Utility of an Untargeted Metabolomics Approach Using a 2D GC-GC-MS Platform to Distinguish Relapsing and Progressive Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
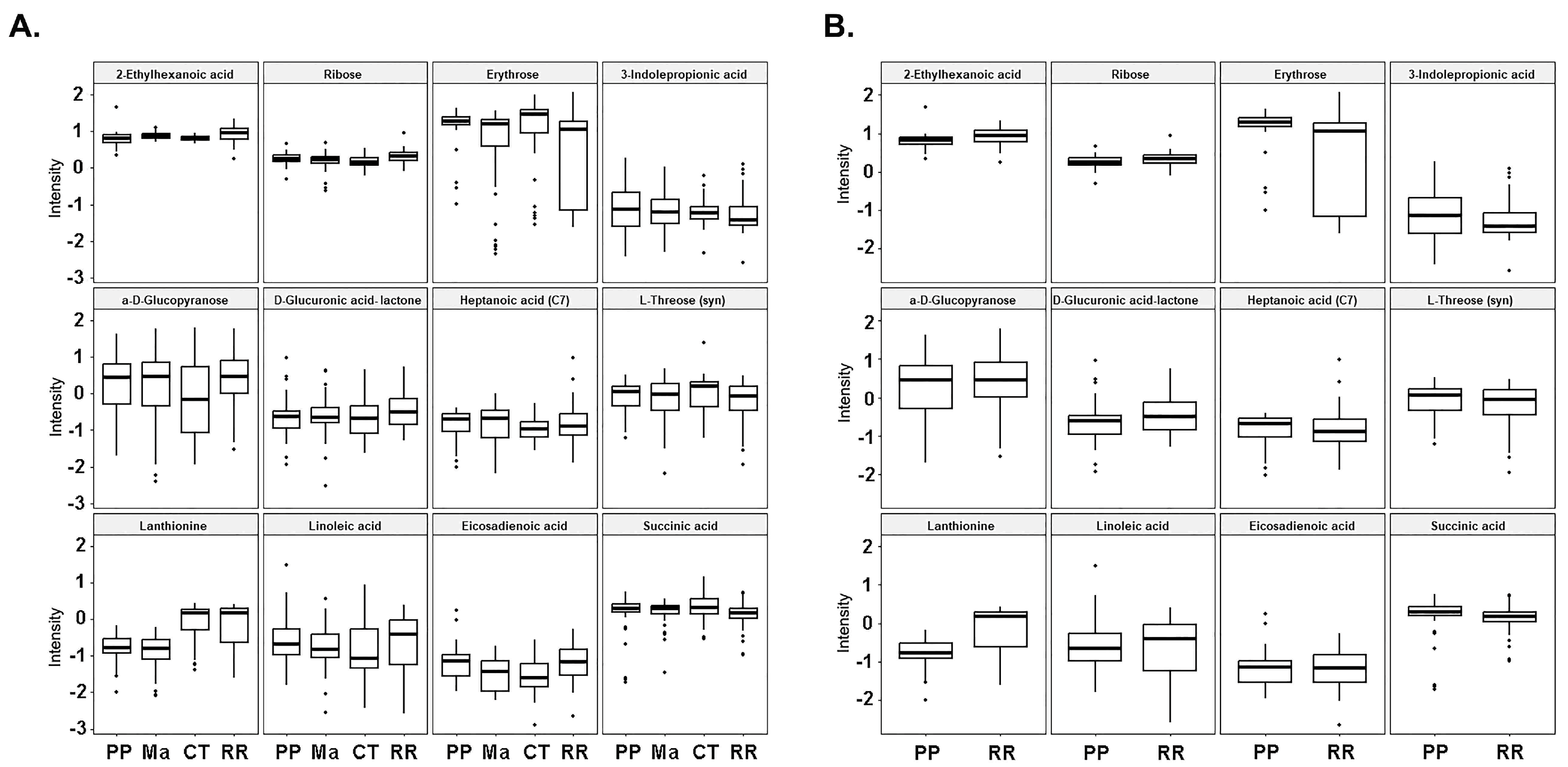
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucchinetti, C.; Bruck, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Smith, T.W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Tuddenham, J.F.; Sadiq, S.A. Biomarkers of multiple sclerosis: Current findings. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Rui, B.; Khan, J.; Datta, I.; Giri, S. An emerging potential of metabolomics in multiple sclerosis: A comprehensive overview. Cell. Mol. Life Sci. 2021, 78, 3181–3203. [Google Scholar] [CrossRef] [PubMed]
- Datta, I.; Zahoor, I.; Ata, N.; Rashid, F.; Cerghet, M.; Rattan, R.; Poisson, L.M.; Giri, S. Utility of an untargeted metabolomics approach using a 2D GC-GC-MS platform to distinguish relapsing and progressive multiple sclerosis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mangalam, A.; Poisson, L.; Nemutlu, E.; Datta, I.; Denic, A.; Dzeja, P.; Rodriguez, M.; Rattan, R.; Giri, S. Profile of Circulatory Metabolites in a Relapsing-remitting Animal Model of Multiple Sclerosis using Global Metabolomics. J. Clin. Cell. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Zahoor, I.; Suhail, H.; Datta, I.; Ahmed, M.E.; Poisson, L.M.; Waters, J.; Rashid, F.; Bin, R.; Singh, J.; Cerghet, M.; et al. Blood-based untargeted metabolomics in relapsing-remitting multiple sclerosis revealed the testable therapeutic target. Proc. Natl. Acad. Sci. USA 2022, 119, e2123265119. [Google Scholar] [CrossRef]
- Storey, J.D.; Bass, A.J.; Dabney, A.; Robinson, D. qvalue: Q-Value Estimation for False Discovery Rate Control. R Package Version 2220. 2020. Available online: https://github.com/StoreyLab/qvalue (accessed on 9 December 2020).
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.F.; Claudino, R.F.; Dutra, R.C.; Marcon, R.; Calixto, J.B. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J. Immunol. 2011, 187, 1957–1969. [Google Scholar] [CrossRef]
- Rinaudo, P.; Boudah, S.; Junot, C.; Thevenot, E.A. biosigner: A New Method for the Discovery of Significant Molecular Signatures from Omics Data. Front. Mol. Biosci. 2016, 3, 26. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Ravera, S.; Panfoli, I. Role of myelin sheath energy metabolism in neurodegenerative diseases. Neural Regen. Res. 2015, 10, 1570–1571. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin Resistance and Neurodegeneration: Progress towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino Acid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef]
- Hellmuth, C.; Kirchberg, F.F.; Lass, N.; Harder, U.; Peissner, W.; Koletzko, B.; Reinehr, T. Tyrosine Is Associated with Insulin Resistance in Longitudinal Metabolomic Profiling of Obese Children. J. Diabetes Res. 2016, 2016, 2108909. [Google Scholar] [CrossRef]
- Blankfield, A. A Brief Historic Overview of Clinical Disorders Associated with Tryptophan: The Relevance to Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). Int. J. Tryptophan Res. 2012, 5, 27–32. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R. L-tryptophan in neuropsychiatric disorders: A review. Int. J. Neurosci. 1992, 67, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Smith, M.D.; Kim, S.; Sotirchos, E.S.; Kornberg, M.D.; Douglas, M.; Nourbakhsh, B.; Graves, J.; Rattan, R.; Poisson, L.; et al. Multi-omic evaluation of metabolic alterations in multiple sclerosis identifies shifts in aromatic amino acid metabolism. Cell Rep. Med. 2021, 2, 100424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, Y.; Zhang, J.; Li, Y.; Chen, J.; Huang, R.; Ma, G.; Wang, L.; Zhang, Y.; Nie, K.; et al. L-Asparaginase Exerts Neuroprotective Effects in an SH-SY5Y-A53T Model of Parkinson’s Disease by Regulating Glutamine Metabolism. Front. Mol. Neurosci. 2020, 13, 563054. [Google Scholar] [CrossRef] [PubMed]
- Manoli, I.; Venditti, C.P. Disorders of branched chain amino acid metabolism. Transl. Sci. Rare Dis. 2016, 1, 91–110. [Google Scholar] [CrossRef]
- Nasaruddin, M.L.; Pan, X.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Holscher, C.; Graham, S.F.; Green, B.D. Evidence That Parietal Lobe Fatty Acids May Be More Profoundly Affected in Moderate Alzheimer’s Disease (AD) Pathology Than in Severe AD Pathology. Metabolites 2018, 8, 69. [Google Scholar] [CrossRef]
- Huang, Y.S.; Huang, W.C.; Li, C.W.; Chuang, L.T. Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Mol. Cell. Biochem. 2011, 358, 85–94. [Google Scholar] [CrossRef]
- Yu, H.; Bai, S.; Hao, Y.; Guan, Y. Fatty acids role in multiple sclerosis as “metabokines”. J. Neuroinflammation 2022, 19, 157. [Google Scholar] [CrossRef]
- Gibson, G.E.; Hirsch, J.A.; Cirio, R.T.; Jordan, B.D.; Fonzetti, P.; Elder, J. Abnormal thiamine-dependent processes in Alzheimer’s Disease. Lessons from diabetes. Mol. Cell. Neurosci. 2013, 55, 17–25. [Google Scholar] [CrossRef]
- Poisson, L.M.; Suhail, H.; Singh, J.; Datta, I.; Denic, A.; Labuzek, K.; Hoda, M.N.; Shankar, A.; Kumar, A.; Cerghet, M.; et al. Untargeted Plasma Metabolomics Identifies Endogenous Metabolite with Drug-like Properties in Chronic Animal Model of Multiple Sclerosis. J. Biol. Chem. 2015, 290, 30697–30712. [Google Scholar] [CrossRef]
- Zahoor, I.; Nematullah, M.; Ahmed, M.E.; Fatma, M.; Mir, S.; Ayasolla, K.; Cerghet, M.; Palaniyandi, S.; Ceci, V.; Carrera, G.; et al. Maresin-1 promotes neuroprotection and prevents disease progression in experimental models of multiple sclerosis through metabolic reprogramming and shaping innate and adaptive disease-associated cell types. bioRxiv 2024. [Google Scholar] [CrossRef]
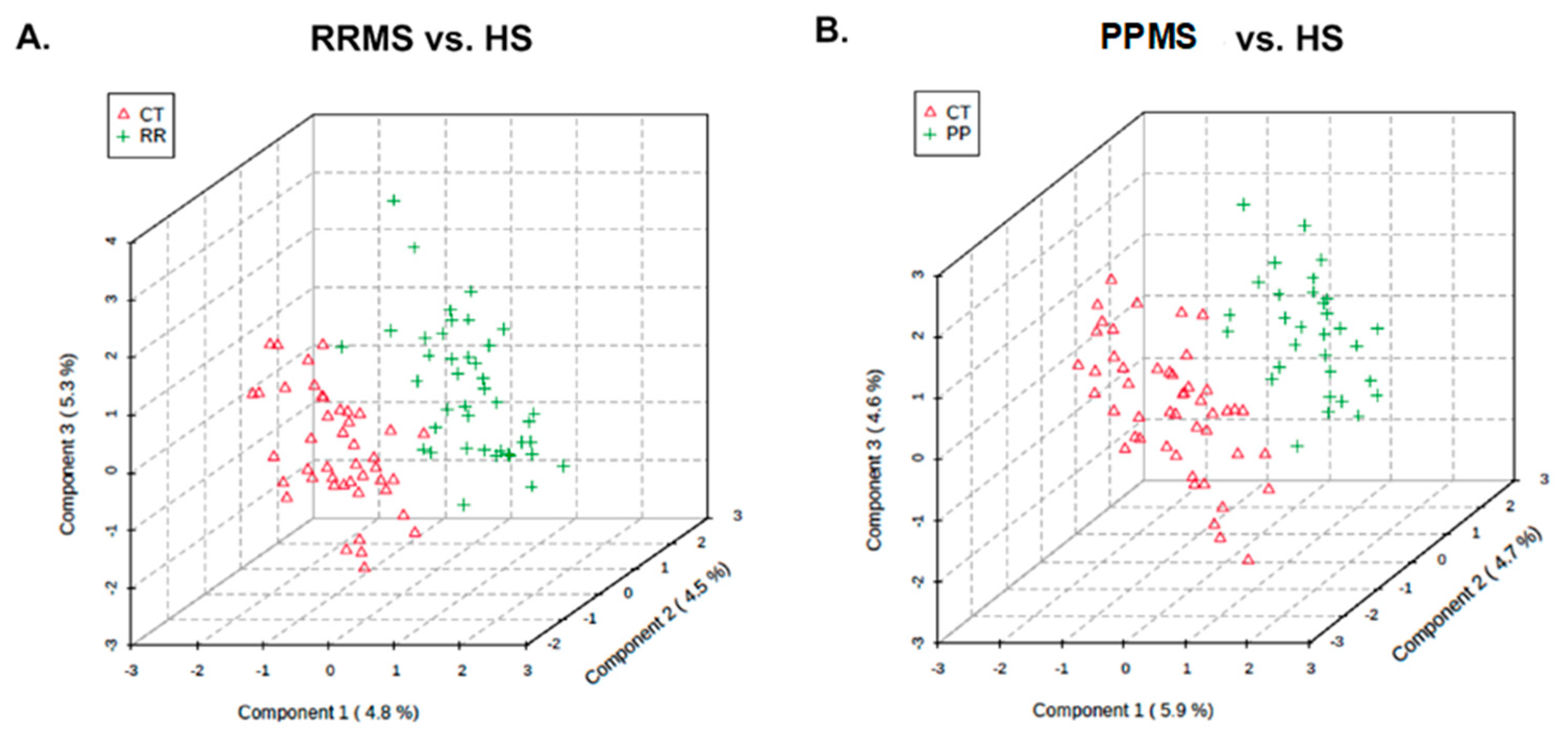
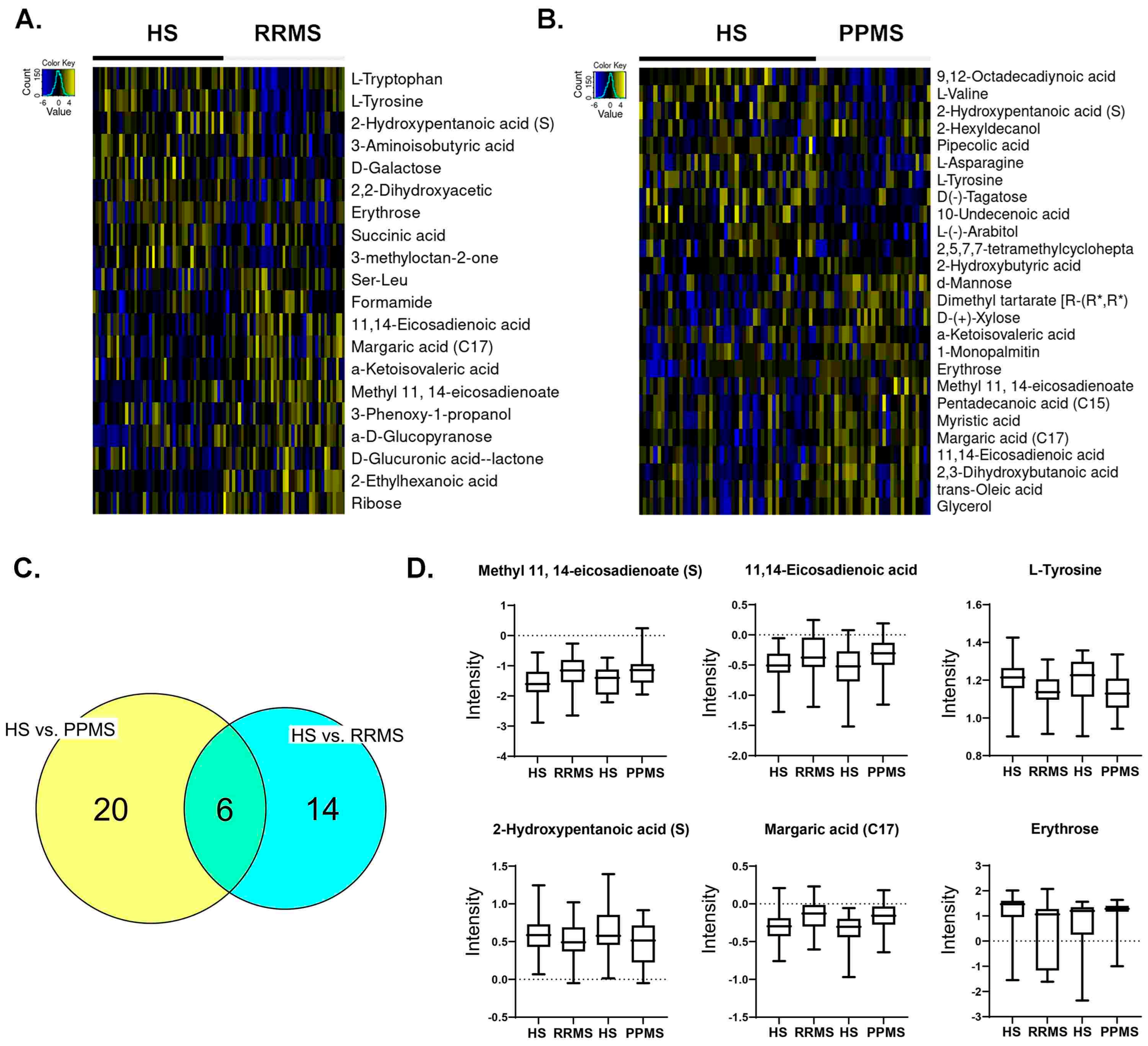
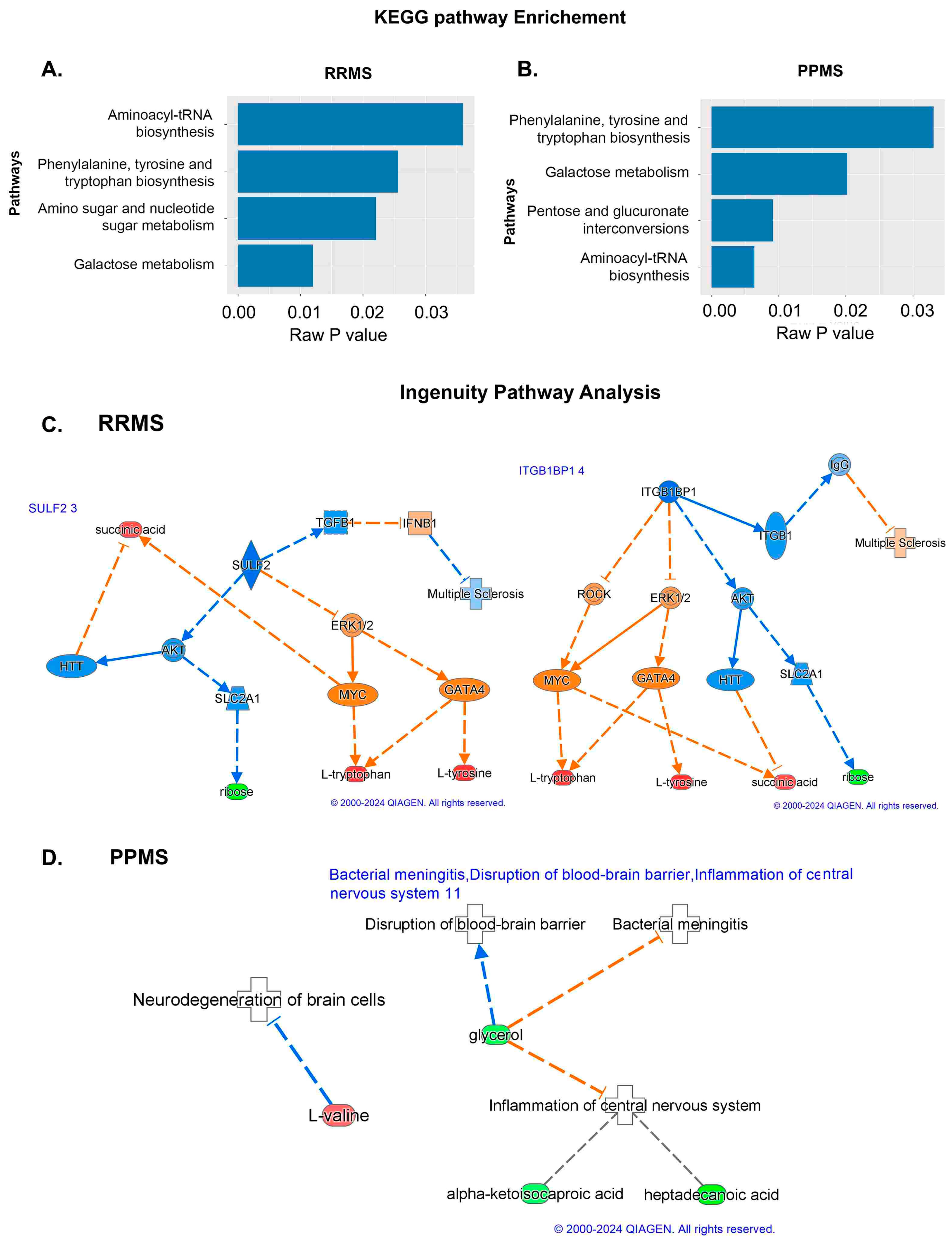
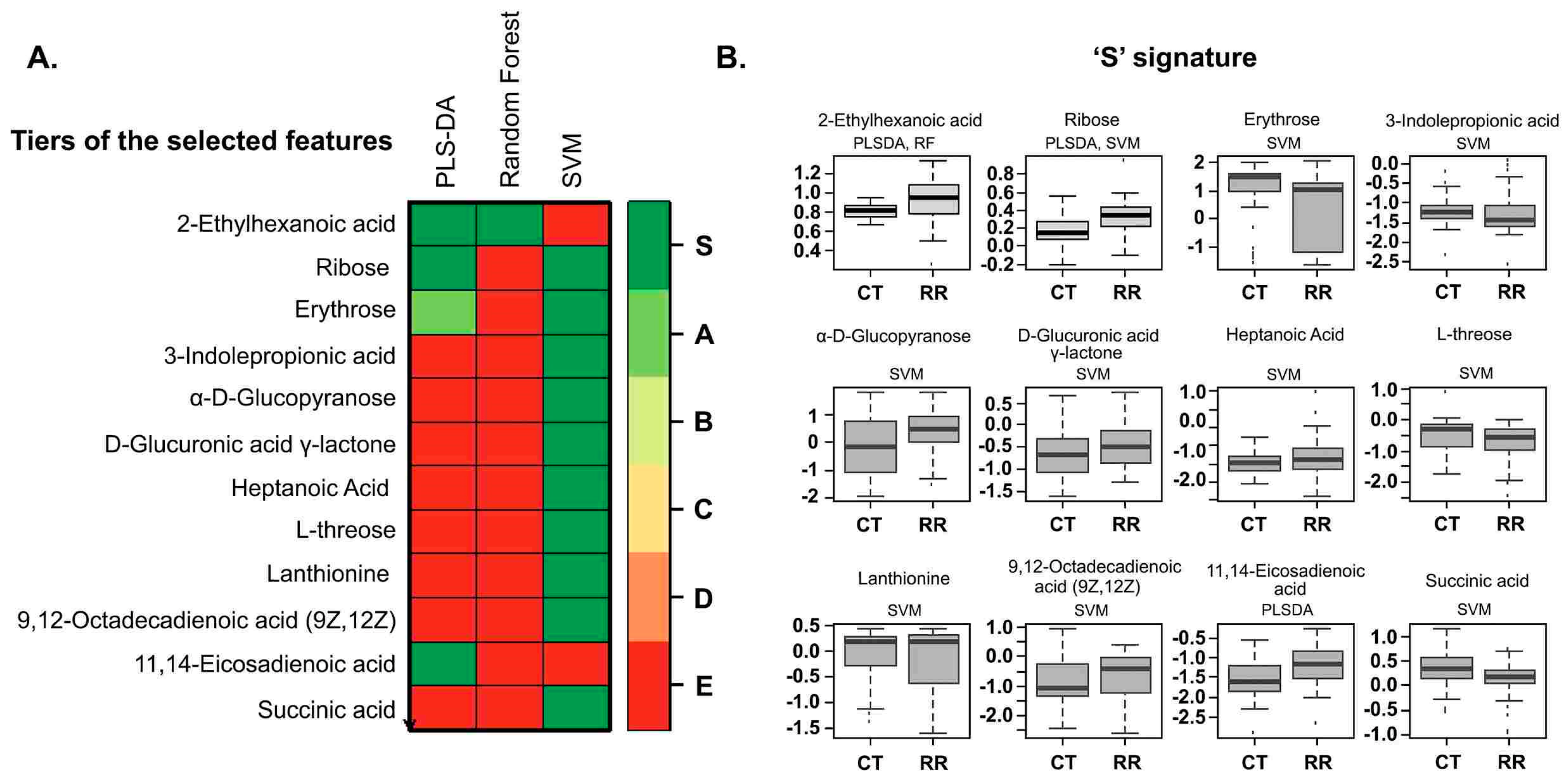
| Variable | RRMS | CTRL for RRMS | PPMS | CTRL for PPMS | |
|---|---|---|---|---|---|
| N | 41 | 44 | 31 | 47 | |
| Age, median [IQR] | 39 [34, 48] | 39.5 [33.75, 49] | 49 [46, 58.5] | 53 [46.5, 60.5] | |
| Sex, N (%) | Male | 12 (29.3) | 13 (29.5) | 9 (29.0) | 12 (25.5) |
| Female | 29 (70.7) | 31 (70.5) | 22 (71.0) | 35 (74.5) | |
| Race +, N (%) | Black | 4 (9.8) | 4 (9.1) | 1 (3.3) | 1 (2.1) |
| White | 35 (85.4) | 39 (88.6) | 28 (90.3) | 44 (93.6) | |
| Other * | 2 (4.8) | 1 (2.3) | 2 (6.4) | 2 (4.3) | |
| Name of Classifier | PLS-DA | Random Forest | SVM |
|---|---|---|---|
| Accuracy | 73% | 75% | 77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datta, I.; Zahoor, I.; Ata, N.; Rashid, F.; Cerghet, M.; Rattan, R.; Poisson, L.M.; Giri, S. Utility of an Untargeted Metabolomics Approach Using a 2D GC-GC-MS Platform to Distinguish Relapsing and Progressive Multiple Sclerosis. Metabolites 2024, 14, 493. https://doi.org/10.3390/metabo14090493
Datta I, Zahoor I, Ata N, Rashid F, Cerghet M, Rattan R, Poisson LM, Giri S. Utility of an Untargeted Metabolomics Approach Using a 2D GC-GC-MS Platform to Distinguish Relapsing and Progressive Multiple Sclerosis. Metabolites. 2024; 14(9):493. https://doi.org/10.3390/metabo14090493
Chicago/Turabian StyleDatta, Indrani, Insha Zahoor, Nasar Ata, Faraz Rashid, Mirela Cerghet, Ramandeep Rattan, Laila M. Poisson, and Shailendra Giri. 2024. "Utility of an Untargeted Metabolomics Approach Using a 2D GC-GC-MS Platform to Distinguish Relapsing and Progressive Multiple Sclerosis" Metabolites 14, no. 9: 493. https://doi.org/10.3390/metabo14090493
APA StyleDatta, I., Zahoor, I., Ata, N., Rashid, F., Cerghet, M., Rattan, R., Poisson, L. M., & Giri, S. (2024). Utility of an Untargeted Metabolomics Approach Using a 2D GC-GC-MS Platform to Distinguish Relapsing and Progressive Multiple Sclerosis. Metabolites, 14(9), 493. https://doi.org/10.3390/metabo14090493







