The Urinary Metabolome of Newborns with Perinatal Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Sample Collection
2.3. Metabolite Measurement
LC/DFI-MS/MS Analysis
2.4. Statistical Analysis
3. Results
3.1. Healthy and NICU Newborns: Clinical and Metabolic Characteristics
3.2. Clinical Characteristics, Perinatal Outcomes, and Maternal History of Newborns with Bronchopulmonary Dysplasia (BPD)
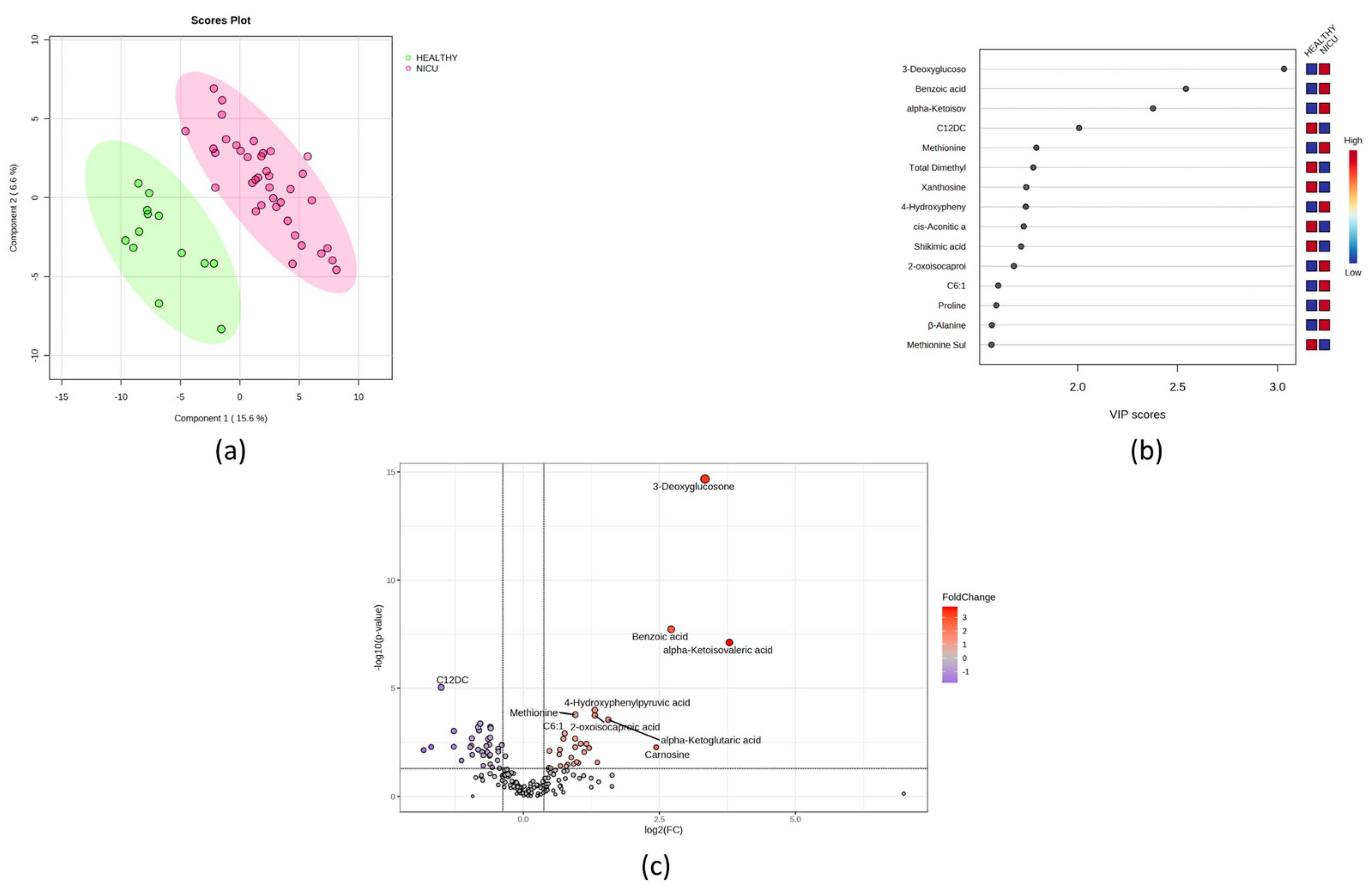
3.3. Comparison between Healthy and NICU Newborns
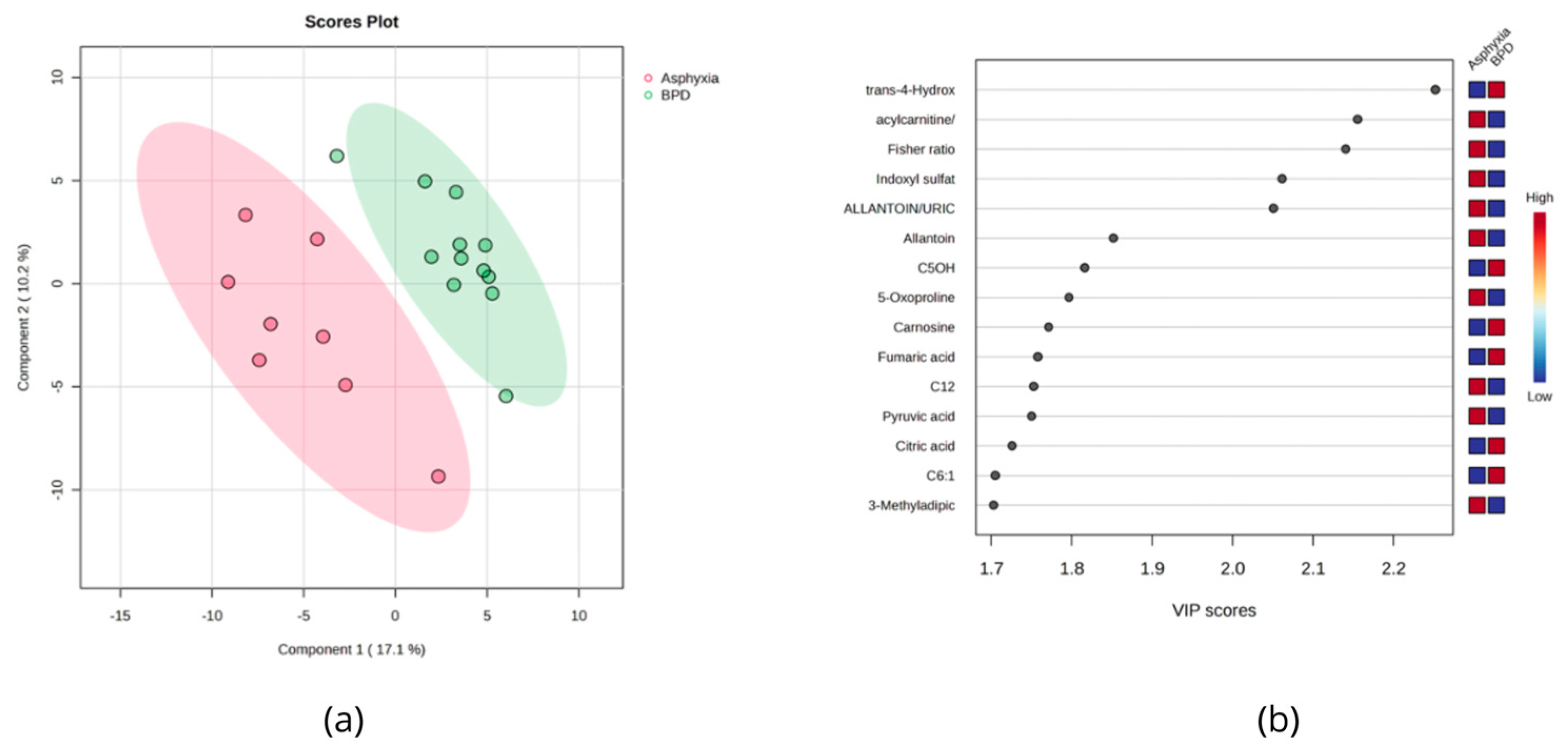
3.4. Urinary Metabolites Altered in BPD and Asphyxiated Newborns
3.5. NICU Newborns Exposed to SARS-CoV-2 during Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Newborn Mortality; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Li, Z.; Kapoor, M.; Kim, R.; Subramanian, S. V Association of Maternal History of Neonatal Death with Subsequent Neonatal Death across 56 Low- and Middle-Income Countries. Sci. Rep. 2021, 11, 19919. [Google Scholar] [CrossRef]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw. Open 2022, 5, e2215787. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary Dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78. [Google Scholar] [CrossRef]
- Joseph Pellissery, A.; Gopika Vinayamohan, P.; Susan Viju, L.; Joseph, D.; Venkitanarayanan, K. Application of Urine Metabolomics as a Marker in Health and Disease. In Advances and Challenges in Urine Laboratory Analysis [Working Title]; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; Blanco-Sandate, J.O.; Van Oostdam, A.S.H.; Zheng, J.; Guo, A.C.; Lima-Rogel, V.; Rajabzadeh, R.; Salgado-Bustamante, M.; Adrian-Lopez, J.; et al. The Urinary Metabolome of Healthy Newborns. Metabolites 2020, 10, 165. [Google Scholar] [CrossRef]
- Ariagno, R.L. National Institute of Child Health and Human Development (NICHD) and American Academy of Pediatrics (AAP) Workshop on Research in Neonatal and Perinatal Medicine. J. Perinatol. 2006, 26 (Suppl. 2), S3–S4. [Google Scholar] [CrossRef][Green Version]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A Systematic Review and Meta-Analysis to Revise the Fenton Growth Chart for Preterm Infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef]
- Lentner, C. Geigy Scientific Tables; Lentner, C., Ed.; Ciba-Geigy: Basle, Switzerland, 1981. [Google Scholar]
- BC Children’s Hospital Biochemical Genetics Lab. Available online: http://www.bcchildrens.ca (accessed on 30 May 2023).
- Piñeiro-Ramos, J.D.; Cascant, M.M.; Núñez-Ramiro, A.; López-Gonzálvez, Á.; Solaz-García, Á.; Albiach-Delgado, A.; Martínez-Rodilla, J.; Llorens-Salvador, R.; Sanjuan-Herraez, D.; Quintás, G.; et al. Noninvasive Monitoring of Evolving Urinary Metabolic Patterns in Neonatal Encephalopathy. Pediatr. Res. 2022, 91, 598–605. [Google Scholar] [CrossRef]
- Illsinger, S.; Schmidt, K.-H.; Lücke, T.; Vaske, B.; Bohnhorst, B.; Das, A.M. Plasma and Urine Amino Acid Pattern in Preterm Infants on Enteral Nutrition: Impact of Gestational Age. Amino Acids 2010, 38, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.M.; Krywawych, S.; Petros, A.J. Asymmetric Dimethyl Arginine and Symmetric Dimethyl Arginine Levels in Infants with Persistent Pulmonary Hypertension of the Newborn. Pediatr. Crit. Care Med. 2004, 5, 517–520. [Google Scholar] [CrossRef]
- Buck, A.; Kayacelebi, A.A.; Chobanyan-Jürgens, K.; Illsinger, S.; Bohnhorst, B.; Beckmann, B.; Hanff, E.; Das, A.M.; Tsikas, D.; Lücke, T. Comprehensive Analysis of the L-Arginine/l-Homoarginine/Nitric Oxide Pathway in Preterm Neonates: Potential Roles for Homoarginine and Asymmetric Dimethylarginine in Foetal Growth. Amino Acids 2017, 49, 783–794. [Google Scholar] [CrossRef]
- Kelley, R.I.; Robinson, D.; Puffenberger, E.G.; Strauss, K.A.; Morton, D.H. Amish Lethal Microcephaly: A New Metabolic Disorder with Severe Congenital Microcephaly and 2-Ketoglutaric Aciduria. Am. J. Med. Genet. 2002, 112, 318–326. [Google Scholar] [CrossRef]
- Forde, D.; Deming, D.D.; Tan, J.C.; Phillips, R.M.; Fry-Bowers, E.K.; Barger, M.K.; Bahjri, K.; Angeles, D.M.; Boskovic, D.S. Oxidative Stress Biomarker Decreased in Preterm Neonates Treated With Kangaroo Mother Care. Biol. Res. Nurs. 2020, 22, 188–196. [Google Scholar] [CrossRef]
- Allegri, G.; Fernandes, M.J.; Scalco, F.B.; Correia, P.; Simoni, R.E.; Llerena, J.C.; de Oliveira, M.L.C. Fumaric Aciduria: An Overview and the First Brazilian Case Report. J. Inherit. Metab. Dis. 2010, 33, 411–419. [Google Scholar] [CrossRef]
- Al Morsy, E.A.; Mokhtar, E.R.; Ibrahim, G.E.; El-Nasser, A.M.; Ebrahem, E.E.; Elattar, S. Urinary Metabolomic Profiles and Netrin-1 as Diagnostics and Predictors of Acute Kidney Injury in Preterm Neonates. Am. J. Med. Med. Sci. 2018, 8, 79–90. [Google Scholar] [CrossRef]
- Embade, N.; Cannet, C.; Diercks, T.; Gil-Redondo, R.; Bruzzone, C.; Ansó, S.; Echevarría, L.R.; Ayucar, M.M.M.; Collazos, L.; Lodoso, B.; et al. NMR-Based Newborn Urine Screening for Optimized Detection of Inherited Errors of Metabolism. Sci. Rep. 2019, 9, 13067. [Google Scholar] [CrossRef]
- Cucchi, M.L.; Frattini, P.; Santagostino, G.; Preda, S.; Orecchia, G. Catecholamines Increase in the Urine of Non-Segmental Vitiligo Especially During Its Active Phase. Pigment Cell Res. 2003, 16, 111–116. [Google Scholar] [CrossRef]
- Cole, D.E.C.; Farag, S.; Dooley, K.C. Ethanolaminuria: A Non-Specific Laboratory Finding in the Seriously III Infant. Clin. Biochem. 1988, 21, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Häberle, J.; Görg, B.; Rutsch, F.; Schmidt, E.; Toutain, A.; Benoist, J.-F.; Gelot, A.; Suc, A.-L.; Höhne, W.; Schliess, F.; et al. Congenital Glutamine Deficiency with Glutamine Synthetase Mutations. N. Engl. J. Med. 2005, 353, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Monostori, P.; Klinke, G.; Hauke, J.; Richter, S.; Bierau, J.; Garbade, S.F.; Hoffmann, G.F.; Langhans, C.-D.; Haas, D.; Okun, J.G. Extended Diagnosis of Purine and Pyrimidine Disorders from Urine: LC MS/MS Assay Development and Clinical Validation. PLoS ONE 2019, 14, e0212458. [Google Scholar] [CrossRef]
- Van Gennip, A.H.; Abeling, N.G.G.M.; Stroomer, A.E.M.; Overmars, H.; Bakker, H.D. The Detection of Molybdenum Cofactor Deficiency: Clinical Symptomatology and Urinary Metabolite Profile. J. Inherit. Metab. Dis. 1994, 17, 142–145. [Google Scholar] [CrossRef]
- Swarna, M.; Jyothy, A.; Rani, P.U.; Reddy, P.P. Amino Acid Disorders in Mental Retardation: A Two-Decade Study from Andhra Pradesh. Biochem. Genet. 2004, 42, 85–98. [Google Scholar] [CrossRef]
- Sewell, A.C.; Krille, M.; Wilhelm, I. Sarcosinaemia in a Retarded, Amaurotic Child. Eur. J. Pediatr. 1986, 144, 508–510. [Google Scholar] [CrossRef]
- Abeling, N.G.G.M.; van Gennip, A.H.; Barth, P.G.; van Cruchten, A.; Westra, M.; Wijburg, F.A. Aromatic L-Amino Acid Decarboxylase Deficiency: A New Case with a Mild Clinical Presentation and Unexpected Laboratory Findings. J. Inherit. Metab. Dis. 1998, 21, 240–242. [Google Scholar] [CrossRef]
- Baykal, T.; Karaaslan, I.; Gokcay, G.; Demir, F.; Laleli, Y.; Demirkol, M. Hyperhydroxyprolinaemia Detected in Newborn Screening with Tandem Mass Spectrometry. J. Inherit. Metab. Dis. 2004, 27, 781–782. [Google Scholar] [CrossRef]
- Guneral, F.; Bachmann, C. Age-Related Reference Values for Urinary Organic Acids in a Healthy Turkish Pediatric Population. Clin. Chem. 1994, 40, 862–866. [Google Scholar] [CrossRef]
- James, A.W.; Miranda, S.G.; Culver, K.; Hall, B.D.; Golabi, M. DOOR Syndrome: Clinical Report, Literature Review and Discussion of Natural History. Am. J. Med. Genet. A 2007, 143A, 2821–2831. [Google Scholar] [CrossRef]
- Chu, C.Y.; Xiao, X.; Zhou, X.G.; Lau, T.K.; Rogers, M.S.; Fok, T.F.; Law, L.K.; Pang, C.P.; Wang, C.C. Metabolomic and Bioinformatic Analyses in Asphyxiated Neonates. Clin. Biochem. 2006, 39, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Shinka, T.; Inoue, Y.; Mitsubuchi, H.; Ishimatsu, J.; Yoshino, M.; Kuhara, T. Asymptomatic A-ketoadipic Aciduria Detected during a Pilot Study of Neonatal Urine Screening. Acta Paediatr. 1999, 88, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Tserng, K.-Y.; Jin, S.-J.; Kerr, D.S.; Hoppel, C.L. Urinary 3-Hydroxydicarboxylic Acids in Pathophysiology of Metabolic Disorders with Dicarboxylic Aciduria. Metabolism 1991, 40, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, F.; Cassanello, M.; Enea, A.; Poma, F.; D’Onofrio, V.; Guala, G.; Garrone, G.; Puccinelli, P.; Caruso, U.; Porta, F.; et al. A Neonatal Case of 3-Hydroxy-3-Methylglutaric-Coenzyme A Lyase Deficiency. Ital. J. Pediatr. 2013, 39, 33. [Google Scholar] [CrossRef] [PubMed]
- Mönch, E.; Kneer, J.; Jakobs, C.; Arnold, M.; Diehl, H.; Batzler, U. Examination of Urine Metabolites in the Newborn Period and during Protein Loading Tests at 6 Months of Age—Part 1. Eur. J. Pediatr. 1990, 149, 17–24. [Google Scholar] [CrossRef]
- Hoehn, T.; Janssen, S.; Mani, A.R.; Brauers, G.; Moore, K.P.; Schadewaldt, P.; Mayatepek, E. Urinary Excretion of the Nitrotyrosine Metabolite 3-Nitro-4-Hydroxyphenylacetic Acid in Preterm and Term Infants. Neonatology 2008, 93, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Abdenur, J.E.; Abeling, N.; Specola, N.; Jorge, L.; Schenone, A.B.; van Cruchten, A.C.; Chamoles, N.A. Aromatic L-Aminoacid Decarboxylase Deficiency: Unusual Neonatal Presentation and Additional Findings in Organic Acid Analysis. Mol. Genet. Metab. 2006, 87, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Mayatepek, E. 5-Oxoprolinuria in Patients with and without Defects in the -Glutamyl Cycle. Eur. J. Pediatr. 1999, 158, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, S.; Salter, W.; Thorburn, D.R.; Durkie, M.; Panayi, M.; Wu, J.Y.; Easterbrook, A.; Coman, D.J. Fumarase Deficiency in Dichorionic Diamniotic Twins. Twin Res. Hum. Genet. 2013, 16, 1117–1120. [Google Scholar] [CrossRef]
- Shoemaker, J.D.; Elliott, W.H. Automated Screening of Urine Samples for Carbohydrates, Organic and Amino Acids after Treatment with Urease. J. Chromatogr. B Biomed. Sci. Appl. 1991, 562, 125–138. [Google Scholar] [CrossRef]
- Oglesbee, D.; He, M.; Majumder, N.; Vockley, J.; Ahmad, A.; Angle, B.; Burton, B.; Charrow, J.; Ensenauer, R.; Ficicioglu, C.H.; et al. Development of a Newborn Screening Follow-up Algorithm for the Diagnosis of Isobutyryl-CoA Dehydrogenase Deficiency. Genet. Med. 2007, 9, 108–116. [Google Scholar] [CrossRef]
- Bonnefont, J.-P.; Chretien, D.; Rustin, P.; Robinson, B.; Vassault, A.; Aupetit, J.; Charpentier, C.; Rabier, D.; Saudubray, J.-M.; Munnich, A. Alpha-Ketoglutarate Dehydrogenase Deficiency Presenting as Congenital Lactic Acidosis. J. Pediatr. 1992, 121, 255–258. [Google Scholar] [CrossRef]
- Amendt, B.A.; Greene, C.; Sweetman, L.; Cloherty, J.; Shih, V.; Moon, A.; Teel, L.; Rhead, W.J. Short-Chain Acyl-Coenzyme A Dehydrogenase Deficiency. Clinical and Biochemical Studies in Two Patients. J. Clin. Investig. 1987, 79, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Matalon, R.; Michaels, K.; Kaul, R.; Whitman, V.; Rodriguez-Novo, J.; Goodman, S.; Thorburn, D. Malonic Aciduria and Cardiomyopathy. J. Inherit. Metab. Dis. 1993, 16, 571–573. [Google Scholar] [CrossRef]
- Kopin, A.S.; McBride, E.W.; Chen, C.; Freidinger, R.M.; Chen, D.; Zhao, C.-M.; Beinborn, M. Identification of a Series of CCK-2 Receptor Nonpeptide Agonists: Sensitivity to Stereochemistry and a Receptor Point Mutation. Proc. Natl. Acad. Sci. USA 2003, 100, 5525–5530. [Google Scholar] [CrossRef]
- Ombrone, D.; Salvatore, F.; Ruoppolo, M. Quantitative Liquid Chromatography Coupled with Tandem Mass Spectrometry Analysis of Urinary Acylglycines: Application to the Diagnosis of Inborn Errors of Metabolism. Anal. Biochem. 2011, 417, 122–128. [Google Scholar] [CrossRef]
- Scolamiero, E.; Cozzolino, C.; Albano, L.; Ansalone, A.; Caterino, M.; Corbo, G.; di Girolamo, M.G.; Di Stefano, C.; Durante, A.; Franzese, G.; et al. Targeted Metabolomics in the Expanded Newborn Screening for Inborn Errors of Metabolism. Mol. Biosyst. 2015, 11, 1525–1535. [Google Scholar] [CrossRef]
- Aukett, A.; Bennett, M.J.; Hosking, G.P. Molybdenum Co-Factor Deficiency: An Easily Missed Inborn Error of Metabolism. Dev. Med. Child. Neurol. 1988, 30, 531–535. [Google Scholar] [CrossRef]
- Van Coster, R.N.; Gerlo, E.A.; Giardina, T.G.; Engelke, U.F.; Smet, J.E.; De Praeter, C.M.; Meersschaut, V.A.; De Meirleir, L.J.; Seneca, S.H.; Devreese, B.; et al. Aminoacylase I Deficiency: A Novel Inborn Error of Metabolism. Biochem. Biophys. Res. Commun. 2005, 338, 1322–1326. [Google Scholar] [CrossRef]
- Blau, N. Physician’s Guide to the Laboratory Diagnosis of Metabolic Diseases, 2nd ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Haybrard, J.; Simon, N.; Danel, C.; Pinçon, C.; Barthélémy, C.; Tessier, F.J.; Décaudin, B.; Boulanger, E.; Odou, P. Factors Generating Glucose Degradation Products In Sterile Glucose Solutions For Infusion: Statistical Relevance Determination Of Their Impacts. Sci. Rep. 2017, 7, 11932. [Google Scholar] [CrossRef] [PubMed]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the Paediatric Population: A Review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Zea-Rey, A.V.; Cruz-Camino, H.; Vazquez-Cantu, D.L.; Gutiérrez-García, V.M.; Santos-Guzmán, J.; Cantú-Reyna, C. The Incidence of Transient Neonatal Tyrosinemia Within a Mexican Population. J. Inborn Errors Metab. Screen. 2017, 5, 232640981774423. [Google Scholar] [CrossRef]
- Sternowsky, H.J.; Heigl, K. Tyrosine and Its Metabolites in Urine and Serum of Premature and Mature Newborns: Increased Values during Formula versus Breast Feeding. Eur. J. Pediatr. 1979, 132, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Millán, I.; Piñero-Ramos, J.; Lara, I.; Parra-Llorca, A.; Torres-Cuevas, I.; Vento, M. Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting. Antioxidants 2018, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.; Rafii, M.; Sgro, M.; Ball, R.O.; Pencharz, P. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates. Pediatr. Res. 2011, 69, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M. The Regulatory Mechanisms of Proline and Hydroxyproline Metabolism: Recent Advances in Perspective. Front. Oncol. 2023, 12, 1118675. [Google Scholar] [CrossRef]
- Buller, K.M.; Wixey, J.A.; Reinebrant, H.E. Disruption of the Serotonergic System after Neonatal Hypoxia-Ischemia in a Rodent Model. Neurol. Res. Int. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Lammertink, F.; Vinkers, C.H.; Tataranno, M.L.; Benders, M.J.N.L. Premature Birth and Developmental Programming: Mechanisms of Resilience and Vulnerability. Front. Psychiatry 2021, 11, 531571. [Google Scholar] [CrossRef]
- Vine, T.; Brown, G.M.; Frey, B.N. Melatonin Use during Pregnancy and Lactation: A Scoping Review of Human Studies. Braz. J. Psychiatry 2022, 44, 342–348. [Google Scholar] [CrossRef]
- Butler, J.; Kelly, S.D.; Muddiman, K.J.; Besinis, A.; Upton, M. Hospital Sink Traps as a Potential Source of the Emerging Multidrug-Resistant Pathogen Cupriavidus Pauculus: Characterization and Draft Genome Sequence of Strain MF1. J. Med. Microbiol. 2022, 71. [Google Scholar] [CrossRef]
- Koopman, F.; Wierckx, N.; de Winde, J.H.; Ruijssenaars, H.J. Identification and Characterization of the Furfural and 5-(Hydroxymethyl)Furfural Degradation Pathways of Cupriavidus Basilensis HMF14. Proc. Natl. Acad. Sci. USA 2010, 107, 4919–4924. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Macfarlane, G.T. Enumeration of Human Colonic Bacteria Producing Phenolic and Indolic Compounds: Effects of PH, Carbohydrate Availability and Retention Time on Dissimilatory Aromatic Amino Acid Metabolism. J. Appl. Bacteriol. 1996, 81, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Y.; Wang, G.; Zheng, X.; Hao, H. Gut Microbial Metabolites of Aromatic Amino Acids as Signals in Host–Microbe Interplay. Trends Endocrinol. Metab. 2020, 31, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, T.D.; Beaulieu, J.-M.; Espinoza, S.; Masri, B.; Zhang, X.; Salahpour, A.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. The Dopamine Metabolite 3-Methoxytyramine Is a Neuromodulator. PLoS ONE 2010, 5, e13452. [Google Scholar] [CrossRef]
- Saugstad, O.D. Bronchopulmonary Dysplasia—Oxidative Stress and Antioxidants. Semin. Neonatol. 2003, 8, 39–49. [Google Scholar] [CrossRef]
- Kumar, H.S.; Caffeine, V. Bronchopulmonary Dysplasia and Neurodevelopmental Outcomes in Premature Infants. Pediatr. Neonatal. Nurs. 2018, 5, 29–36. [Google Scholar] [CrossRef]
- Hakobyan, M.; Dijkman, K.P.; Laroche, S.; Naulaers, G.; Rijken, M.; Steiner, K.; van Straaten, H.L.M.; Swarte, R.M.C.; ter Horst, H.J.; Zecic, A.; et al. Outcome of Infants with Therapeutic Hypothermia after Perinatal Asphyxia and Early-Onset Sepsis. Neonatology 2019, 115, 127–133. [Google Scholar] [CrossRef]
- Denihan, N.M.; Boylan, G.B.; Murray, D.M. Metabolomic Profiling in Perinatal Asphyxia: A Promising New Field. Biomed Res. Int. 2015, 2015, 254076. [Google Scholar] [CrossRef]
- Locci, E.; Bazzano, G.; Demontis, R.; Chighine, A.; Fanos, V.; d’Aloja, E. Exploring Perinatal Asphyxia by Metabolomics. Metabolites 2020, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Noto, A.; Puddu, M.; Pomero, G.; Demontis, R.; Dalmazzo, C.; Delogu, A.; Fanos, V.; d’Aloja, E.; Gancia, P. A Longitudinal 1H-NMR Metabolomics Analysis of Urine from Newborns with Hypoxic-Ischemic Encephalopathy Undergoing Hypothermia Therapy. Clinical and Medical Legal Insights. PLoS ONE 2018, 13, e0194267. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, K.; Efstathiou, N.; Begou, O.; Soubasi, V.; Agakidou, E.; Gika, E.; Theodoridis, G.; Drossou, V. Urine Metabolomic Profile in Neonates with Hypoxic-Ischemic Encephalopa-Thy. Hippokratia 2017, 21, 80–84. [Google Scholar] [PubMed]
- Longini, M.; Giglio, S.; Perrone, S.; Vivi, A.; Tassini, M.; Fanos, V.; Sarafidis, K.; Buonocore, G. Proton Nuclear Magnetic Resonance Spectroscopy of Urine Samples in Preterm Asphyctic Newborn: A Metabolomic Approach. Clin. Chim. Acta 2015, 444, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.C.; Sirich, T.L. Indoxyl Sulfate-Review of Toxicity and Therapeutic Strategies. Toxins 2016, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Stockard, B.; Gauldin, C.; Truog, W.; Lewis, T. Pharmacometabolomics Profiling of Preterm Infants Validates Patterns of Metabolism Associated With Response to Dexamethasone Treatment for Bronchopulmonary Dysplasia. Front. Pediatr. 2022, 10, 898806. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Gouyon, J.; Cottenet, J.; Bechraoui-Quantin, S.; Rozenberg, P.; Mariet, A.; Quantin, C. Impact of SARS-CoV-2 Infection on Risk of Prematurity, Birthweight and Obstetric Complications: A Multivariate Analysis from a Nationwide, Population-based Retrospective Cohort Study. BJOG 2022, 129, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, O.; Rodriguez, P.P.; Hernandez, M.M.; Pardilla, M.B.E.; Perez, N.P.; Hernandez, M.R.V.; Yarza, A.V.; Velasco, O.N.; Fernandez, P.G.D.B.; Acebal, L.F.; et al. The Association between SARS-CoV-2 Infection and Preterm Delivery: A Prospective Study with a Multivariable Analysis. BMC Pregnancy Childbirth 2021, 21, 273. [Google Scholar] [CrossRef]
- Turkoglu, O.; Alhousseini, A.; Sajja, S.; Idler, J.; Stuart, S.; Ashrafi, N.; Yilmaz, A.; Wharton, K.; Graham, S.F.; Bahado-Singh, R.O. Fetal Effects of Mild Maternal COVID-19 Infection: Metabolomic Profiling of Cord Blood. Metabolomics 2023, 19, 41. [Google Scholar] [CrossRef]
- Kontou, A.; Virgiliou, C.; Mouskeftara, T.; Begou, O.; Meikopoulos, T.; Thomaidou, A.; Agakidou, E.; Gika, H.; Theodoridis, G.; Sarafidis, K. Plasma Lipidomic and Metabolomic Profiling after Birth in Neonates Born to SARS-CoV-19 Infected and Non-Infected Mothers at Delivery: Preliminary Results. Metabolites 2021, 11, 830. [Google Scholar] [CrossRef]

| Maternal Conditions | N (%) | Neonatal Conditions | N (%) |
|---|---|---|---|
| Preeclampsia | 5 (13) | Transient Tachypnea | 17 (44.7) |
| Gestational Diabetes | 6 (15.8) | Intrauterine Pneumonia | 17 (44.7) |
| Obesity | 1 (2.6) | Persistent Pulmonary Hypertension | 6 (15.8) |
| Drug Abuse | 3 (7.9) | Pneumothorax | 2 (5.3) |
| AIDS | 1 (2.6) | Respiratory Hypoxemic Failure | 1 (2.6) |
| Anemia | 2 (5.3) | Early Onset Sepsis | 8 (21.1) |
| Urinary Infection | 13 (34.2) | Necrotizing Enterocolitis 1 A | 2 (5.3) |
| Premature Labor | 26 (68.4) | Meconium Aspiration Syndrome | 3 (7.9) |
| Placenta Previa | 4 (10.5) | Neonatal Depression by Anesthetics | 3 (7.9) |
| Loss of Fetal Reactivity | 8 (21.1) | Hypoxic Ischemic Encephalopathy stage II with seizures | 3 (7.9) |
| Chorioamnionitis | 1 (2.6) | Hypoxic Ischemic Encephalopathy stage II without seizures | 6 (15.8) |
| Premature Rupture of Membranes | 4 (10.5) | Intrauterine Growth Restriction | 3 (7.9) |
| Multiple Pregnancies | 9 (23.7) | Liver Hematoma | 2 (5.3) |
| Abruptio Placentae | 1 (2.6) | Patent Ductus Arteriosus | 5 (13.2) |
| Achondroplasya | 1 (2.6) | Distress Respiratory Syndrome | 6 (15.8) |
| Cervicitis | 6 (15.8) | ||
| Obstetric Trauma | 1 (2.6) | ||
| Asthma | 1 (2.6) |
| Variable | Healthy (N = 13) | NICU (N = 38) | p-Value * |
|---|---|---|---|
| Male Gender, n (%) | 7 (63.6) | 29 (76.3) | 0.4016 |
| Vaginal Birth, n (%) | 3 (27.3) | 20 (52.6) | 0.1805 |
| Gestational Age (wks) | 38.1 (1.2) | 35.6 (3) | 0.007 |
| APGAR (1 min) | 8 (8–8) | 7 (5.5–8) | 0.0005 |
| APGAR (5 min) | 9 (9–9) | 9 (7–9) | 0.0188 |
| Weight (g) | 2750 (2628–3273) | 1935 (1650–2773) | 0.002 |
| Length (cm) | 50 (49–50) | 44 (42–48.3) | <0.0001 |
| Cephalic Perimeter (cm) | 35 (34–35) | 31 (29.4–34) | <0.0001 |
| Temperature (°C) | 36.7 (36.6–36.8) | 36.4 (36–36.9) | 0.1865 |
| Cardiac Rate (bpm) | 140 (135.8–144.8) | 152 (145–160) | 0.003 |
| Respiratory Rate (bpm) | 45.3 (3.7) | 57 (9.9) | 0.0002 |
| SaO2 (%) | 98 (97–98.8) | 96.5 (94–99) | 0.1651 |
| FiO2 (%) | 21 (21–21) | 35 (30–100) | <0.0001 |
| Hemoglobin (g/dL) | NA | 16.9 +/− 2.1 | NA |
| Ht (%) | NA | 50.4 +/− 7 | NA |
| Leukocytes (×109/L) | NA | 10,550 (7450–14,050) | NA |
| Lymphocytes (×109/L) | NA | 3145 (2158–5148) | NA |
| Monocytes (×109/L) | NA | 899 +/− 482.6 | NA |
| Platelets | NA | 212,586 +/− 56,030 | NA |
| Neutrophils (×109/L) | NA | 6030 +/− 3008 | NA |
| Glucose (mg/dL) | NA | 73.7 (59.2–100.1) | NA |
| Creatinine (mg/dL) | NA | 0.7 (0.6–0.8) | NA |
| Urea (mg/dL) | NA | 16.4 (11.6–21.6) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Hernández, Y.; Lima-Rogel, V.; Mandal, R.; Zheng, J.; Zhang, L.; Oler, E.; García-López, D.A.; Torres-Calzada, C.; Mejía-Elizondo, A.R.; Poelzer, J.; et al. The Urinary Metabolome of Newborns with Perinatal Complications. Metabolites 2024, 14, 41. https://doi.org/10.3390/metabo14010041
López-Hernández Y, Lima-Rogel V, Mandal R, Zheng J, Zhang L, Oler E, García-López DA, Torres-Calzada C, Mejía-Elizondo AR, Poelzer J, et al. The Urinary Metabolome of Newborns with Perinatal Complications. Metabolites. 2024; 14(1):41. https://doi.org/10.3390/metabo14010041
Chicago/Turabian StyleLópez-Hernández, Yamilé, Victoria Lima-Rogel, Rupasri Mandal, Jiamin Zheng, Lun Zhang, Eponine Oler, David Alejandro García-López, Claudia Torres-Calzada, Ana Ruth Mejía-Elizondo, Jenna Poelzer, and et al. 2024. "The Urinary Metabolome of Newborns with Perinatal Complications" Metabolites 14, no. 1: 41. https://doi.org/10.3390/metabo14010041
APA StyleLópez-Hernández, Y., Lima-Rogel, V., Mandal, R., Zheng, J., Zhang, L., Oler, E., García-López, D. A., Torres-Calzada, C., Mejía-Elizondo, A. R., Poelzer, J., López, J. A., Zubkowski, A., & Wishart, D. S. (2024). The Urinary Metabolome of Newborns with Perinatal Complications. Metabolites, 14(1), 41. https://doi.org/10.3390/metabo14010041









