Effects of E-Cigarettes on the Lung and Systemic Metabolome in People with HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Data Collection Instruments
2.3. Sample Collection
2.3.1. Exhaled Breath Condensate and Oropharyngeal Gargles
2.3.2. Serum and Plasma
2.3.3. Fecal Material
2.4. Metabolite Extractions
2.5. UHPLC-HRMS
2.6. Metabolomics Data Processing
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Substance Use
3.3. HIV-Related Variables
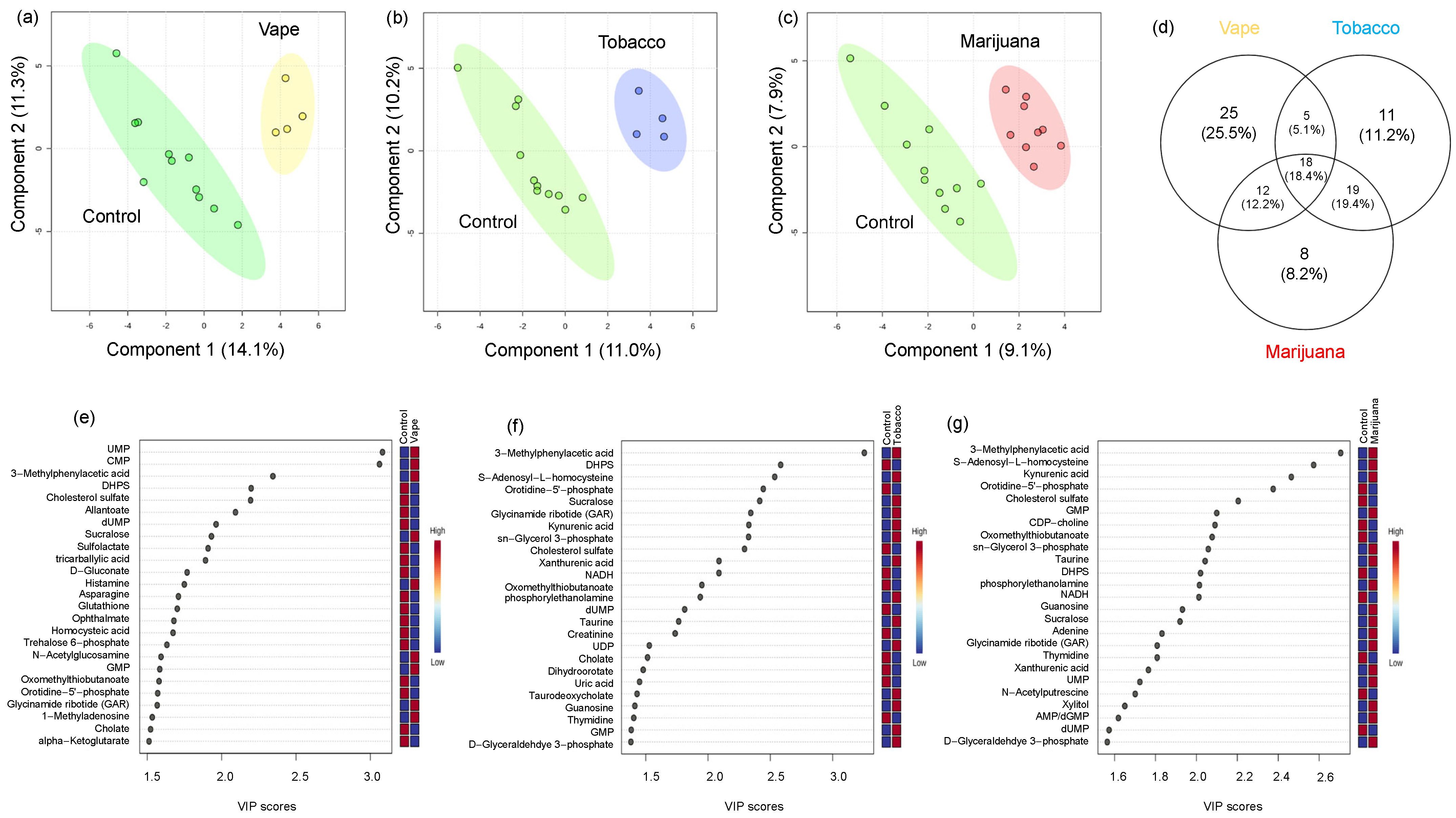
3.4. Global Metabolomics
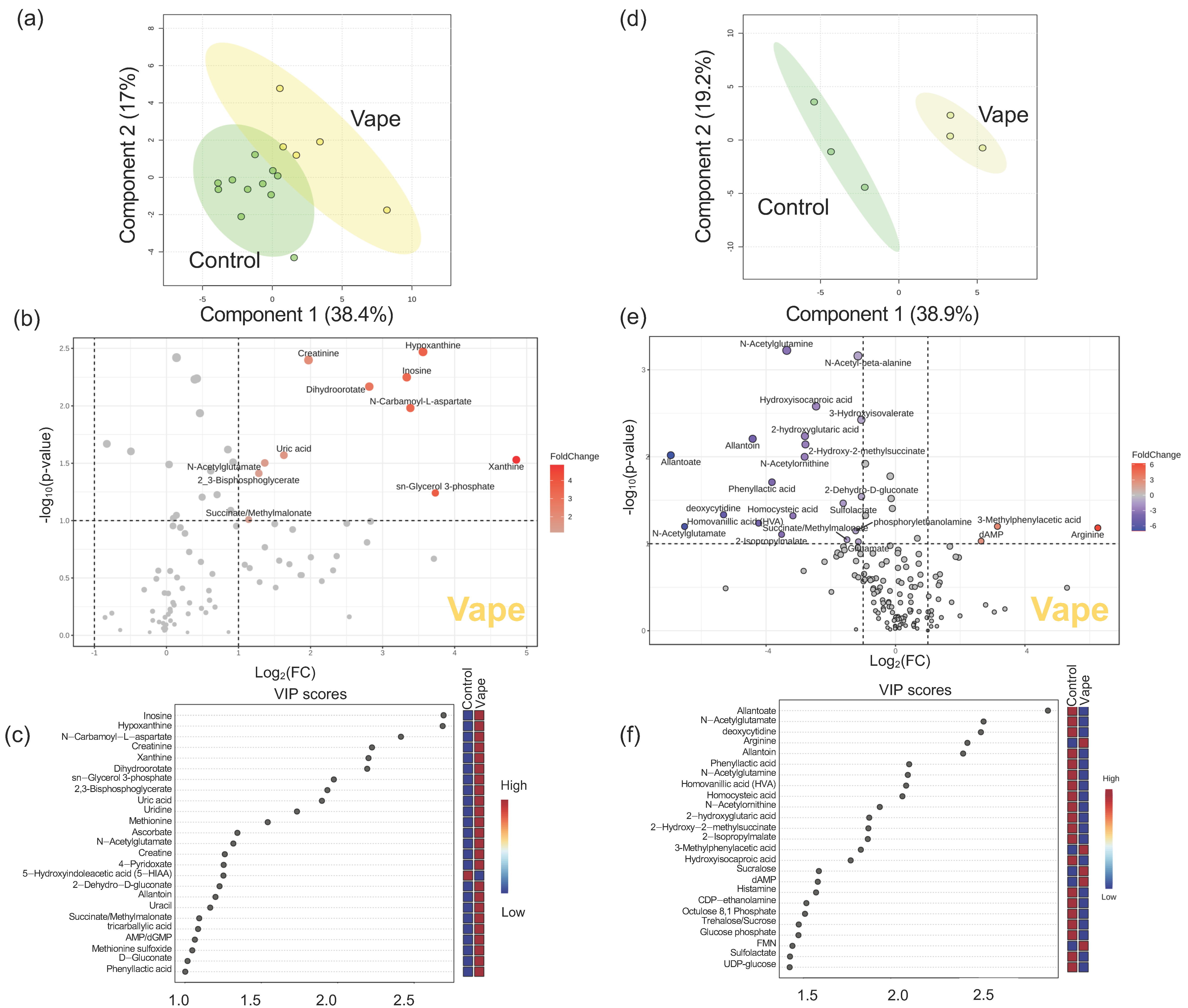
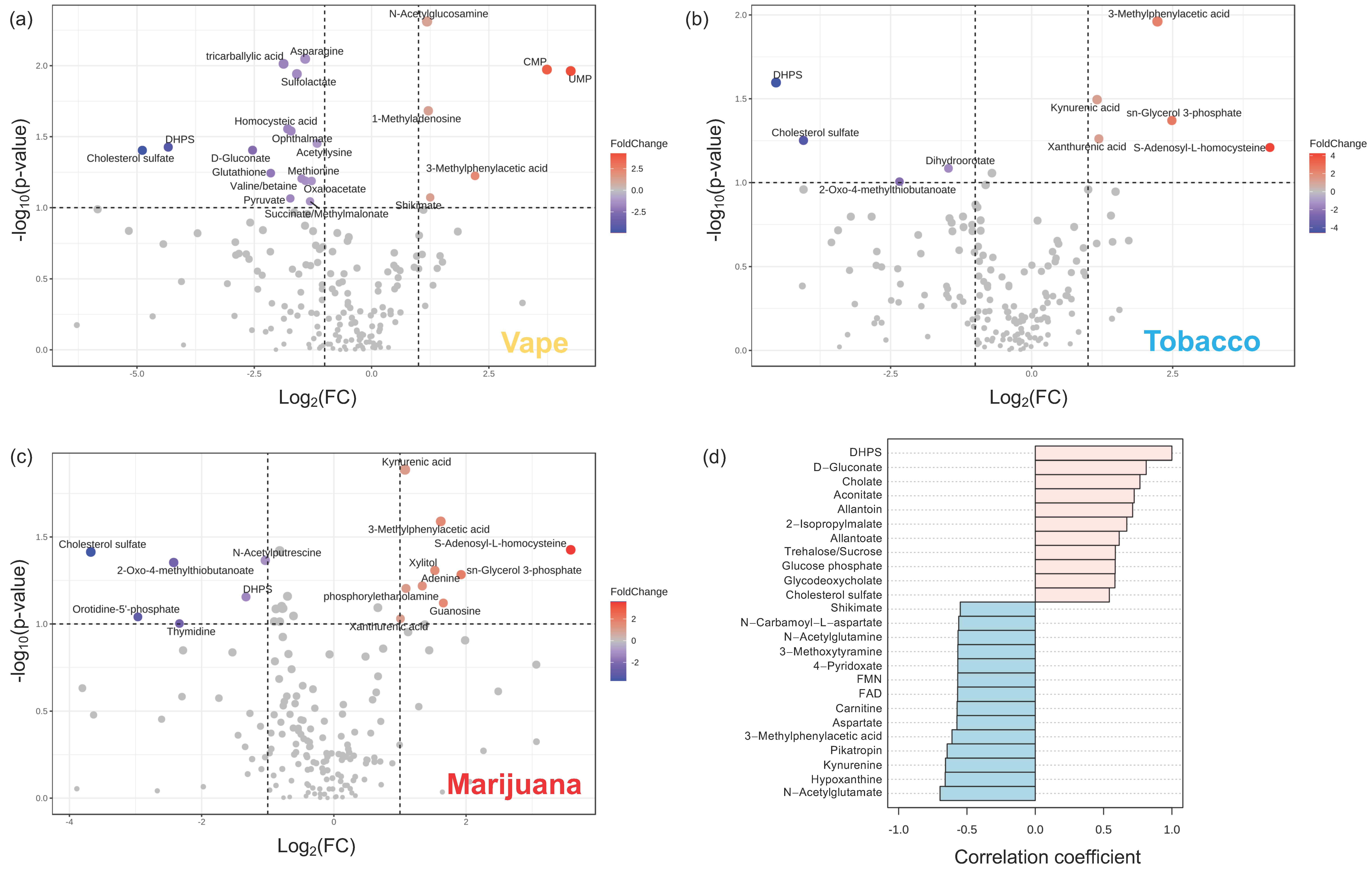
3.4.1. Exhaled Breath Condensate Metabolome
3.4.2. Oropharyngeal Metabolome
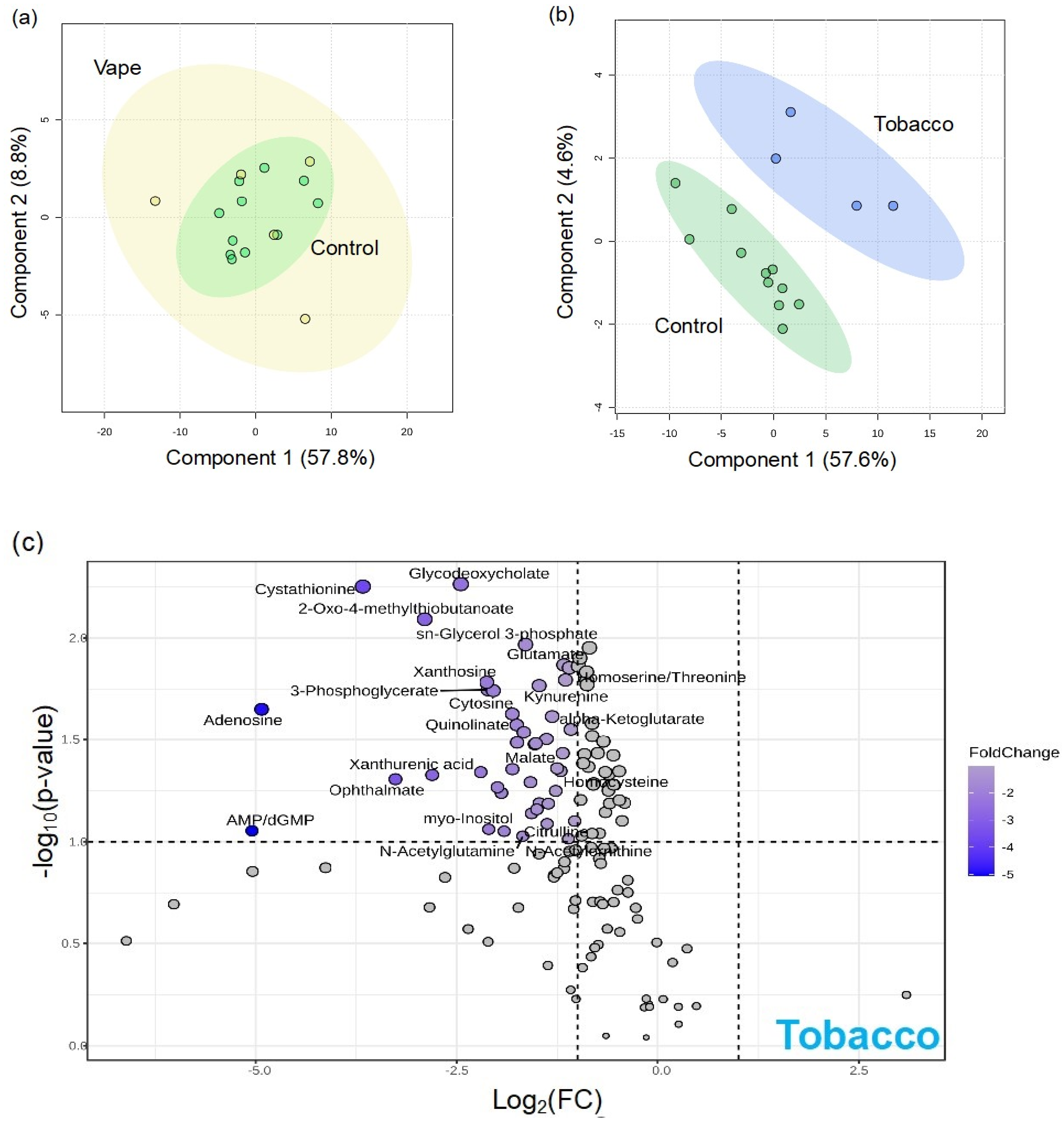
3.4.3. Peripheral Blood Metabolome
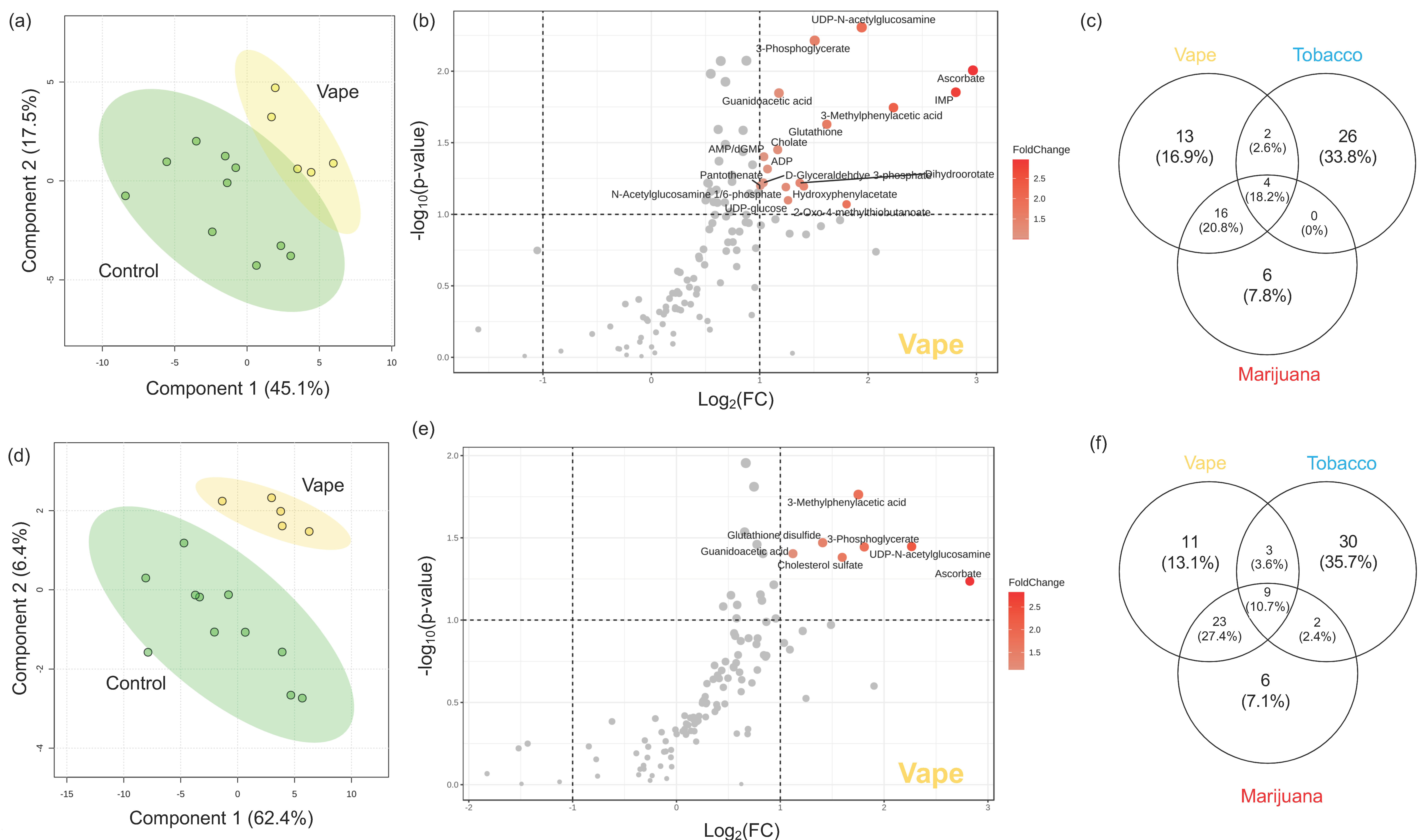
3.4.4. Gut Metabolome
4. Discussion
4.1. Vaping Induces Considerable Changes in the Upper Respiratory Metabolome
4.2. Availability of Nutrients in Circulation Are Affected by Vaping
4.3. Vaping-Induced Metabolic Dyshomeostasis Is Most Evident in the Gut Metabolome
4.4. Vaping Leads to Systemic Metabolome Alterations as Well as Distinctive Metabolic Patterns Not Observed in Tobacco or Marijuana Smoking
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ware, O.D.; Srivastava, A.; Masa, R.; Baca-Atlas, S.N.; Chowa, G. HIV Prevention Services in Residential Substance Use Disorder Treatment Facilities in the United States. AIDS Educ. Prev. 2023, 35, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Lifson, A.R.; Neuhaus, J.; Arribas, J.R.; Berg-Wolf, M.v.D.; Labriola, A.M.; Read, T.R.; INSIGHT SMART Study Group. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am. J. Public Health 2010, 100, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Savage, C.L.; Sanchez, M. Alcohol and Substance Use Disorder Screening, Brief Intervention, and Referral to Treatment Among People Living with HIV/AIDS. J. Addict. Nurs. 2016, 27, 214–217. [Google Scholar] [CrossRef]
- Asfar, T.; Perez, A.; Shipman, P.; Carrico, A.W.; Lee, D.J.; Alcaide, M.L.; Jones, D.L.; Brewer, J.; Koru-Sengul, T. National Estimates of Prevalence, Time-Trend, and Correlates of Smoking in US People Living with HIV (NHANES 1999–2016). Nicotine Tob. Res. 2021, 23, 1308–1317. [Google Scholar] [CrossRef]
- Services USDoHaH. HIV.gov: HIV and Smoking. Updated 2024. Available online: https://www.hiv.gov/hiv-basics/staying-in-hiv-care/other-related-health-issues/smoking (accessed on 22 April 2024).
- Rahmanian, S.; Wewers, M.E.; Koletar, S.; Reynolds, N.; Ferketich, A.; Diaz, P. Cigarette smoking in the HIV-infected population. Proc. Am. Thorac. Soc. 2011, 8, 313–319. [Google Scholar] [CrossRef]
- Ye, Y.; Shrestha, S.; Burkholder, G.; Bansal, A.; Erdmann, N.; Wiener, H.; Tang, J. Rates and Correlates of Incident Type 2 Diabetes Mellitus Among Persons Living with HIV-1 Infection. Front. Endocrinol. 2020, 11, 555401. [Google Scholar] [CrossRef]
- Thet, D.; Siritientong, T. Antiretroviral Therapy-Associated Metabolic Complications: Review of the Recent Studies. HIV/AIDS Res. Palliat. Care 2020, 12, 507–524. [Google Scholar] [CrossRef]
- Koethe, J.R.; Lagathu, C.; Lake, J.E.; Domingo, P.; Calmy, A.; Falutz, J.; Brown, T.T.; Capeau, J. HIV and antiretroviral therapy-related fat alterations. Nat. Rev. Dis. Primers 2020, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Cummins, N.W. Metabolic Complications of Chronic HIV Infection: A Narrative Review. Pathogens 2022, 11, 197. [Google Scholar] [CrossRef]
- Hammond, S.; Phillips, J. E-Cigarettes and Vaping. Workplace Health Saf. 2020, 68, 301. [Google Scholar] [CrossRef]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The rise of e-cigarettes, pod mod devices, and JUUL among youth: Factors influencing use, health implications, and downstream effects. Drug Alcohol. Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Osei, A.D.; Mirbolouk, M.; Orimoloye, O.A.; Dzaye, O.; Uddin, S.M.I.; Benjamin, E.J.; Hall, M.E.; DeFilippis, A.P.; Stokes, A.; Bhatnagar, A.; et al. Association between E-Cigarette Use and Cardiovascular Disease Among Never and Current Combustible-Cigarette Smokers. Am. J. Med. 2019, 132, 949–954.e2. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 2013, 8, e57987. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Kistler, K.A.; Gillman, G.; Voudris, V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob. Res. 2014, 17, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ellington, S.; Salvatore, P.P.; Ko, J.; Danielson, M.; Kim, L.; Cyrus, A.; Wallace, M.; Board, A.; Krishnasamy, V.; King, B.A.; et al. Update: Product, Substance-Use, and Demographic Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury-United States, August 2019–January 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Moritz, E.D.; Zapata, L.B.; Lekiachvili, A.; Glidden, E.; Annor, F.B.; Werner, A.K.; Ussery, E.N.; Hughes, M.M.; Kimball, A.; DeSisto, C.L.; et al. Update: Characteristics of Patients in a National Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injuries-United States, October 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 985–989. [Google Scholar] [CrossRef]
- Rebuli, M.E.; Rose, J.J.; Noël, A.; Croft, D.P.; Benowitz, N.L.; Cohen, A.H.; Goniewicz, M.L.; Larsen, B.T.; Leigh, N.; McGraw, M.D.; et al. The E-cigarette or Vaping Product Use-Associated Lung Injury Epidemic: Pathogenesis, Management, and Future Directions: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2023, 20, 1–17. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Sankari, A.; Rahman, O. Vaping-Associated Pulmonary Injury; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Iacob, A.M.; Martínez, M.F.E.; Castro, E.B.; Olay, S.J.; García, S.O.; Gutiérrez, L.M.J. Effects of Vape Use on Oral Health: A Review of the Literature. Medicina 2024, 60, 365. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.K.; Meyers, M.C.; Taylor, C.M.; Luo, M.; E Dowd, S.; Yue, X.; O Byerley, L. Sex-Dependent Effects of Inhaled Nicotine on the Gut Microbiome. Nicotine Tob. Res. 2022, 24, 1363–1370. [Google Scholar] [CrossRef]
- August, A.; Altraja, S.; Kilk, K.; Porosk, R.; Soomets, U.; Altraja, A. E-Cigarette Affects the Metabolome of Primary Normal Human Bronchial Epithelial Cells. PLoS ONE 2015, 10, e0142053. [Google Scholar]
- Yang, I.; Rodriguez, J.; Wright, C.Y.; Hu, Y. Oral microbiome of electronic cigarette users: A cross-sectional exploration. Oral Dis. 2022, 29, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Hwang, B.-O.; Lim, M.; Ok, S.-H.; Lee, S.-K.; Chun, K.-S.; Park, K.-K.; Hu, Y.; Chung, W.-Y.; Song, N.-Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, J.; Fonseca, A.G.; Moshensky, A.; Kothari, T.; Sayed, I.M.; Ibeawuchi, S.-R.; Pranadinata, R.F.; Ear, J.; Sahoo, D.; et al. E-cigarettes compromise the gut barrier and trigger inflammation. Iscience 2021, 24, 102035. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-C.; Matulewicz, R.S.; Sherman, S.E.; Jaspers, I.; Weitzman, M.L.; Gordon, T.; Liu, C.-W.; Yang, Y.; Lu, K.; Bjurlin, M.A. Untargeted Metabolomics to Characterize the Urinary Chemical Landscape of E-Cigarette Users. Chem. Res. Toxicol. 2023, 36, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Boasso, A.; Shearer, G.M.; Chougnet, C. Immune dysregulation in human immunodeficiency virus infection: Know it, fix it, prevent it? J. Intern. Med. 2008, 265, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Richey, L.; Schroeder, J.; Apolzan, J.; Mistretta, K.; Holloway, K.; Lin, H.-Y.; Arnold, C.; Welsh, D. The Relationship between Modifiable Health Behaviors and Health Literacy in People with HIV is Complicated by Multiple Factors. Open Forum Infect. Dis. 2022, 9 (Suppl. S2), 1247. [Google Scholar] [CrossRef]
- Adamson, S.J.; Kay-Lambkin, F.J.; Baker, A.L.; Lewin, T.J.; Thornton, L.; Kelly, B.J.; Sellman, J.D. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010, 110, 137–143. [Google Scholar] [CrossRef]
- Joshua, D.; Rabinowitz, E.K. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal. Chem. 2007, 79, 6167–6173. [Google Scholar]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Bazurto, J.V.; Dearth, S.P.; Tague, E.D.; Campagna, S.R.; Downs, D.M. Untargeted metabolomics confirms and extends the understanding of the impact of aminoimidazole carboxamide ribotide (AICAR) in the metabolic network of Salmonella enterica. Microb. Cell 2017, 5, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Tang, W.H.; Römpp, A.; Neumann, S.; Pizarro, A.D.; et al. mzML—A community standard for mass spectrometry data. Mol. Cell Proteom. 2011, 10, R110.000133. [Google Scholar] [CrossRef]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, 37, 14.11.1–14.11.23. [Google Scholar] [CrossRef] [PubMed]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic Analysis and Visualization Engine for LC−MS Data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef] [PubMed]
- Byerley, L.O.; Gallivan, K.M.; Christopher, C.J.; Taylor, C.M.; Luo, M.; Dowd, S.E.; Davis, G.M.; Castro, H.F.; Campagna, S.R.; Ondrak, K.S. Gut Microbiome and Metabolome Variations in Self-Identified Muscle Builders Who Report Using Protein Supplements. Nutrients 2022, 14, 533. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Sotelo-Orozco, J.; Chen, S.-Y.; Hertz-Picciotto, I.; Slupsky, C.M. A Comparison of Serum and Plasma Blood Collection Tubes for the Integration of Epidemiological and Metabolomics Data. Front. Mol. Biosci. 2021, 8, 682134. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tackett, A.P.; Berlowitz, J.B.; Harlow, A.F.; Kathuria, H.; Galiatsatos, P.; Fetterman, J.L.; Cho, J.; Blaha, M.J.; Hamburg, N.M.; et al. Association of Electronic Cigarette Use with Respiratory Symptom Development among U.S. Young Adults. Am. J. Respir. Crit. Care Med. 2022, 205, 1320–1329. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Fraiman, J.B. Cardiovascular effects of electronic cigarettes. Nat. Rev. Cardiol. 2017, 14, 447–456. [Google Scholar] [CrossRef]
- Chaiton, M.; Pienkowski, M.; Musani, I.; Bondy, S.J.; Cohen, J.E.; Dubray, J.; Eissenberg, T.; Kaufman, P.; Stanbrook, M.; Schwartz, R. Smoking, e-cigarettes and the effect on respiratory symptoms among a population sample of youth: Retrospective cohort study. Tob. Induc. Dis. 2023, 21, 08. [Google Scholar] [CrossRef]
- Szafran, D.; Görig, T.; Vollstädt-Klein, S.; Grundinger, N.; Mons, U.; Lohner, V.; Schneider, S.; Andreas, M. Addictive Potential of e-Cigarettes as Reported in e-Cigarette Online Forums: Netnographic Analysis of Subjective Experiences. J. Med. Internet Res. 2023, 25, e41669. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.; Samburova, V.; Son, Y.; Cappelli, D.; Demopoulos, C.; Capurro, A.; Pinto, A.; Chrzan, B.; Kingsley, K.; Howard, K.; et al. Harmful chemicals emitted from electronic cigarettes and potential deleterious effects in the oral cavity. Tob. Induc. Dis. 2020, 18, 41. [Google Scholar] [CrossRef]
- Sun, R.; Méndez, D.; Warner, K.E. Association of Electronic Cigarette Use by US Adolescents with Subsequent Persistent Cigarette Smoking. JAMA Netw Open 2023, 6, e234885. [Google Scholar] [CrossRef]
- Thorne, S.L.; Caraballo, R.S.; Tie, Y.; Harris, N.S.; Shouse, R.L.; Brooks, J.T. E-Cigarette Use Among persons with Diagnosed HIV in the U.S. AJPM Focus 2023, 2, 100056. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Rhoads, J.M.; Satterfield, M.C.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2008, 37, 153–168. [Google Scholar] [CrossRef]
- Maki, K.A.; Ganesan, S.M.; Meeks, B.; Farmer, N.; Kazmi, N.; Barb, J.J.; Joseph, P.V.; Wallen, G.R. The role of the oral microbiome in smoking-related cardiovascular risk: A review of the literature exploring mechanisms and pathways. J. Transl. Med. 2022, 20, 584. [Google Scholar] [CrossRef]
- Menzies, K.J.; Zhang, H.; Katsyuba, E.; Auwerx, J. Protein acetylation in metabolism metabolites and cofactors. Nat. Rev. Endocrinol. 2015, 12, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.D.; Ford, L.; Wittmann, B.; Conner, J.; Wulff, J.; Mitchell, M.; Evans, A.M.; Toal, D.R. Global biochemical analysis of plasma, serum and whole blood collected using various anticoagulant additives. PLoS ONE 2021, 16, e0249797. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Žitňanová, I.; Korytár, P.; Aruoma, O.I.; Sustrová, M.; Garaiová, I.; Muchová, J.; Kalnovicová, T.; Pueschel, S.; Duracková, Z. Uric acid and allantoin levels in Down syndrome: Antioxidant and oxidative stress mechanisms? Clin. Chim. Acta 2004, 341, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Carr, T.; Oke, O.; Jaunky, T.; Breheny, D.; Lowe, F.; Gaça, M. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol. Mech. Methods 2016, 26, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Assiri, M.A.; Al Jumayi, S.R.; Alsuhaymi, S.; Emwas, A.-H.; Jaremko, M.; Alsaleh, N.B.; Al Mutairi, M.M.; Alshamrani, A.A.; Sobeai, H.A.; Alghibiwi, H. Electronic cigarette vapor disrupts key metabolic pathways in human lung epithelial cells. audi Pharm. J. 2023, 32, 101897. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.H.; Sun, B.; Zhao, T.; Shu, S.; Chang, C.H.; Messadi, D.; Xia, T.; Zhu, Y.; Hu, S. Characterization of electronic cigarette aerosol and its induction of oxidative stress response in oral keratinocytes. PLoS ONE 2016, 11, e0154447. [Google Scholar]
- Jung, S.; Kim, M.K.; Choi, B.Y. The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 806–816. [Google Scholar] [CrossRef]
- Xu, D.; Ma, R.; Ju, Y.; Song, X.; Niu, B.; Hong, W.; Wang, R.; Yang, Q.; Zhao, Z.; Zhang, Y.; et al. Cholesterol sulfate alleviates ulcerative colitis by promoting cholesterol biosynthesis in colonic epithelial cells. Nat. Commun. 2022, 13, 4428. [Google Scholar] [CrossRef]
- Strott, C.A.; Higashi, Y. Cholesterol sulfate in human physiology: What’s it all about? J. Lipid Res. 2003, 44, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Zoli, M.; Picciotto, M.R. Nicotinic regulation of energy homeostasis. Nicotine Tob. Res. 2012, 14, 1270–1290. [Google Scholar] [CrossRef] [PubMed]
- Maddatu, J.; Anderson-Baucum, E.; Evans-Molina, C. Smoking and the risk of type 2 diabetes. Transl. Res. 2017, 184, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.T.; Kits, K.D.; Löffler, J.; Burrichter, A.G.; Fiedler, A.; Denger, K.; Frommeyer, B.; Herbold, C.W.; Rattei, T.; Karcher, N.; et al. Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut. ISME J. 2021, 15, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Frommeyer, B.; Fiedler, A.W.; Oehler, S.R.; Hanson, B.T.; Loy, A.; Franchini, P.; Spiteller, D.; Schleheck, D. Environmental and Intestinal Phylum Firmicutes Bacteria Metabolize the Plant Sugar Sulfoquinovose via a 6-Deoxy-6-sulfofructose Transaldolase Pathway. iScience 2020, 23, 101510. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, Y.; Lin, L.; Teng, L.; Yin, J.; Lu, Q.; Chen, J.; Zheng, Y.; Li, Y.; Xu, R.; et al. Two radical-dependent mechanisms for anaerobic degradation of the globally abundant organosulfur compound dihydroxypropanesulfonate. Proc. Natl. Acad. Sci. USA 2020, 117, 15599–15608. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Tong, Y.; Zhang, Y. New mechanisms for bacterial degradation of sulfoquinovose. Biosci. Rep. 2022, 42, 314. [Google Scholar] [CrossRef] [PubMed]
- Denger, K.; Weiss, M.; Felux, A.-K.; Schneider, A.; Mayer, C.; Spiteller, D.; Huhn, T.; Cook, A.M.; Schleheck, D. Sulphoglycolysis in Escherichia coli K-12 closes a gap in the biogeochemical sulphur cycle. Nature 2014, 507, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, R.; Wu, L.; Yang, G. Hydrogen sulfide signaling in regulation of cell behaviors. Nitric Oxide 2020, 103, 9–19. [Google Scholar] [CrossRef]
- Murros, K.E. Hydrogen Sulfide Produced by Gut Bacteria May Induce Parkinson’s Disease. Cells 2022, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Fakharian, F.; Thirugnanam, S.; Welsh, D.A.; Kim, W.-K.; Rappaport, J.; Bittinger, K.; Rout, N. The Role of Gut Dysbiosis in the Loss of Intestinal Immune Cell Functions and Viral Pathogenesis. Microorganisms 2023, 11, 1849. [Google Scholar] [CrossRef]
- Crakes, K.R.; Jiang, G. Gut Microbiome Alterations During HIV/SIV Infection: Implications for HIV Cure. Front. Microbiol. 2019, 10, 1104. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New Insights into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef]
- Brunius, C.; Vidanarachchi, J.K.; Tomankova, J.; Lundström, K.; Andersson, K.; Zamaratskaia, G. Skatole metabolites in urine as a biological marker of pigs with enhanced hepatic metabolism. Animal 2016, 10, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-J.; Hwang, Y.-J.; Yun, M.-O.; Kim, J.-H.; Oh, G.-S.; Park, J.-H. Indoxyl 3-sulfate stimulates Th17 differentiation enhancing phosphorylation of c-Src and STAT3 to worsen experimental autoimmune encephalomyelitis. Toxicol. Lett. 2013, 220, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Banoglu, E.; Jha, G.G.; King, R.S. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2001, 26, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Piquereau, J.; Boitard, S.E.; Ventura-Clapier, R.; Mericskay, M. Metabolic Therapy of Heart Failure: Is There a Future for B Vitamins? Int. J. Mol. Sci. 2021, 23, 30. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Jiang, H. Gut microbiota in atherosclerosis: Focus on trimethylamine N-oxide. Apmis 2020, 128, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shao, W.; Liu, Q.; Liu, N.; Wang, Q.; Xu, J.; Zhang, X.; Weng, Z.; Lu, Q.; Jiao, L.; et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat. Commun. 2022, 13, 252. [Google Scholar] [CrossRef]
- Romano, K.; Vivas, E.; Amador-Noguez, D. FE Rey Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.P.; Abdelwahed, S.H.; Fenwick, M.K.; Hazra, A.B.; Taga, M.E.; Zhang, Y.; Ealick, S.E.; Begley, T.P. Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B12 Biosynthesis. J. Am. Chem. Soc. 2015, 137, 10444–10447. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Hazra, A.B.; Abdelwahed, S.; Hilmey, D.G.; Begley, T.P. A “radical dance” in thiamin biosynthesis: Mechanistic analysis of the bacterial hydroxymethylpyrimidine phosphate synthase. Angew. Chem. Int. Ed. Engl. 2010, 49, 8653–8656. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, S.W.; Maity, A.; Oprysko, P.R.; Kachur, A.V.; Ayene, I.S.; Biaglow, J.E.; Koch, C.J. Detection of reactive oxygen species via endogenous oxidative pentose phosphate cycle activity in response to oxygen concentration: Implications for the mechanism of HIF-1α stabilization under moderate hypoxia. J. Biol. Chem. 2007, 282, 36790–36796. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, D.; Fu, X.; Reisz, J.A.; Kanias, T.; Nemkov, T.; Page, G.P.; Dumont, L.; Roubinian, N.; Stone, M.; Kleinman, S.; et al. Nicotine exposure increases markers of oxidant stress in stored red blood cells from healthy donor volunteers. Transfusion 2020, 60, 1160–1174. [Google Scholar] [CrossRef]
- Moon, S.J.; Dong, W.; Stephanopoulos, G.N.; Sikes, H.D. Oxidative pentose phosphate pathway and glucose anaplerosis support maintenance of mitochondrial NADPH pool under mitochondrial oxidative stress. Bioeng. Transl. Med. 2020, 5, e10184. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; Ashfield-Watt, P.A.; Powers, H.J.; Newcombe, R.G.; McDowell, I.F. Effect of riboflavin status on the homocysteine-lowering effect of folate in relation to the MTHFR (C677T) genotype. Clin. Chem. 2003, 49, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Gladyshev, V.N. The biological significance of methionine sulfoxide stereochemistry. Free. Radic. Biol. Med. 2010, 50, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Van Remmen, H.; Richardson, A.; Wehr, N.B.; Levine, R.L. Methionine oxidation and aging. Biochim. Biophys. Acta 2005, 1703, 135–140. [Google Scholar] [CrossRef]
- Kass, A.P.; Overbeek, D.L.; Chiel, L.E.; Boyer, E.W.; Casey, A.M.H. Case series: Adolescent victims of the vaping public health crisis with pulmonary complications. Pediatr. Pulmonol. 2020, 55, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Salzman, G.A. The Vaping Epidemic in Adolescents. Mo. Med. 2020, 117, 56–58. [Google Scholar] [PubMed]
- Arain, M.; Haque, M.; Johal, L.; Mathur, P.; Nel, W.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013, 9, 449–461. [Google Scholar] [PubMed]
| Variable |
Control Group
(n = 10) | Participants Who Smoke Combustible Tobacco and/or Marijuana (n = 7) |
Participants Who Use E-Cigarettes/Vape
(n = 6) |
|---|---|---|---|
| Age (years) | 51 (SD= 7.33) | 48.83 (SD = 14.14) | 43.83 (SD = 11.92) |
| Male | 6 (60%) | 6 (85.71%) | 5 (83.33%) |
| Female | 4 (40%) | 1 (14.28%) | 1 (16.66%) |
| Ethnicity | |||
| African-American | 7 (70%) | 5 (71.42%) | 3 (50%) |
| Caucasian/White | 1 (10%) | 1 (14.28%) | 3 (50%) |
| Hispanic/Latino | 1 (10%) | - | - |
| Did not report | 1 (10%) | 1 (14.28%) | - |
| BMI | 33.84 (SD = 6.56) | 40.53 (SD = 14.06) | 28.52 (SD = 5.93) |
| Variable |
Control Group
(n = 10) | Participants Who Smoke Combustible Tobacco and/or Marijuana (n = 7) |
Participants Who Smoke E-Cigarettes/Vape
(n = 6) |
|---|---|---|---|
| Tobacco smoking | |||
| Number of cigarettes 1 | 0 | 4.83 (SD = 4.57) | 6.5 (SD = 8.8) |
| Smoking Years | - | 16.8 (SD = 13.92) | 24.33 (SD = 13.93) |
| E-cigarettes use (days) 1 | - | - | 20.33 (SD = 14.97) |
|
Marijuana
Lifetime (yes) | 6 (60%) | 7 (100%) | 6 (100%) |
| Days of use 1 | 0 | 6.33 (SD = 11.75) | 21.5 (SD = 13.47) |
| Alcohol use | |||
| Lifetime (yes) | 8 (75%) | 7 (100%) | 6 (100%) |
| Days of use 1 | 8 (SD = 4) | 2 (SD = 0) | 2 (SD = 0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaparte, A.; Christopher, C.J.; Arnold, C.; Richey, L.; Castille, A.; Mistretta, K.; Taylor, C.M.; Lin, H.; Nelson, S.; Kirwan, J.P.; et al. Effects of E-Cigarettes on the Lung and Systemic Metabolome in People with HIV. Metabolites 2024, 14, 434. https://doi.org/10.3390/metabo14080434
Zaparte A, Christopher CJ, Arnold C, Richey L, Castille A, Mistretta K, Taylor CM, Lin H, Nelson S, Kirwan JP, et al. Effects of E-Cigarettes on the Lung and Systemic Metabolome in People with HIV. Metabolites. 2024; 14(8):434. https://doi.org/10.3390/metabo14080434
Chicago/Turabian StyleZaparte, Aline, Courtney J. Christopher, Connie Arnold, Lauren Richey, Adairre Castille, Kyle Mistretta, Christopher M. Taylor, Huiyi Lin, Steve Nelson, John P. Kirwan, and et al. 2024. "Effects of E-Cigarettes on the Lung and Systemic Metabolome in People with HIV" Metabolites 14, no. 8: 434. https://doi.org/10.3390/metabo14080434
APA StyleZaparte, A., Christopher, C. J., Arnold, C., Richey, L., Castille, A., Mistretta, K., Taylor, C. M., Lin, H., Nelson, S., Kirwan, J. P., Apolzan, J. W., Campagna, S. R., & Welsh, D. A. (2024). Effects of E-Cigarettes on the Lung and Systemic Metabolome in People with HIV. Metabolites, 14(8), 434. https://doi.org/10.3390/metabo14080434








