid and Bone Health in Middle-Aged and Elderly Hypertensive Patients: A Potential U-Shaped Association and Implications for Future Fracture Risk. Metabolites. 2025; 15(1):15. https://doi.org/10.3390/metabo15010015
Chicago/Turabian StyleSong, Shuaiwei, Xintian Cai, Junli Hu, Qing Zhu, Di Shen, Huimin Ma, Yingying Zhang, Rui Ma, Pan Zhou, Wenbo Yang, and et al. 2025. "Serum Uric Acid and Bone Health in Middle-Aged and Elderly Hypertensive Patients: A Potential U-Shaped Association and Implications for Future Fracture Risk" Metabolites 15, no. 1: 15. https://doi.org/10.3390/metabo15010015
APA StyleSong, S., Cai, X., Hu, J., Zhu, Q., Shen, D., Ma, H., Zhang, Y., Ma, R., Zhou, P., Yang, W., Hong, J., & Li, N. (2025). Serum Uric Acid and Bone Health in Middle-Aged and Elderly Hypertensive Patients: A Potential U-Shaped Association and Implications for Future Fracture Risk. Metabolites, 15(1), 15. https://doi.org/10.3390/metabo15010015







