The Legacy Effect of Mountain Pine Beetle Outbreaks on the Chemical and Anatomical Defences of Surviving Lodgepole Pine Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Sample Collection
2.3. Chemical Analysis
2.3.1. Monoterpene Analysis
2.3.2. Diterpene Analysis
2.3.3. Non-Structural Carbohydrate (Soluble Sugars and Starch) Analyses
2.4. Anatomical Defence and Growth Characteristics
2.5. Data Analysis
2.5.1. Gradient Analysis
2.5.2. Linear Mixed-Effect Models
2.5.3. Comparative Analyses
3. Results
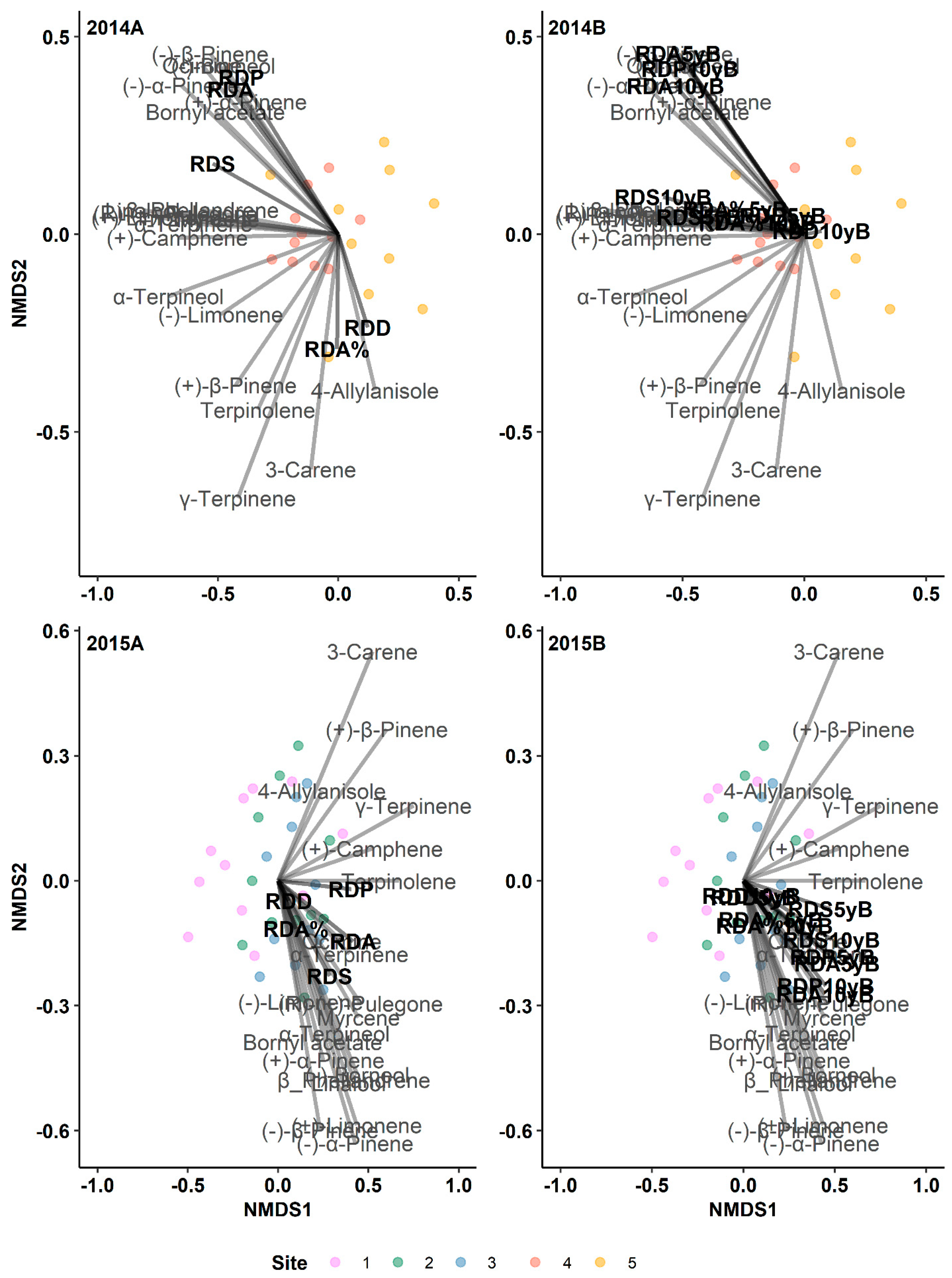
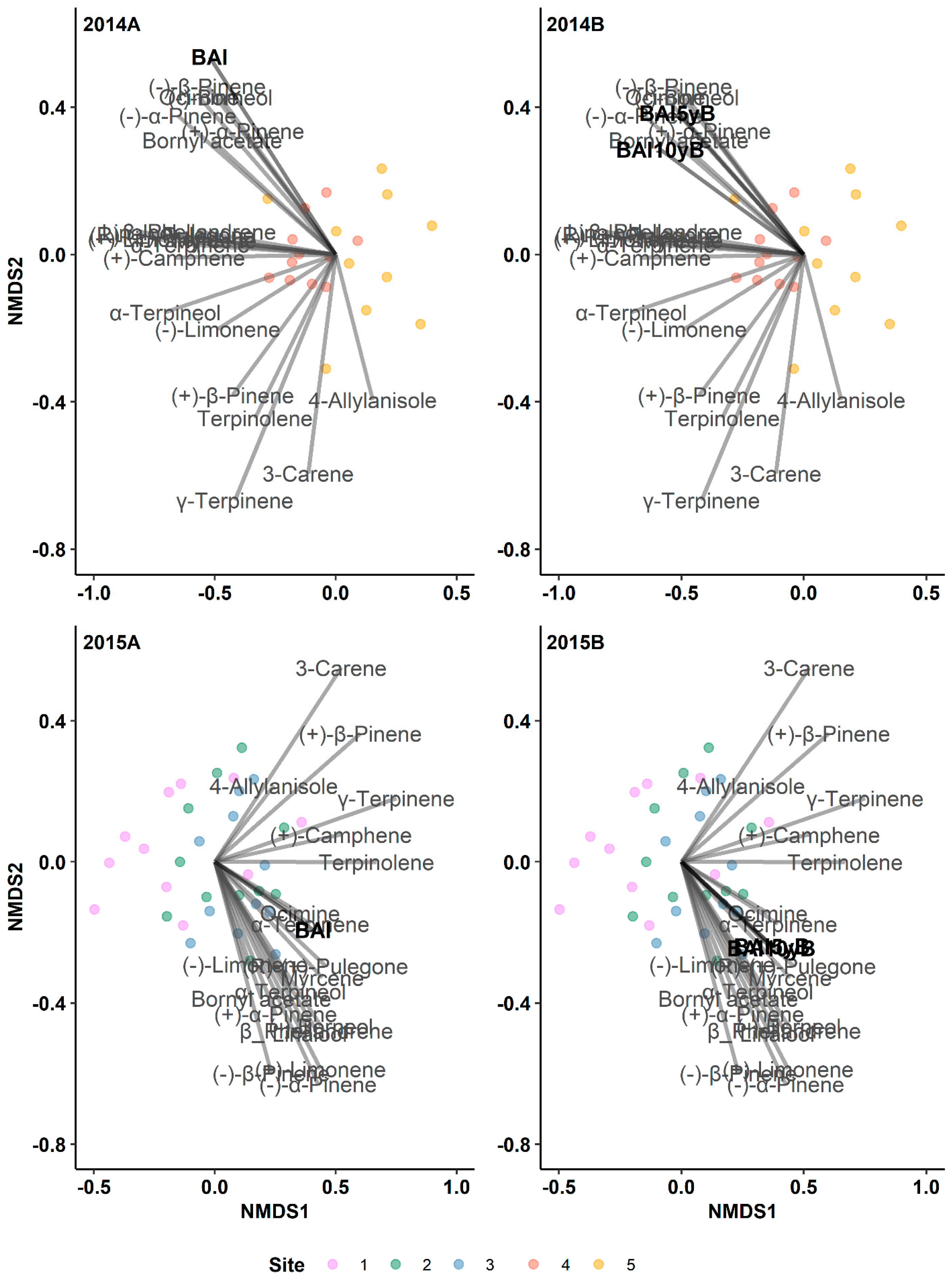
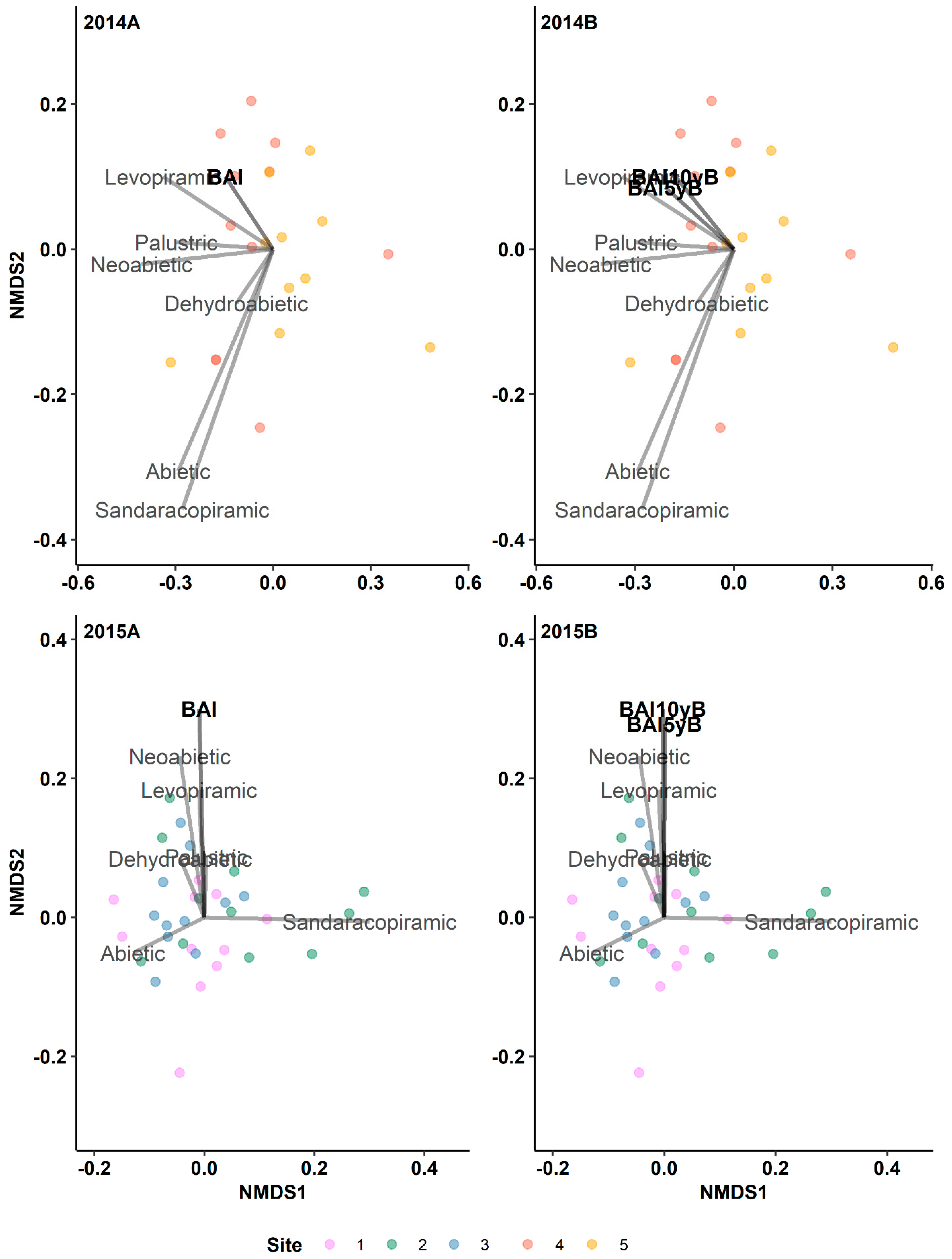
3.1. The Historical Resin Duct Characteristics Affect the Current Year’s Terpenes
3.1.1. Monoterpenes
3.1.2. Diterpenes
3.2. NSCs Affected the Total Terpenes in the 2015 Outbreak but Not in the 2014 Outbreak
3.3. The Radial Growth Characteristics Affect Terpene Production
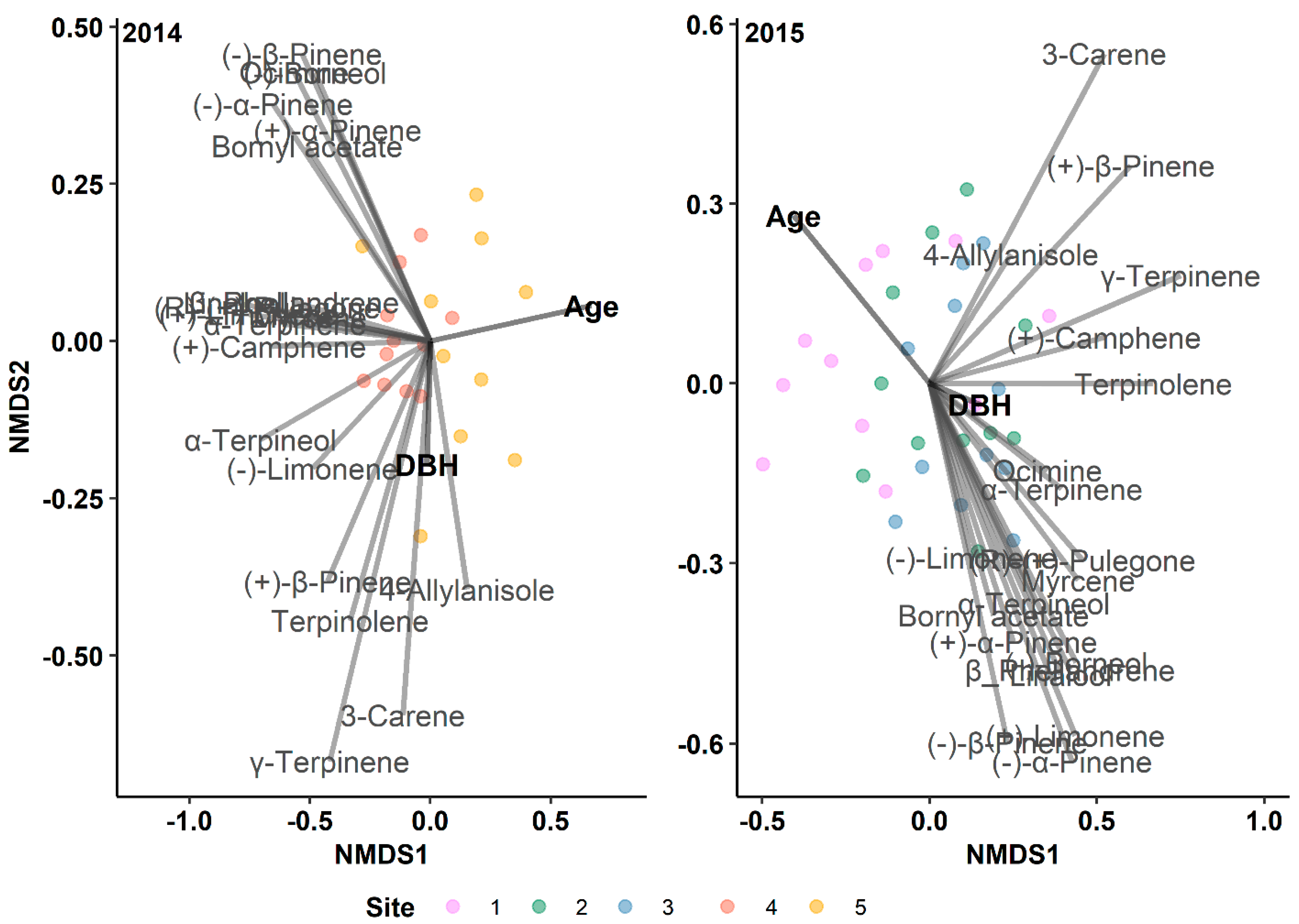
3.4. Tree Age, but Not DBH, Affects Terpene Production
3.5. There Was No Relationship between NSCs and Anatomical Defence Traits, Radial Growth Rate, and Tree Growth Traits
3.6. There Is No Trade-Off between Monoterpenes and Diterpenes
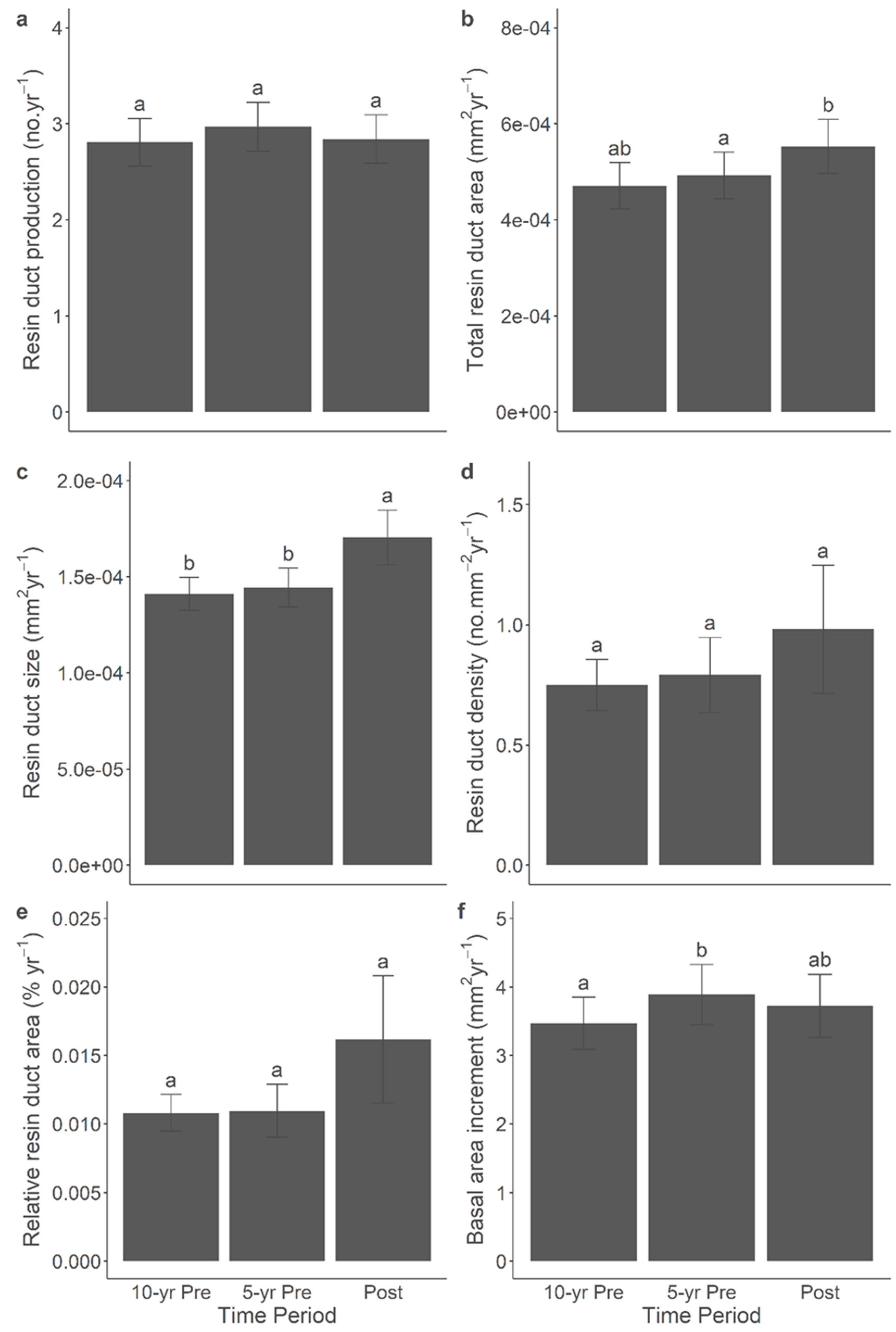
3.7. Resin Duct Characteristics Post-Outbreak Varied with Growth Responses across Time Periods
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAI | Basal area increment |
| DBH | Diameter at breast height |
| DW | Dry weight |
| ELSD | Evaporative light scattering detector |
| GC/MS | Gas chromatograph/mass spectrometer |
| MPB | Mountain pine beetle |
| NMDS | Non-linear multidimensional scaling |
| NSCs | Non-structural carbohydrates |
| UHPLC | Ultra-high performance liquid chromatograph |
References
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Kausrud, K.; Økland, B.; Skarpaas, O.; Grégoire, J.; Erbilgin, N.; Stenseth, N.C. Population dynamics in changing environments: The case of an eruptive forest pest species. Biol Rev. 2011, 87, 34–51. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Chang. 2014, 4, 806–810. [Google Scholar] [CrossRef]
- Ghimire, B.; Williams, C.A.; Collatz, G.J.; Vanderhoof, M.; Rogan, J.; Kulakowski, D.; Masek, J.G. Large carbon release legacy from bark beetle outbreaks across Western United States. Glob. Chang. Biol. 2015, 21, 3087–3101. [Google Scholar] [CrossRef]
- Aldea, J.; Dahlgren, J.; Holmström, E.; Löf, M. Current and future drought vulnerability for three dominant boreal tree species. Glob. Chang. Biol. 2023, 30, e17079. [Google Scholar] [CrossRef]
- Erbilgin, N.; Cale, J.A.; Hussain, A.; Ishangulyyeva, G.; Klutsch, J.G.; Najar, A.; Zhao, S. Weathering the storm: How lodgepole pine trees survive mountain pine beetle outbreaks. Oecologia 2017, 184, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Erbilgin, N. Larger resin ducts are linked to the survival of lodgepole pine trees during mountain pine beetle outbreak. Front Plant Sci. 2019, 10, 1459. [Google Scholar] [CrossRef]
- Amoroso, M.M.; Coates, K.D.; Astrup, R. Stand recovery and self-organization following large-scale mountain pine beetle induced canopy mortality in northern forests. For. Ecol. Manag. 2013, 310, 300–311. [Google Scholar] [CrossRef]
- Perovich, C.; Sibold, J.S. Forest composition change after a mountain pine beetle outbreak, Rocky Mountain National Park, CO, USA. For. Ecol. Manag. 2016, 366, 184–192. [Google Scholar] [CrossRef]
- Morris, J.E.; Buonanduci, M.S.; Agne, M.C.; Battaglia, M.A.; Harvey, B.J. Does the legacy of historical thinning treatments foster resilience to bark beetle outbreaks in subalpine forests? Ecol Appl. 2021, 32, e02474. [Google Scholar] [CrossRef]
- Rodman, K.C.; Andrus, R.A.; Carlson, A.R.; Carter, T.A.; Chapman, T.B.; Coop, J.D.; Fornwalt, P.J.; Gill, N.S.; Harvey, B.J.; Hoffman, A.E.; et al. Rocky Mountain forests are poised to recover following bark beetle outbreaks but with altered composition. J. Ecol. 2022, 110, 2929–2949. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Benedik, J.; Stadt, K.; Macdonald, S.E. Poor regeneration of pine after mountain pine beetle attack in colder boreal regions of Canada. Can. J. For. Res. 2024, 54, 168–191. [Google Scholar] [CrossRef]
- Kichas, N.E.; Trowbridge, A.M.; Raffa, K.F.; Malone, S.C.; Hood, S.M.; Everett, R.G.; McWethy, D.B.; Pederson, G.T. Growth and defense characteristics of whitebark pine (Pinus albicaulis) and lodgepole pine (Pinus contorta var. latifolia) in a high-elevation, disturbance-prone mixed-conifer forest in northwestern Montana, USA. For. Ecol. Manag. 2021, 493, 119286. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Thom, D.; Rammer, W.; Seidl, R. The impact of future forest dynamics on climate: Interactive effects of changing vegetation and disturbance regimes. Ecol. Monogr. 2017, 87, 665–684. [Google Scholar] [CrossRef]
- Sommerfeld, A.; Rammer, W.; Heurich, M.; Hilmers, T.; Müller, J.; Seidl, R. Do bark beetle outbreaks amplify or dampen future bark beetle disturbances in Central Europe? J. Ecol. 2020, 109, 737–749. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- Xu, D.; Xu, L.; Zhou, F.; Wang, B.; Wang, S.; Lu, M.; Sun, J. Gut bacterial communities of Dendroctonus valens and monoterpenes and carbohydrates of Pinus tabuliformis at different attack densities to host pines. Front. Microbiol. 2018, 9, 1251. [Google Scholar] [CrossRef]
- Vázquez-González, C.; Zas, R.; Erbilgin, N.; Ferrenberg, S.; Rozas, V.; Sampedro, L. Resin ducts as resistance traits in conifers: Linking dendrochronology and resin-based defences. Tree Physiol. 2020, 40, 1313–1326. [Google Scholar] [CrossRef]
- Erbilgin, N. Phytochemicals as mediators for host range expansion of a native invasive forest insect herbivore. New Phytol. 2018, 221, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Keefover-Ring, K.; Villari, C.; Klutsch, J.G.; Cook, S.; Bonello, P.; Erbilgin, N.; Raffa, K.F.; Townswend, P.A. Anatomical defenses against bark beetles related to degree of historical exposure between species and are allocated independently of chemical defenses within trees. Plant Cell Environ. 2019, 42, 633–646. [Google Scholar] [CrossRef]
- Nagel, R.; Hammerbacher, A.; Kunert, G.; Phillips, M.A.; Gershenzon, J.; Schmidt, A. Bark beetle attack history does not influence the induction of terpene and phenolic defenses in mature Norway spruce (Picea abies) trees by the bark beetle-associated fungus Endoconidiophora polonica. Front Plant Sci. 2022, 6, 892907. [Google Scholar] [CrossRef] [PubMed]
- Mageroy, M.H.; Nagy, N.E.; Steffenrem, A.; Krokene, P.; Hietala, A.M. Conifer Defences against Pathogens and Pests Mechanisms, Breeding, and Management. Curr. For. Rep. 2023, 9, 429–443. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. Toxicity of pine monoterpenes to mountain pine beetle. Sci. Rep. 2017, 7, 8858. [Google Scholar] [CrossRef]
- Ullah, A.; Klutsch, J.G.; Erbilgin, N. Production of complementary defense metabolites reflects a co-evolutionary arms race between a host plant and a mutualistic bark beetle-fungal complex. Plant Cell Environ. 2021, 44, 3064–3077. [Google Scholar] [CrossRef]
- Chiu, C.C.; Bohlmann, J. Mountain pine beetle epidemic: An interplay of terpenoids in host defense and insect pheromones. Annu. Rev. Plant Biol. 2022, 73, 475–494. [Google Scholar] [CrossRef]
- Schiebe, C.; Hammerbacher, A.; Birgersson, G.; Witzell, J.; Brodelius, P.E.; Gershenzon, J.; Hansson, B.S.; Krokene, P.; Schlyter, F. Inducibility of chemical defenses in Norway spruce bark is correlated with unsuccessful mass attacks by the spruce bark beetle. Oecologia 2012, 170, 183–198. [Google Scholar] [CrossRef]
- Goodsman, D.W.; Lusebrink, I.; Landhäusser, S.M.; Erbilgin, N.; Lieffers, V.J. Variation in carbon availability, defense chemistry and susceptibility to fungal invasion along the stems of mature trees. New Phytol. 2012, 197, 586–594. [Google Scholar] [CrossRef]
- Erbilgin, N.; Zanganeh, L.; Klutsch, J.G.; Chen, S.; Zhao, S.; Ishangulyyeva, G.; Burr, S.J.; Gaylord, M.; Hofstetter, R.; Keefover-Ring, K.; et al. Combined drought and bark beetle attacks deplete non-structural carbohydrates and promote death of mature pine trees. Plant, Cell Environ. 2021, 44, 3866–3881. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of non-structural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Wiley, E.; Rogers, B.J.; Hodgkinson, R.; Landhäusser, S.M. Non-structural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack. New Phytol. 2015, 209, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.B.; Kautz, M.; Trowbridge, A.M.; Hammerbacher, A.; Raffa, K.F.; Adams, H.D.; Goodsman, D.W.; Xu, C.; Meddens, A.J.H.; Kandasamy, D.; et al. Tree defense and bark beetles in a drying world: Carbon partitioning, functioning and modeling. New Phytol. 2020, 225, 26–36. [Google Scholar] [CrossRef]
- Lahr, E.C.; Krokene, P. Conifer stored resources and resistance to a fungus associated with the spruce bark beetle Ips typographus. PLoS ONE 2013, 8, e72405. [Google Scholar] [CrossRef]
- Zas, R.; Moreira, X.; Ramos, M.; Lima, M.R.M.; Da Silva, M.N.; Solla, A.; Vasconcelos, M.W.; Sampedro, L. Intraspecific variation of anatomical and chemical defensive traits in Maritime pine (Pinus pinaster) as factors in susceptibility to the pinewood nematode (Bursaphelenchus xylophilus). Trees—Struct Func. 2014, 29, 663–673. [Google Scholar] [CrossRef]
- Blanche, C.A.; Lorio, P.L.; Sommers, R.A.; Hodges, J.D.; Nebeker, T.E. Seasonal cambial growth and development of loblolly pine: Xylem formation, inner bark chemistry, resin ducts, and resin flow. For. Ecol. Manag. 1992, 49, 151–165. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Ayres, M.P.; Lorio, P.L., Jr.; Ruel, J.J. Environmental effects on constitutive and inducible resin defences of Pinus taeda. Ecol. Lett. 2000, 3, 329–339. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; López, R.; Martín, J.A.; Pinillos, F.; Gil, L. Resin yield in Pinus pinaster is related to tree dendrometry, stand density and tapping-induced systemic changes in xylem anatomy. For. Ecol. Manag. 2014, 313, 47–54. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A. Ponderosa pine resin defenses and growth: Metrics matter. Tree Physiol. 2015, 35, 1223–1235. [Google Scholar] [CrossRef]
- Westbrook, J.W.; Walker, A.R.; Neves, L.G.; Munoz, P.; Resende, M.F., Jr.; Neale, D.B.; Wegrzyn, J.L.; Huber, D.A.; Kirst, M.; Davis, J.M.; et al. Discovering candidate genes that regulate resin canal number in Pinus taeda stems by integrating genetic analysis across environments, ages, and populations. New Phytol. 2015, 205, 627–641. [Google Scholar] [CrossRef]
- Kane, J.M.; Kolb, T.E. Importance of resin ducts in reducing ponderosa pine mortality from bark beetle attack. Oecologia 2010, 164, 601–609. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Kane, J.M.; Mitton, J.B. Resin duct characteristics associated with tree resistance to bark beetles across lodgepole and limber pines. Oecologia 2013, 174, 1283–1292. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A.; Heyerdahl, E.K.; Boutin, M. Low-severity fire increases tree defense against bark beetle attacks. Ecology 2015, 96, 1846–1855. [Google Scholar] [CrossRef]
- Cale, J.A.; Klutsch, J.G.; Dykstra, C.B.; Peters, B.; Erbilgin, N. Pathophysiological responses of pine defensive metabolites largely lack differences between pine species but vary with eliciting ophiostomatoid fungal species. Tree Physiol. 2019, 39, 1121–1135. [Google Scholar] [CrossRef]
- Kersten, P.J.; Kopper, B.J.; Raffa, K.F.; Illman, B.L. Rapid analysis of abietanes in conifers. J. Chem. Ecol. 2006, 22, 2679–2685. [Google Scholar] [CrossRef]
- R Core Team. 2018 R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. Vienna, Austria. Available online: https://wwwR-projectorg/ (accessed on 1 March 2024).
- Harrell, F.E. Hmisc: Harrell Miscellaneous. R Package Version 4.6-0. 2021. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 1 March 2024).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 March 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 1 March 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2014, 67, 1–48. [Google Scholar]
- Erbilgin, N.; Ma, C.; Whitehouse, C.; Shan, B.; Najar, A.; Evenden, M. Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem. New Phytol. 2014, 201, 940–950. [Google Scholar] [CrossRef]
- Moles, A.T.; Peco, B.; Wallis, I.R.; Foley, W.J.; Poore, A.G.B.; Seabloom, E.W.; Vesk, P.A.; Bisigato, A.J.; Cella-Pizarro, L.; Clark, C.J.; et al. Correlations between physical and chemical defences in plants: Tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytol. 2013, 198, 252–263. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Kolb, T.E.; McDowell, N.G. Mechanisms of pion pine mortality after severe drought: A retrospective study of mature trees. Tree Physiol. 2015, 35, 806–816. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Non-structural carbon in woody plans. Ann Rev Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Mullin, M.; Klutsch, J.G.; Cale, J.A.; Hussain, A.; Zhao, S.; Whitehouse, C.; Erbilgin, N. Primary and secondary metabolite profiles of lodgepole pine trees change with elevation, but not with latitude. J. Chem. Ecol. 2021, 47, 280–293. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Kolb, T.; Keefover-Ring, K.; Burr, S.J.; Hofstetter, R.; Gaylord, M.; Raffa, K.F. Drought-mediated changes in tree physiological processes weaken tree defenses to bark beetle attack. J. Chem. Ecol. 2019, 45, 888–900. [Google Scholar] [CrossRef]
- Swihart, R.K.; Bryant, J.P. Importance of biogeography and ontogeny of woody plants in winter herbivory by mammals. J. Mammal. 2001, 82, 1–21. [Google Scholar] [CrossRef][Green Version]
- Shrimpton, D.M. Age-and size-related response of lodgepole pine to inoculation with Europhium clavigerum. Can. J. Bot. 1973, 51, 1155–1160. [Google Scholar] [CrossRef]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef]
- Seybold, S.J.; Huber, D.P.W.; Lee, J.C.; Graves, A.D.; Bohlmann, J. Pine monoterpenes and pine bark beetles: A marriage of convenience for defense and chemical communication. Phytochem. Rev. 2006, 5, 143–178. [Google Scholar] [CrossRef]
- Reid, M.L.; Purcell, J.R.C. Condition-dependent tolerance of monoterpenes in an insect herbivore. Arthropod-Plant Interact. 2011, 5, 331–337. [Google Scholar] [CrossRef]
- Shrimpton, D.M.; Reid, R.W. Change in resistance of lodgepole pine to mountain pine beetle between 1965 and 1972. Can. J. For. Res. 1973, 3, 430–433. [Google Scholar] [CrossRef]


| Outbreak Years | Period | Variables | Total Monoterpenes | ||
|---|---|---|---|---|---|
| Coefficient | t-Value | p-Value | |||
| 2014 | Post-outbreak | Resin duct production | 1.1 × 103 | 4.3 | <0.001 |
| Total resin duct area | 4.8 × 106 | 4.8 | <0.001 | ||
| Individual resin duct size | 4.2 × 107 | 2.4 | 0.029 | ||
| Resin duct density | 1.4 × 103 | 0.5 | 0.626 | ||
| Relative resin duct area (%) | 2.2 × 105 | 1.4 | 0.187 | ||
| 5 years pre-outbreak | Resin duct production | 1.1 × 103 | 3.1 | 0.005 | |
| Total resin duct area | 4.8 × 106 | 3.2 | 0.005 | ||
| Individual resin duct size | 3.0 × 107 | 1.7 | 0.107 | ||
| Resin duct density | 2.7 × 103 | 0.5 | 0.637 | ||
| Relative resin duct area (%) | 4.2 × 105 | 1.4 | 0.189 | ||
| 10 years pre-outbreak | Resin duct production | 1.4 × 103 | 4.2 | <0.001 | |
| Total resin duct area | 6.2 × 106 | 4.1 | <0.001 | ||
| Individual resin duct size | 6.0 × 107 | 2.4 | 0.025 | ||
| Resin duct density | 1.5 × 103 | 0.3 | 0.761 | ||
| Relative resin duct area (%) | 4.2 × 105 | 1.3 | 0.219 | ||
| 2015 | Post-outbreak | Resin duct production | 78.7 | 0.2 | 0.831 |
| Total resin duct area | 3.4 × 106 | 2.1 | 0.045 | ||
| Individual resin duct size | 2.3 × 107 | 4.2 | <0.001 | ||
| Resin duct density | −100.8 | −0.3 | 0.767 | ||
| Relative resin duct area (%) | −1.3 × 103 | −0.1 | 0.946 | ||
| 5 years pre-outbreak | Resin duct production | 584.0 | 1.6 | 0.127 | |
| Total resin duct area | 5.8 × 106 | 3.3 | 0.002 | ||
| Individual resin duct size | 3.0 × 107 | 3.7 | 0.002 | ||
| Resin duct density | −127.4 | −0.2 | 0.830 | ||
| Relative resin duct area (%) | −8.0 × 103 | −0.2 | 0.868 | ||
| 10 years pre-outbreak | Resin duct production | 741.6 | 1.8 | 0.081 | |
| Total resin duct area | 6.2 × 106 | 3.3 | 0.002 | ||
| Individual resin duct size | 4.0 × 107 | 4.1 | <0.001 | ||
| Resin duct density | −280.3 | −0.3 | 0.762 | ||
| Relative resin duct area (%) | −6.8 × 103 | −0.1 | 0.926 | ||
| Monoterpenes | 2014 Outbreak | 2015 Outbreak | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-Outbreak | 5 Years Pre-Outbreak | 10 Years Pre-Outbreak | Post-Outbreak | 5 Years Pre-Outbreak | 10 Years Pre-Outbreak | |||||||||
| RDP | RDA | RDS | RDP | RDA | RDP | RDA | RDS | RDA | RDS | RDA | RDS | RDA | RDS | |
| 4-allylanisole | ||||||||||||||
| (−)-borneol | ++ | ++ | ++ | ++ | + | + | + | + | ++ | + | + | + | ++ | |
| Bornyl acetate | + | + | + | + | + | + | ||||||||
| (+)-camphene | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | + | +++ | +++ | |||
| 3-carene | + | + | + | |||||||||||
| (+)-limonene | +++ | +++ | + | ++ | ++ | +++ | +++ | + | +++ | ++ | ++ | ++ | +++ | |
| (−)-limonene | ||||||||||||||
| Linalool | + | ++ | ++ | +++ | +++ | ++ | ++ | + | ++ | + | ++ | ++ | ++ | |
| Myrcene | ++ | +++ | ++ | ++ | +++ | +++ | + | +++ | ++ | +++ | ++ | ++ | ||
| Ocimene | ++ | ++ | +++ | +++ | +++ | +++ | ||||||||
| β-phellandrene | ++ | ++ | + | + | ++ | +++ | +++ | + | +++ | + | ++ | + | +++ | |
| (+)-α-pinene | + | ++ | + | + | + | |||||||||
| (−)-α-pinene | +++ | +++ | + | +++ | +++ | +++ | +++ | + | + | +++ | +++ | ++ | +++ | +++ |
| (+)-β-pinene | + | ++ | + | + | + | + | + | ++ | + | + | + | + | + | ++ |
| (−)-β-pinene | ++ | +++ | ++ | + | ++ | ++ | ++ | + | ++ | +++ | ||||
| β-phellandrene | ++ | ++ | + | + | ++ | +++ | +++ | + | +++ | + | ++ | + | +++ | |
| (R)-(+)-pulegone | + | + | ++ | ++ | + | ++ | ++ | |||||||
| α-terpinene | + | + | + | |||||||||||
| γ-terpinene | + | + | + | ++ | + | + | ++ | ++ | + | ++ | ||||
| α-terpineol | +++ | ++ | + | +++ | ||||||||||
| Terpinolene | ++ | +++ | + | + | + | |||||||||
| Outbreak Years | BAI | Total Monoterpenes | Total Diterpenes | ||||
|---|---|---|---|---|---|---|---|
| Coefficient | t-Value | p-Value | Coefficient | t-Value | p-Value | ||
| 2014 | Post-outbreak | 532.4 | 2.7 | 0.015 | −0.8 | −0.5 | 0.608 |
| 5 years pre-outbreak | 549.9 | 2.6 | 0.015 | −0.8 | −0.5 | 0.658 | |
| 10 years pre-outbreak | 665.1 | 3.2 | 0.004 | −1.2 | −0.7 | 0.516 | |
| 2015 | Post-outbreak | 485.9 | 2.6 | 0.013 | 4.9 | 2.7 | 0.012 |
| 5 years pre-outbreak | 686.8 | 3.7 | 0.002 | 4.9 | 2.4 | 0.024 | |
| 10 years pre-outbreak | 791.7 | 3.7 | 0.001 | 6.3 | 2.6 | 0.013 | |
| Terpene Classes | 2014 Outbreak | 2015 Outbreak | ||||
|---|---|---|---|---|---|---|
| Post-Outbreak BAI | 5-Year Pre-Outbreak BAI | 10-Year Pre-Outbreak BAI | Post-Outbreak BAI | 5-Year Pre-Outbreak BAI | 10-Year Pre-Outbreak BAI | |
| Monoterpenes | ||||||
| 4-allylanisole | ||||||
| (−)-borneol | +++ | + | ++ | |||
| Bornyl acetate | ++ | + | + | |||
| (+)-camphene | ++ | ++ | ++ | + | + | + |
| 3-carene | ||||||
| (+)-limonene | + | + | ++ | ++ | +++ | +++ |
| (−)-Limonene | ||||||
| linalool | +++ | +++ | +++ | +++ | ++ | +++ |
| Myrcene | ++ | ++ | +++ | ++ | +++ | +++ |
| Ocimene | + | + | ++ | |||
| β-phellandrene | + | ++ | ++ | ++ | ++ | +++ |
| (+)-α-pinene | ++ | + | ||||
| (−)-α-pinene | +++ | ++ | +++ | ++ | +++ | +++ |
| (+)-β-pinene | + | + | + | + | + | + |
| (−)-β-pinene | ++ | + | ++ | ++ | ++ | |
| (R)-(+)-Pulegone | ++ | +++ | +++ | |||
| α-terpinene | ||||||
| γ-terpinene | + | ++ | ++ | |||
| α-terpineol | + | +++ | +++ | +++ | ||
| Terpinolene | + | + | + | |||
| Diterpenes | ||||||
| Abietic | + | + | + | |||
| Dehydroabietic | ++ | ++ | +++ | |||
| Levopiramic | +++ | +++ | +++ | |||
| Neoabietic | +++ | +++ | +++ | |||
| Palustric | ++ | ++ | +++ | |||
| Sandaracopiramic | ||||||
| Tree Age | ||
|---|---|---|
| Monoterpenes | 2014 Outbreak | 2015 Outbreak |
| 4-allylanisole | ||
| (−)-borneol | ||
| Bornyl acetate | ||
| (+)-camphene | - | |
| 3-carene | ||
| (+)-limonene | - | -- |
| (−)-limonene | ||
| Linalool | --- | -- |
| Myrcene | - | |
| Ocimene | ||
| β-phellandrene | - | |
| (+)-α-pinene | ||
| (−)-α-pinene | - | -- |
| (+)-β-pinene | - | |
| (−)-β-pinene | - | |
| (R)-(+)-pulegone | - | |
| α-terpinene | - | |
| γ-terpinene | -- | -- |
| α-terpineol | -- | |
| Terpinolene | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, G.; Zhao, S.; Klutsch, J.G.; Ishangulyyeva, G.; Erbilgin, N. The Legacy Effect of Mountain Pine Beetle Outbreaks on the Chemical and Anatomical Defences of Surviving Lodgepole Pine Trees. Metabolites 2024, 14, 472. https://doi.org/10.3390/metabo14090472
Baker G, Zhao S, Klutsch JG, Ishangulyyeva G, Erbilgin N. The Legacy Effect of Mountain Pine Beetle Outbreaks on the Chemical and Anatomical Defences of Surviving Lodgepole Pine Trees. Metabolites. 2024; 14(9):472. https://doi.org/10.3390/metabo14090472
Chicago/Turabian StyleBaker, Gigi, Shiyang Zhao, Jennifer G. Klutsch, Guncha Ishangulyyeva, and Nadir Erbilgin. 2024. "The Legacy Effect of Mountain Pine Beetle Outbreaks on the Chemical and Anatomical Defences of Surviving Lodgepole Pine Trees" Metabolites 14, no. 9: 472. https://doi.org/10.3390/metabo14090472
APA StyleBaker, G., Zhao, S., Klutsch, J. G., Ishangulyyeva, G., & Erbilgin, N. (2024). The Legacy Effect of Mountain Pine Beetle Outbreaks on the Chemical and Anatomical Defences of Surviving Lodgepole Pine Trees. Metabolites, 14(9), 472. https://doi.org/10.3390/metabo14090472







