Abstract
Atherosclerotic cardiovascular disease poses a significant global health issue, with dyslipidemia standing out as a major risk factor. In recent decades, lipid-lowering therapies have evolved significantly, with statins emerging as the cornerstone treatment. These interventions play a crucial role in both primary and secondary prevention by effectively reducing cardiovascular risk through lipid profile enhancements. Beyond their primary lipid-lowering effects, extensive research indicates that these therapies exhibit pleiotropic actions, offering additional health benefits. These include anti-inflammatory properties, improvements in vascular health and glucose metabolism, and potential implications in cancer management. While statins and ezetimibe have been extensively studied, newer lipid-lowering agents also demonstrate similar pleiotropic effects, even in the absence of direct cardiovascular benefits. This narrative review explores the diverse pleiotropic properties of lipid-modifying therapies, emphasizing their non-lipid effects that contribute to reducing cardiovascular burden and exploring emerging benefits for non-cardiovascular conditions. Mechanistic insights into these actions are discussed alongside their potential therapeutic implications
1. Introduction
Dyslipidemia represents a significant global health challenge and is widely acknowledged as a pivotal risk factor for atherosclerotic cardiovascular disease (ASCVD), contributing to approximately 3.78 million deaths from ischemic heart disease (IHD) [1]. Over the years, a plethora of lipid-modifying therapies have been developed to address this critical issue, demonstrating efficacy in reducing not only lipid levels but also in exerting pleiotropic effects that extend beyond cardiovascular health.
Foremost among these therapies are statins, which inhibit 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) to lower low-density lipoprotein cholesterol (LDL-C) levels. Their distinctive mechanism of action offers various other pleiotropic benefits, as evidenced in the literature. Statins are categorized by intensity, with rosuvastatin and atorvastatin recognized as the most potent [2] and by lipophilicity, influencing their cellular effects. Current evidence indicates that lipophilic statins exhibit more pleiotropic effects compared with hydrophilic statins because of their enhanced ability to penetrate cell membranes [3]. Ezetimibe complements statin treatment by reducing intestinal cholesterol absorption [4]. Recent guidelines advocate for the immediate initiation of ezetimibe as an adjunct to high-intensity statin therapy in individuals with ASCVD and LDL-C levels exceeding 110 mg/dL [5]. Both statins and ezetimibe have shown promise in mitigating inflammation and improving endothelial function impairment. However, statins are associated with diabetogenic effects with conflicting evidence regarding their role in malignancy, whereas the impact of ezetimibe on glucose metabolism and cancer risk remains a subject of debate [6,7].
In addition to the well-established benefits of traditional lipid-lowering therapies in reducing cardiovascular disease (CVD) risk, recent years have witnessed the emergence of novel agents. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, such as monoclonal antibodies evolocumab and alirocumab, along with the synthesis inhibitor inclisiran, have significantly advanced hypercholesterolemia treatment. PCSK9 inhibition increases LDL receptor (LDL-R) density in liver cells, enhancing LDL-C particle clearance and markedly lowering LDL-C levels [8]. Another promising agent, bempedoic acid (BA), has emerged as a prominent therapeutic for managing dyslipidemia, particularly in cases of statin intolerance. BA inhibits cholesterol biosynthesis by targeting adenosine triphosphate-citrate lyase (ACLY), an enzyme upstream of HMGCR. As a pro-drug, BA is primarily converted into its active metabolite by very-long-chain acyl-CoA synthetase 1, predominantly found in the liver, thereby mitigating the risk of myopathy [9]. Evidence for both PCSK9 inhibitors and BA suggests encouraging effects on inflammation and malignancy. However, PCSK9 inhibitors may lead to mild hyperglycemia, although evidence is conflicting [10,11]. On the contrary, BA shows promise in enhancing insulin sensitivity [12].
In addition to traditional and novel agents for dyslipidemia treatment, recent years have seen the introduction of alternative therapeutic approaches, particularly for refractory cases of familial hypercholesterolemia (FH). Two notable agents in this field are mipomersen and lomitapide. Mipomersen, a second-generation antisense oligonucleotide, selectively degrades apolipoprotein B100 (ApoB100) mRNA [13]. Lomitapide, an oral inhibitor of microsomal triglyceride transfer protein (MTP), primarily affects very low-density lipoprotein cholesterol (VLDL) production containing ApoB100. Despite their initial promising lipid-lowering efficacy, their use is now limited because of concerns regarding hepatotoxicity [13,14]. Therefore, mipomersen and lomitapide have not been extensively investigated for their pleiotropic effects, although recent experimental studies note the potential beneficial effects of lomitapide in both inflammation and malignancy [15].
In 2018, the Food and Drug Administration (FDA) approved a novel class of cholesterol-lowering medications known as angiopoietin-like 3 protein (ANGPTL3) inhibitors, namely evinacumab and vupanorsen. These innovative compounds target ANGPTL3, enhancing lipoprotein lipase (LPL) and endothelial lipase (EL) activities. This mechanism results in significant LDL-C reductions independent of the LDL-R activity [16]. Beyond their impact on lipid-lowering, several studies have demonstrated their potential benefits in inflammation and cancer [17]. Additionally, individuals with familial hypercholesterolemia may benefit from lipoprotein apheresis (LA), a therapeutic modality designed to selectively eliminate ApoB-containing lipoproteins from circulation [18]. Despite its questionable role in mitigating CVD risk, LA has demonstrated impressive pleiotropic benefits, including anti-inflammatory effects and improvements in vascular health [19].
In the last few years, novel pharmaceutical agents have emerged that enhance the levels of high-density lipoprotein cholesterol (HDL-C). These agents work either by mimicking the properties of natural HDL-C particles (recombinant HDL-C particles) or by inhibiting cholesteryl ester transfer protein (CETP). CETP, a glycoprotein primarily produced by the liver, facilitates the transfer of cholesteryl esters from HDL-C to VLDL, chylomicrons, and LDL-C while simultaneously transferring triglycerides (TGs) from these particles to HDL-C. Although these drugs significantly increase HDL-C concentrations, they have not delivered the anticipated cardiovascular benefits. Nevertheless, their ability to enhance HDL-C and its associated apolipoproteins has prompted further investigations into their broader impacts beyond traditional lipid management. Emerging evidence from preclinical and clinical studies supports the beneficial effects of CETP inhibitors and recombinant HDL-C (rHDL) particles in treating inflammatory diseases, improving glucose metabolism, and potentially combating certain cancers [20,21]. Similarly, niacin, a potent HDL-C-raising drug with a favorable impact on lowering LDL-C and TGs, has shown disappointing results in reducing CVD risk but exhibits diverse pleiotropic effects [22].
Another area of interest lies in elucidating the pleiotropic properties of agents that ameliorate hypertriglyceridemia, particularly peroxisome proliferator-activated receptor alpha (PPARa) agonists such as fibrates. Clinical studies have shown that fibrates, such as fenofibrate, offer benefits by improving endothelial function, mitigating inflammation, and enhancing insulin sensitivity. Moreover, they hold promise in cancer therapy through their influence on various pathways involved in tumor suppression and apoptosis [23]. Beyond fibrates, the management of hypertriglyceridemia includes the prescription of omega-3 fatty acids (FAs), typically as supplementary treatment, and the use of apolipoprotein CIII (ApoCIII) inhibitors, particularly Volanesorsen, especially in severe cases. Both treatment approaches have shown promising pleiotropic effects, with a reduction in inflammation being central to these benefits [24,25].
This review aims to delve into the diverse pleiotropic effects of lipid-modifying agents beyond their primary role in lowering lipid levels, emphasizing their potential implications in various health issues, with a focus on inflammation, vascular function, glucose metabolism, and malignancy.
2. Literature Search Methodology
For this narrative review, we systematically searched the PubMed database using a comprehensive array of keywords related to lipid-lowering therapies and their mechanisms. Specifically, our search terms encompassed “statins”, “ezetimibe”, “PCSK9 inhibitors”, “bempedoic acid”, “mipomersen”, “lomitapide”, “ANGPTL3 inhibitors”, “lipoprotein apheresis”, “CETP inhibitors”, “recombinant HDL-C”, “niacin”, “fibrates”, “omega-3 fatty acids”, “ApoCIII inhibitors”, and “pleiotropic properties”. Our primary objective was to compile pertinent research articles, randomized clinical trials, and meta-analyses. Moreover, we meticulously examined the references cited within these articles to identify the relevant supplementary literature. It is acknowledged, however, that due to the substantial volume of retrieved manuscripts, this review may not encompass the entirety of the available literature on the subject.
3. Traditional and Novel Lipid-Modifying Treatments That Primary Reduce LDL-C
3.1. Statins
Statins are pivotal in the management of hyperlipidemia, leveraging their unique mechanism of inhibiting HMGCR. This action primarily targets a reduction in ApoB-containing lipoproteins, notably LDL-C. Statins achieve this by either curbing ApoB synthesis in the liver or boosting the clearance of LDL through increased expression of LDL-R on liver cells [26]. Rosuvastatin, the most potent statin at a maximal dose of 40 mg, can reduce LDL-C by up to 55%, lower TGs by 25%, and increase HDL-C by up to 8% [27]. Currently, statins remain the cornerstone of lipid-lowering treatment, offering numerous benefits beyond cardiovascular health as they demonstrate a wide range of pleiotropic effects [26].
3.1.1. Inflammation
In addition to their lipid-lowering effects, the inhibition of HMGCR by statins also augments their anti-inflammatory properties. HMGCR activation has been reported to initiate inflammatory responses through various pathways. Typically, HMGCR upregulates Toll-like receptors (TLRs), thereby elevating levels of pro-inflammatory mediators and leukocyte adhesion molecules [28]. This upregulation further boosts major histocompatibility complex II (MHC)-II expression, prompting T-cell activation and facilitating the secretion of pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [29]. Consequently, inhibiting HMGCR via statins diminishes TLR4 expression on immune cells, a pivotal regulator of the NLR family pyrin domain containing 3 (NLRP3) inflammasome. Recent research has spotlighted the NLRP3 inflammasome and its downstream mediators as potential targets for statins [30].
The antioxidant benefits of statins, propelled by HMGCR, have also been extensively documented and are linked to their capacity to enhance crucial antioxidant enzymes via the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway, consequently curtailing the generation of reactive oxygen species (ROS) [31]. Meta-analyses have underscored that statin therapy bolsters antioxidant properties by markedly elevated circulating levels of glutathione peroxidase (GPx) and superoxide dismutase (SOD) [32].
Moreover, statins are recognized for their prowess in scavenging oxygen free radicals, a function associated with the stabilization of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) inhibitor protein, IκBα [33]. Evidence also indicates that statins curb the production of inflammatory cytokines by downregulating the transcription factor NF-κB within the mevalonate pathway [34]. Last, recent research has highlighted the potential role of statins in inducing ferroptosis through the mevalonate pathway, which is critical for synthesizing GPx4 and forming coenzyme Q10 (CoQ10) [35]. Moreover, they inhibit pyroptosis, a form of programmed cell death associated with inflammation, by blocking the long noncoding RNA nexilin F-actin binding protein antisense RNA 1/nexilin F-actin binding protein (lncRNA NEXNAS1/NEXN) pathway, reducing the release of pro-inflammatory cytokines such as IL-1 and IL-18 [36].
Numerous studies have also confirmed the effectiveness of statins in reducing C-reactive protein (CRP) and high-sensitivity CRP (hs-CRP) levels. CRP is an inflammatory marker indicative of CVD risk, with statins reportedly lowering CRP levels by up to 60% [37]. A recent meta-analysis corroborated the beneficial effects of statins on reducing CRP and hs-CRP levels, independent of the statin’s intensity or the type of CVD. This analysis found that treatment durations exceeding 10 weeks led to decreased hs-CRP levels, although only high-intensity statin regimens marginally reduced CRP serum concentrations in individuals with CVD [38]. Additionally, another meta-analysis provided guidance for selecting statin therapy based on LDL-C and CRP levels, concluding that simvastatin at 40 mg/day was the most effective, while atorvastatin at 80 mg/day offered the best long-term outcomes [39].
Clinically, the anti-inflammatory effects of statins facilitated through HMGCR inhibition have proven advantageous in treating various conditions, including Alzheimer’s disease. HMGCR assumes a pivotal role in the development of neuroinflammation [40] while also provoking microglial proliferation, which can lead to synaptic damage and neuronal apoptosis, primarily via activation of the NF-κB signaling pathway [41]. Additionally, HMGCR facilitates the deposition of amyloid-beta (Aβ), thereby contributing to cognitive deficits [42]. As for Parkinson’s disease (PD), the literature presents conflicting evidence. While some studies suggest that statins exert a positive impact by regulating inflammatory and lysosomal signaling pathways, others argue that they may elevate PD risk by reducing levels of the antioxidant CoQ10 [43].
Statins have demonstrated anti-inflammatory benefits in individuals with chronic kidney disease (CKD) as well. A recent meta-analysis revealed significant anti-inflammatory properties associated with statin use, leading to improvements in complications, slowing CKD progression, and reducing mortality rates. Notably, subgroup analyses indicated a substantial reduction in CRP levels among both predialysis CKD and dialysis patients receiving short- or long-term statin therapy [44]. Moreover, there is emerging evidence suggesting potential benefits of statin treatment for individuals with inflammatory bowel disease (IBD), attributed to statins’ immunomodulatory properties, which include inhibiting T-cell activation, antigen-presenting function, and leukocyte tissue infiltration [45]. A population-based case-control study conducted in Sweden found that statin prescription might be associated with a decreased risk of Crohn’s disease, although not ulcerative colitis [46].
3.1.2. Vascular Health
Statins have the potential to improve endothelial function by inhibiting the NLRP3 inflammasome. Specifically, they may reduce the activation of the NLRP3 inflammasome triggered by oxidized LDL (OxLDL) or TNFα in vascular endothelial cells via a mechanism dependent on the pregnane X receptor (PXR) [47]. PXR is a nuclear receptor that regulates the expression of genes involved in drug metabolism and transport, thereby aiding in the detoxification and elimination of xenobiotics and endotoxins from the body [48]. Furthermore, statins are widely recognized for their beneficial impact on vascular health, partly because of their ability to modulate pro-oxidant enzymes and enhance endothelial nitric oxide synthase (eNOS) functionality. This modulation includes mitigating the activity of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase, a primary source of ROS in the vasculature, and promoting eNOS expression, activity, and enzymatic coupling [49,50]. Current evidence supports that statins can reduce NADPH oxidase activity in both endothelial cells and vascular smooth muscle cells, thereby decreasing ROS production [51]. Additionally, statins enhance eNOS activity by increasing its phosphorylation at specific activation sites, which in turn boosts nitric oxide (NO) availability and improves endothelium-dependent vasorelaxation [52]. Statins have also been shown to elevate heme oxygenase-1 (HO-1) activity through p38 and PI3K/Akt-dependent mechanisms. HO-1 is a critical factor with antioxidative, anti-inflammatory, anti-proliferative, and anti-apoptotic properties in the vasculature [53,54].
The antithrombotic properties of statins are largely due to their ability to inhibit platelet activation and exert anticoagulant effects [55]. Statins achieve this by upregulating eNOS and downregulating cyclooxygenase-1 activation, thereby inhibiting platelet activation [56]. They also reduce tissue factor (TF) activity by downregulating geranylgeranylated proteins, which play a significant role in reducing TF [57]. Furthermore, statins enhance thrombomodulin mRNA levels in a dose-dependent manner by inhibiting the geranylgeranylation of Rho subfamily proteins, such as NF-κB, ultimately activating the protein C pathway and exhibiting anticoagulant activity [58]. A meta-analysis by Kunutsor et al. found that statin use is associated with a significant reduction in deep vein thrombosis risk, with rosuvastatin showing the lowest risk among statins [59].
Recent research has also highlighted the epigenetic modulatory effects of statins, particularly in preventing endothelial-to-mesenchymal transition, which is a process linked to endothelial dysfunction. Simvastatin has been shown to significantly improve the function of human-induced pluripotent stem cell-derived endothelial cells under both normal and diabetic conditions [60].
3.1.3. Glucose Metabolism
Despite their groundbreaking role in mitigating CVD risk, statins have been associated with insulin resistance and the development of new-onset diabetes mellitus (NODM). It is known that statin use can elevate fasting glucose and insulin levels and increase insulin resistance, particularly in a dose-dependent manner [61]. Notably, these adverse effects are more commonly observed with high-intensity statin therapy [6]. The molecular mechanisms underlying statin-induced NODM are not fully understood, although several have been proposed. One mechanism involves the inhibition of the HMGCR receptor and the disruption of intracellular calcium (Ca2+) concentration, primarily regulated by calcium channels, which can significantly impair glucose homeostasis [62].
Statins have also been shown to induce insulin resistance by reducing the translocation of glucose transporter type 4 (GLUT4) to the cell membrane, thereby decreasing peripheral insulin-mediated glucose uptake [63]. Additionally, statins may lower adiponectin levels in adipose tissue, diminishing its beneficial effects on reducing hepatic gluconeogenesis and its protective and regenerative effects on pancreatic beta cells [64]. Recently, Henriksbo et al. have identified p38 and mammalian target of rapamycin (mTOR) as mediators of statin-induced insulin resistance in adipose tissue, demonstrating the ability of statins to activate the NLRP3 inflammasome [65]. Finally, statins might influence genetic and epigenetic mechanisms, including changes in microRNAs, which are known to have diabetogenic effects due to their impact on gene expression in dysfunctional beta cells and insulin-resistant tissues [62].
3.1.4. Malignancy
The anti-cancer benefits of statins have been acknowledged since the 1990s, though they remain a topic of debate. Findings from epidemiological and clinical studies on the relationship between statins and cancer are mixed. A large 15-year observational study in Denmark found reduced mortality for 13 types of cancer among statin users compared with non-users, although it did not find a dose-dependent relationship [66]. Certain studies have shown the benefits of statin use in specific populations, such as postmenopausal women and men with prostate cancer [67]. The duration of statin use also appears to have varying effects on different cancers. For instance, a Japanese study of 67,768 participants found that using statins for more than five years was associated with a reduced risk of liver cancer but an increased risk of pancreatic cancer [68]. Conversely, an 8.8-year Finnish study involving nearly 900,000 people found no link between statin use and cancer [69].
Meta-analyses also offer conflicting results regarding the anti-cancer effects of statins. One meta-analysis involving over a million cancer patients suggested that statin use could reduce cancer-specific mortality by 40% [70]. In contrast, another meta-analysis of 27 clinical trials with 175,000 patients found no evidence that a five-year statin prescription reduced cancer prevalence or mortality [71]. Furthermore, long-term studies exceeding five years did not demonstrate a significant association between statin use and the incidence of overall or specific cancers [72].
The mechanisms through which statins may exert anti-cancer effects have been demonstrated in both in vitro and in vivo studies. In vitro evidence indicates that statins inhibit HMGCG, which is essential for regulating metalloproteinase synthesis through its influence on geranylgeranyl pyrophosphate (GGPP) production [73]. Statins such as simvastatin and fluvastatin reduce matrix metalloproteinase (MMP) 9 release in murine and human macrophages [74]. In vivo evidence indicates that simvastatin may reduce bone metastasis and tumor growth in a mouse model of human lung cancer xenograft by decreasing mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) activity [75]. Simvastatin also promotes eNOS phosphorylation, prevents apoptosis, and enhances angiogenesis via an Akt-dependent mechanism [76]. Pitavastatin can inhibit subcutaneous glioma cell growth [77], while the combination of fluvastatin with gemcitabine can delay and weaken tumor growth in a pancreatic cancer xenograft [78]. Notably, lipophilic statins appear to be more effective in treating cancer than hydrophilic statins because of their higher proapoptotic and cytotoxic potential [79].
3.2. Ezetimibe
Ezetimibe, an azetidine derivative, selectively inhibits Niemann-Pick C1-like protein 1 (NPC1L1), which blocks intestinal cholesterol absorption. This action reduces the incorporation of cholesterol into chylomicrons and subsequently decreases the intrahepatic cholesterol pool. As a result, LDL-R expression is upregulated, enhancing the clearance of ApoB100-containing lipoproteins from the plasma [80]. In clinical settings, ezetimibe is often used as an adjunct to statins for patients who do not reach their LDL-C targets with optimal statin therapy alone. Notably, the combination of ezetimibe with a high-intensity statin can lead to a significant reduction in LDL-C levels, potentially achieving a decrease of around 70% [81].
3.2.1. Inflammation
Beyond its notable efficacy in reducing LDL-C levels, ezetimibe’s anti-inflammatory properties have garnered recognition for several years, prompting numerous studies to investigate the potential underlying mechanisms of action. The influence of ezetimibe on CRP levels has sparked ongoing discourse within the scientific community, particularly in its role as an adjunct therapy for individuals undergoing statin treatment. A comprehensive analysis conducted by Pearson et al. revealed that ezetimibe administration led to a notable 6% decrease in CRP levels compared with a placebo. Furthermore, the addition of ezetimibe to statin therapy exhibited a substantial supplementary reduction in CRP, with a treatment disparity of 10% [82]. In a meta-analysis conducted by the same researchers, the impact of simvastatin monotherapy was juxtaposed with combined ezetimibe/simvastatin treatment regarding CRP levels. The results indicated that ezetimibe/simvastatin combination therapy induced significantly greater reductions in CRP when compared with simvastatin alone, showcasing reductions of 31.0% versus 14.3%, respectively, even at varying simvastatin doses [83].
However, conflicting results emerged from a study conducted by Oh et al., which demonstrated that in hypercholesterolemic subjects, co-administration of ezetimibe and statins failed to yield significant reductions in CRP levels, despite a marked decrease in LDL-C concentrations [84]. These observations were echoed by a meta-analysis encompassing 23 controlled trials, which found no significant advantage in incorporating ezetimibe alongside maximal statin therapy concerning IL-6 levels, the primary driver of CRP synthesis. Nevertheless, this same meta-analysis highlighted that ezetimibe/statin co-administration enhanced TNF-α-lowering effects compared with statin monotherapy [85]. These contrasting findings suggest that the anti-inflammatory effects of ezetimibe may be mediated through mechanisms independent of LDL-C reduction. This hypothesis gains further credence from a recent meta-analysis encompassing 171,668 subjects from 53 randomized controlled trials (RCTs). This study scrutinized the impact of various lipid-lowering agents on both LDL-C and CRP levels and revealed that ezetimibe elicited a significant 28% reduction in CRP levels, irrespective of LDL-C modifications [86].
Exploring alternative potential mechanisms, independent of LDL-C, through which ezetimibe exerts its anti-inflammatory role suggests that the drug might achieve this effect by engaging various intracellular signaling pathways. In particular, diminished NF-κB activation has been reported upon promoting IκB degradation through the MAPK pathway [87]. This modulation of intracellular signaling pathways by ezetimibe suggests emerging favorable outcomes regarding certain pathological conditions. In ischemic stroke, ezetimibe demonstrates potential benefits by reducing inflammation and oxidative stress via the adenosine monophosphate-activated protein kinase(AMPK)/Nrf2/TXNIP pathway, as observed in an experimental model of middle cerebral artery occlusion [88].
Additionally, preliminary evidence indicates that ezetimibe may offer advantages in treating steatohepatitis by inducing autophagy, an effect mediated by AMPK activation and inhibition of the NLRP3 inflammasome [89]. Furthermore, ezetimibe has been reported to exhibit anti-inflammatory properties in individuals with chronic inflammatory conditions, such as ankylosing spondylitis (AS). In vitro data indicate that ezetimibe could serve as an effective therapy for AS patients by mitigating the expression of Th17 differentiation-related genes, including IL-23R and IL-1R [90].
3.2.2. Vascular Health
The impact of ezetimibe on endothelial function has been debated, with conflicting evidence from both experimental and clinical studies. Several clinical trials have not shown significant benefits of ezetimibe, even as an add-on to statin therapy, and its lack of pleiotropic efficacy may be understood in the context of its pharmacokinetic profile and mechanism of action. Ezetimibe is rapidly absorbed, with about 80% of the administered dose eliminated in the feces, resulting in decreased systemic exposure [91,92,93].
Experimental studies suggest that ezetimibe administration can enhance endothelial function in atherosclerotic mouse models. Ezetimibe may improve acetylcholine-mediated vasodilatory responses and boost eNOS expression, thereby positively impacting cytokine expression and oxidative stress [94]. Furthermore, ezetimibe may exhibit antioxidant and anti-thrombotic properties by reducing platelet aggregation, urokinase-type plasminogen activator expression, and LDL-C peroxidation in endothelial cells [95,96].
Similar beneficial effects have been reported in the clinical setting. In cases where ezetimibe improves endothelial function, the effect is primarily attributed to its significant cholesterol-lowering ability rather than the direct cellular effects seen with statin therapy [97]. The lipid-lowering properties of ezetimibe have been associated with improvements in endothelial function. Ezetimibe monotherapy ameliorates postprandial hyperlipidemia, which is closely linked to transient endothelial dysfunction, as evidenced by increased brachial artery flow-mediated dilation (FMD) [98]. Additionally, in obese subjects with metabolic syndrome and coronary artery disease (CAD), ezetimibe improves postprandial hyperinsulinemia, thereby positively impacting endothelial function [99].
When ezetimibe is co-administered with statins, it demonstrates notable benefits for endothelial function. The CuVIC trial highlighted that the adjunctive administration of ezetimibe to statin therapy improved endothelial function in stented coronary arteries more effectively than statin monotherapy, attributed to greater reductions in OxLDLs and oxysterol levels [100]. Notably, in patients with hypercholesterolemia, heart failure, and CAD, ezetimibe-containing regimens seem to be less effective than statin monotherapy for improving endothelial function when both treatments achieve equivalent cholesterol reductions [97].
In conclusion, current evidence suggests that ezetimibe monotherapy is less effective than statin monotherapy in improving endothelial function [101]. However, a low-dose statin combined with ezetimibe may offer comparable or superior benefits to high-dose statin therapy alone [102,103].
3.2.3. Glucose Metabolism
The effects of ezetimibe on glucose metabolism are conflicting. Some studies report that ezetimibe improves insulin resistance and reduces visceral fat, while others suggest that it may cause hyperglycemia. Various mechanisms might explain the beneficial effects of ezetimibe, particularly in individuals with diabetes mellitus type 2 (T2DM). One notable mechanism is the increased expression of NPC1L1 mRNA in the duodenum of T2DM patients since NPC1L1 is the primary target of ezetimibe [104]. Experimental evidence in high-fat diet-fed diabetic mouse models shows that ezetimibe may enhance active glucagon-like peptide-1 levels in the intestine, reduce adipocyte size in visceral fat, lower serum levels of free fatty acids, induce fatty acid oxidation, improve adipocytic inflammation, and partially improve glycemic index values [105].
Research has also explored the impact of combining ezetimibe with statins on insulin sensitivity in T2DM patients [106]. It is suggested that replacing high-dose statin treatment with ezetimibe, either alone or combined with low-dose statins, may reduce the risk of hyperglycemia. A meta-analysis of 16 RCTs found that add-on ezetimibe therapy in patients on low-dose statins for over three months significantly reduced fasting glucose levels compared with high-dose statin therapy [107]. However, recent evidence indicates that while the rosuvastatin/ezetimibe combination therapy is more effective at reducing LDL-C levels than rosuvastatin alone, it does not significantly enhance insulin sensitivity or reduce vascular inflammatory responses. Markers of insulin resistance and oxidative stress (HOMA-IR and PRDX4) did not differ significantly between the two groups after 12 weeks of treatment [108].
3.2.4. Malignancy
The combination of ezetimibe and statins has been associated with a heightened malignancy risk [7]. Nevertheless, recent years have seen a surge in preclinical evidence suggesting that ezetimibe could confer advantages in the onset and advancement of diverse cancer types, including breast cancer [109], gastrointestinal tumors [108], and renal cell carcinoma [110]. These positive anti-cancer outcomes of ezetimibe are believed to stem from its anti-inflammatory characteristics, as well as its beneficial effects on stem cell suppression, cellular proliferation, immune modulation, and angiogenesis [108].
In an experimental mouse model of hypercholesterolemic urinary bladder cancer, ezetimibe decreased the proportion of cancer cells expressing CK5, CK14, and p-STAT3, as well as cancer stemness markers such as ALDH1A1 and CD44 [111]. Furthermore, recent data support that ezetimibe promotes antitumor immunity and mitigates prostate tumor growth and metastasis by inhibiting protein kinase B (Akt) phosphorylation and mammalian target of rapamycin complex 2 (mTORC2) signaling in lymphocytes [110]. Notably, in models of steatohepatitis-related hepatocellular carcinoma, ezetimibe exhibits anti-cancer properties by lowering serum and liver cholesterol levels and inhibiting angiogenesis induced by CD31 and vascular endothelial growth factor (VEGF) [112].
3.3. PCSK9 Inhibitors
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease known for its role in regulating LDL-R levels in hepatocytes [113]. PCSK9 inhibitors, such as monoclonal antibodies such as evolocumab and alirocumab, as well as synthesis inhibitors such as inclisiran, form a diverse group of medications primarily prescribed for treating primary hypercholesterolemia or mixed dyslipidemia. These inhibitors function by increasing LDL-R density in hepatocytes, which enhances the clearance of LDL-C particles and reduces plasma LDL-C levels [114]. Clinical trials consistently show that PCSK9 inhibition, when added to maximally tolerated statin treatment, reduces serum LDL-C levels by 50 to 65%, regardless of the specific agent or dosage regimen used [115]. Despite their relatively recent introduction, numerous studies have also highlighted the anti-inflammatory benefits of these agents.
3.3.1. Inflammation
Traditional LDL-C-lowering agents, particularly statins, have been proven to reduce hsCRP levels notably. However, randomized clinical trials involving PCSK9 inhibitors have not shown a similar effect. In the FOURIER trial, which included 27,564 patients with stable ASCVD and LDL-C levels over 70 mg/dL despite statin treatment, participants were given either evolocumab or a placebo. Over a median period of 2.2 years, the trial assessed evolocumab’s impact on cardiovascular outcomes, including cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization. The analysis, stratified by baseline hsCRP levels, demonstrated that evolocumab reduced CVD events across all hsCRP levels, with more significant reductions seen in those with higher baseline hsCRP levels [116]. In contrast, a meta-analysis from five years ago involving 4,198 participants found no significant effect of PCSK9 monoclonal antibodies (PCSK9-mAbs) on circulating hsCRP levels. This finding remained consistent across participant characteristics, PCSK9-mAb types, determination methods, and treatment duration [117].
In an observational study involving 645 patients who had been on stable therapy for at least six months and were undergoing carotid endarterectomy, researchers examined the effects of PCSK9 inhibitors on the expression of various inflammation-associated factors within atheromatous plaques. This study revealed that patients treated with PCSK9 inhibitors exhibited reduced expression of pro-inflammatory proteins, such as IL-1β and TNFα, despite similar levels of circulating hs-CRP. This effect was consistent even in subgroups with LDL-C levels below 100 mg/dL, suggesting an independent anti-inflammatory action of PCSK9 inhibitors [118]. As for inclisiran, it appears to exert its anti-inflammatory properties by inhibiting IL-1α, IL-6, and TNF-α in OxLDL-stimulated THP-1-derived macrophages through the suppression of NF-κB nuclear translocation [119]. Additionally, although not statistically significant for individual doses, inclisiran at 300 mg and 500 mg may lead to a reduction in hs-CRP levels (16.2% and 19.8%, respectively) [120].
In recent years, several experimental studies have illuminated the potential anti-inflammatory capacity of PCSK9 inhibition by regulating specific intracellular signaling pathways. A growing body of evidence indicates an interplay between PCSK9 expression and the activation of NLRP3 inflammasome signaling, emphasizing NLRP3 inhibition as a prospective therapeutic target of PCSK9 inhibition. Specifically, PCSK9 governs caspase-1-dependent pyroptosis by instigating mitochondrial DNA damage and activating NLRP3 inflammasome signaling, while the NLRP3 inflammasome, in turn, exerts its influence via IL-1β to regulate PCSK9 secretion [121,122,123]. Recently, Shin et al. have unveiled the PCSK9-CAP1-Syk/PKCδ pathway as a potential novel target of PCSK9 inhibitor activity. Their study outcomes revealed that PCSK9 might contribute to atherosclerosis by inducing NF-κB and inflammatory genes in monocytes, independent of LDL-R involvement within this experimental framework, PCSK9 bonded to cyclase-associated actin cytoskeleton regulatory protein 1 (CAP1), its primary binding partner, which is crucial for the inflammatory characteristics of PCSK9, thereby leading to escalated cytokine production and the activation of TLR4 and scavenger receptors, thereby fostering the uptake of OxLDLs by macrophages [124].
3.3.2. Vascular Health
Mounting evidence highlights the connection between serum PCSK9 and endothelial dysfunction, shedding light on the favorable outcomes associated with PCSK9 inhibition [125]. The precise mechanism through which PCSK9 inhibitors enhance endothelial function remains a subject of ongoing research. However, existing insights suggest that this positive impact could stem from a reduction in LDL-C levels or the mitigation of PCSK9’s capacity to incite a macrophage-mediated inflammatory response. PCSK9’s role in upregulating VEGF-A and intercellular adhesion molecule (ICAM-1) expression promotes endothelial cell activation and facilitates monocyte/macrophage migration, thereby fostering an inflammatory milieu conducive to atherosclerosis [126]. Both in vivo and ex vivo data indicate that PCSK9 inhibitors may mitigate endothelial dysfunction by suppressing endothelial chemokine production and reducing leukocyte-endothelium interactions [127].
Additionally, research suggests that PCSK9 monoclonal antibodies may influence circulating endothelial progenitor cells [128], crucial players in vascular repair following endothelial injury [129]. Experimental sepsis models have shown promising results, indicating that PCSK9 inhibitors counteract sepsis-induced inflammation and endothelial dysfunction driven by heightened PCSK9 expression, mediated through the TLR4/MyD88/NF-κB and NLRP3 pathways [130]. Furthermore, PCSK9 silencing appears to mitigate endothelial cell apoptosis via the MAPK signaling pathway [131]. Clinical studies corroborate these findings, demonstrating that a two-month treatment with evolocumab at 140 mg can improve endothelial function in individuals at elevated cardiovascular risk, as evidenced by enhancements in mean brachial artery diameter, velocity time integral, and FMD [132].
Recent knowledge has underscored the potential of PCSK9 to influence platelet function, suggesting that its inhibition might mitigate this effect [133]. In vivo research indicates that the pro-thrombotic properties of PCSK9 are primarily linked to its interaction with the CD36 receptor on platelets. This receptor recognizes specific oxidized phospholipids and lipoproteins, initiating signaling pathways that promote platelet activation and thrombosis [134]. Serum PCSK9 interferes with the CD36 receptor, leading to the activation of ROS-upregulating enzymes such as Src kinase, MAPK, extracellular signal-regulated kinase 5, and c-Jun N-terminal kinase [135].
Interestingly, recent data suggest that platelets might produce PCSK9, creating a feedback loop that enhances platelet aggregation and thrombus formation [136]. Notably, the anti-thrombotic properties of PCSK9 inhibitors could partly be due to their ability to lower serum lipoprotein(a) [Lp(a)] levels, which has been associated with a 31% reduction in the relative risk of venous thromboembolism [137,138]. Last, PCSK9 inhibition may offer anti-thrombotic benefits because of its interaction with factors related to coagulopathy. Specifically, PCSK9 inhibitor treatment may lead to a reduction in plasminogen activator inhibitor-1 (PAI-1) levels, resulting in enhanced fibrinolytic activity [139]. Additionally, PCSK9 silencing may positively impact the levels of TF, further contributing to its anti-thrombotic effects [140].
3.3.3. Glucose Metabolism
Safety outcomes from significant cardiovascular trials, such as the ODYSSEY trial for alirocumab [141] and the FOURIER trial for evolocumab [142], have not indicated an increased occurrence of NODM. Similarly, meta-analyses have not identified a heightened risk of NODM in patients treated with PCSK9 inhibitors. For instance, a meta-analysis of RCTs assessing the effects of statins and statins combined with PCSK9 inhibitors in non-diabetic participants assigned to either more intensive or less intensive lipid-lowering therapies revealed that neither LDL-C reduction nor the use of PCSK9 inhibitors was associated with the onset of NODM [143].
Conversely, Mendelian randomization data have shown an increased likelihood of developing NODM among individuals with genetic variants at the PCSK9 locus. Supporting this, Mbikay et al. have found that PCSK9-deficient mice demonstrate impaired glucose tolerance and altered glucose-stimulated insulin secretion, mainly due to reduced insulin secretion rather than peripheral insulin resistance [144]. These results are consistent with post-marketing safety data, indicating that PCSK9 monoclonal antibodies may be linked to mild hyperglycemia, particularly within the first six months of treatment [145].
3.3.4. Malignancy
PCSK9 overexpression has been identified in various solid tumors and hematological malignancies [146], including breast cancer, hepatocellular carcinoma, colorectal cancer, and lymphoblastic leukemia [147,148,149,150]. Elevated PCSK9 levels are correlated with increased cancer severity and poorer prognosis, whereas lower PCSK9 levels are associated with improved outcomes and reduced tumor growth. These observations have led to recent research focusing on the potential of PCSK9 inhibition to debilitate malignancy progression.
Experimental models, both in vitro and in vivo, have elucidated several mechanisms by which PCSK9 inhibition may promote apoptosis in cancer cells. Specifically, PCSK9 inhibition can interfere with the progression of the cell cycle, inhibiting cell proliferation and inducing endoplasmic reticulum (ER) stress, leading to apoptotic cell death. Furthermore, PCSK9 inhibition affects mitochondrial pathways by activating pro-apoptotic molecules such as caspase-3 and downregulating anti-apoptotic proteins such as phosphorylated Akt (p-Akt) and survivin [151]. Additionally, PCSK9 inhibitors can downregulate pathways crucial for cancer progression, including the MAPK pathway, the KRAS/MEK/ERK signaling pathway, and the Jak2/STAT3 signaling pathway [152,153].
The anti-cancer potential of cholesterol depletion through PCSK9 inhibition represents another mechanism of action against tumor progression. A high-cholesterol diet was shown to reverse the protective effects of PCSK9 knockout against melanoma metastasis in the liver [154]. Recent findings additionally underscore the role of PCSK9 inhibition in bolstering the antitumor immune response. Specifically, reducing PCSK9 levels may result in heightened expression of MHC-I on tumor cell surfaces [155] and augmentation of CD8+ T cell infiltration within tumors [156]. This increase in MHC-I expression hinders cancer cells from evading immune detection [157], while the rise in CD8+ T cell infiltration suggests decreased cancer proliferation and a lowered likelihood of metastasis and recurrence [158].
However, the impact of PCSK9 inhibition may vary across different cancers. For instance, anti-PCSK9 vaccination in mice with melanoma tumors did not result in reduced tumor growth or improved survival outcomes [159]. Notably, PCSK9 siRNA was reported to protect prostate cancer cells from ionizing radiation-induced damage because of its anti-apoptotic properties [160]. Moreover, we should keep in mind that PCSK9 overexpression has been associated with pro-angiogenic features in vitro, as it may promote the release of VEGF. This is a significant drawback regarding their potential beneficial role in malignancy [161]. In conclusion, while PCSK9 inhibition shows promise as a therapeutic strategy against cancer, its effects are complex and may differ depending on the type of cancer and specific cellular contexts.
3.4. Bempedoic Acid
Bempedoic acid is a novel hypolipidemic agent approved by the Food and Drug Administration (FDA) in 2020 for treating dyslipidemia. It acts by inhibiting ACLY, resulting in LDL-C level reductions [162]. As a monotherapy, BA lowers LDL-C by up to 30% and boosts the effects of high-intensity statins by approximately 15%. A daily combination of BA at 180 mg, ezetimibe at 10 mg, and atorvastatin at 20 mg results in an LDL-C decrease of nearly 60% in hypercholesterolemia patients [163]. BA is also an alternative option for individuals who exhibit intolerance to statins, as it does not cause myopathy [164].
3.4.1. Inflammation
Bempedoic acid offers CVD reduction, likely due to both its lipid-lowering and anti-inflammatory properties. The anti-inflammatory features of BA are primarily achieved through the upregulation of AMPK in immune cells, which attenuates the activation of MAPK pro-inflammatory pathways. This results in a reduction in the production of chemokines, cytokines, and adhesion molecules, thereby decreasing leukocyte accumulation and activation in the artery subendothelium and visceral adipose tissues [165].
Experimental evidence from diet-induced obese mice has demonstrated that BA administration can restore AMPK activity in adipose tissue, leading to a reduction in IL-6 production and tissue inflammation [166]. Given that IL-6 promotes the production of CRP by the liver, it is not surprising that individuals receiving BA may exhibit a 20–30% reduction in hsCRP levels [167]. A pooled analysis of four CLEAR randomized trials evaluating BA in subjects with hypercholesterolemia and ASCVD, whether on statin therapy or statin-intolerant, revealed a significant reduction in hs-CRP levels. The decrease was 18.1% in individuals on statin treatment and 27.4% in statin-intolerant patients, indicating a potent anti-inflammatory effect [168]. Furthermore, the BA-induced inhibition of ACLY appears to facilitate a reduction in prostaglandin E2 production, further alleviating the inflammatory response [169].
3.4.2. Vascular Health
Bempedoic acid has been associated with potential endothelial dysfunction because of its ability to cause hyperuricemia. Elevated uric acid levels can contribute to hypertension by increasing systemic vascular resistance and decreasing NO availability [170]. However, recently, it has been reported that bempedoic acid may have unexpected antihypertensive effects and improve vascular health. This potential benefit could be due to its capacity to counteract the harmful effects of chronic activation of the Ang II receptor 1 (AT-1R) on cellular protection and survival. BA activates the adenosine monophosphate-activated AMPK signaling pathway, leading to higher NO production and potential reduction in ER stress. Additionally, bempedoic acid might exhibit anti-fibrotic properties and prevent vascular remodeling by inhibiting the extracellular signal-regulated kinase (ERK)/transforming growth factor-β fibrotic signaling pathway [171].
3.4.3. Glucose Metabolism
Bempedoic acid may positively affect glucose metabolism and insulin sensitivity due to its combined impact on ACLY inhibition and AMPK activation. ACLY inhibition reduces glucose synthesis by limiting oxaloacetate availability for gluconeogenesis [12,172,173]. Additionally, AMPK activation regulates glucose production and energy balance by downregulating key enzymes involved in glucose production, such as glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK). G6Pase hydrolyzes glucose-6-phosphate to produce free glucose for release from the cell, while PEPCK catalyzes the initial step of gluconeogenesis, converting oxaloacetate into phosphoenolpyruvate [173,174].
3.4.4. Malignancy
ACLY is often overexpressed in many cancers and plays a central role in cancer metabolism by depleting cytosolic citrate, which enhances glycolysis through increased PFK1 and PFK2 activity and activates oncogenic drivers such as PI3K/AKT. Therefore, inhibiting ACLY with BA may help mitigate tumor development [175]. Recent evidence suggests that BA may be a potential treatment for head and neck squamous cell carcinoma (HNSCC). This may be attributed to its ability to inhibit ACLY, which is abundant in small extracellular vesicles (sEVs) derived from interleukin-8-activated CXCR1High cells. ACLY promotes the transformation of CXCR1Low to CXCR1High cells, aiding HNSCC progression by acetylating NF-κB p65 and facilitating its nuclear translocation to transcribe CXCR1 [176].
Furthermore, BA has shown promise for individuals undergoing treatment for breast and pancreatic cancer with palbociclib, a CDK4/6 inhibitor. When palbociclib activates ACLY, combining it with BA inhibits ACLY, reducing tumor cell viability and enhancing apoptosis, thereby impeding cell invasion [177]. Interestingly, BA has been linked to alterations in intracellular isoprenoid composition, which can significantly impact protein prenylation. As such, bempedoic acid may function as a prenyltransferase inhibitor. PTases serve as posttranslational modifiers of proteins involved in various cellular processes, rendering them potential drug targets for a broad spectrum of disorders, including malignancy and AD [178].
In conclusion, the landscape of lipid-lowering treatments that primarily reduce LDL-C has been extensively explored, with statins and ezetimibe being the most researched. Statins are known for their multiple beneficial effects, including enhancing vascular function and reducing inflammation, although they have diabetogenic concerns and mixed evidence regarding cancer risk. Ezetimibe also contributes significantly to cardiovascular health beyond cholesterol reduction, showing anti-inflammatory benefits and potential improvements in vascular health, albeit with inconsistent effects on glucose metabolism and cancer outcomes. PCSK9 inhibitors emerge as powerful agents, excelling in LDL-C reduction and exhibiting anti-inflammatory and potential anticancer properties. Last, bempedoic acid is recognized for its effectiveness in managing dyslipidemia, along with promising roles in addressing inflammation, vascular health, glucose metabolism, and malignancy, underscoring its versatility in contemporary therapeutic strategies. Figure 1 illustrates different anti-inflammatory mechanisms through which the aforementioned agents exert their pleiotropic effects, as evidenced by experimental studies.
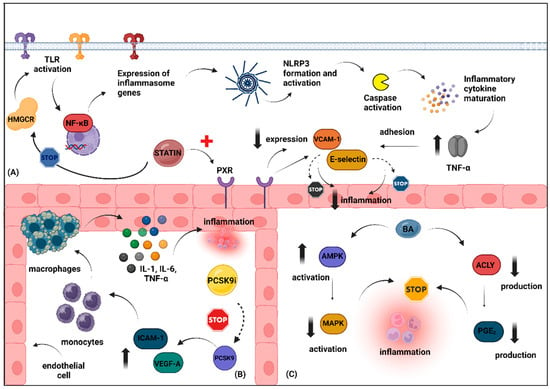
Figure 1.
Schematic illustration depicting the potential anti-inflammatory effects of hypolipidemic agents primarily targeting LDL-C reduction. (A). Typically, HMGCR facilitates the activation of TLRs, which subsequently triggers the expression of inflammasome-related genes through the NF-kB signaling pathway. This leads to inflammasome formation and the activation of caspase-1 protein, resulting in the maturation of several pro-inflammatory cytokines, including TNF-a. TNF-a promotes the adhesion of various molecules, such as VCAM-1 and E-selectin, contributing to endothelial dysfunction. Statins may exert anti-inflammatory effects either by inhibiting HMGCR or by enhancing the expression of PXR, a receptor commonly found in the vasculature. Activation of PXR reduces the expression of these adhesion molecules, thus promoting vascular detoxification and reducing vascular inflammation. (B). PCSK9 increases the expression of VEGF-A and ICAM-1 adhesion molecules, which leads to the migration of monocytes and macrophages, thereby boosting the production of pro-inflammatory cytokines and causing endothelial dysfunction. Silencing PCSK9 may counteract these effects. (C). Bempedoic acid exerts its anti-inflammatory effects primarily by upregulating AMPK, which subsequently reduces the activation of MAPK pro-inflammatory pathways. Additionally, bempedoic acid may improve the inflammatory environment by decreasing the production of prostaglandin E2 through the inhibition of ACLY. Abbreviations: ACLY, ATP-citrate lyase; AMPK, AMP-activated protein kinase; BA, bempedoic acid; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; ICAM-1, intercellular adhesion molecule-1; LDL-C, low-density lipoprotein cholesterol; MAPK, mitogen-activated protein kinase; NF-kB, nuclear factor kappa B; PCSK9, proprotein convertase subtilisin/kexin type 9; PCSK9 i, proprotein convertase subtilisin/kexin type 9 inhibitor; PXR, pregnane X receptor; TLR, toll-like receptors; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule-1; VEGF-A, vascular endothelial growth factor A. Created with www.BioRender.com. (assessed on 14 July 2024).
4. Thinking Pleiotropic in Familial Hypercholesterolemia
4.1. Mipomersen and Lomitapide
Mipomersen and lomitapide are lipid-lowering agents primarily recommended for individuals with homozygous familial hypercholesterolemia (HoFH). Mipomersen, a second-generation antisense oligonucleotide inhibitor of ApoB100, promotes the selective degradation of ApoB100 mRNA, independently of LDL-R function, thereby reducing the production of ApoB100-enriched lipoproteins, including LDL-C, VLDL-C, and Lp(a) [13]. Lomitapide, on the other hand, is an oral selective inhibitor of MTP, which affects the production of VLDL lipoproteins that primarily contain ApoB100. Comparatively, lomitapide is thought to exhibit more robust lipid-lowering effects, reducing LDL-C by about 50%, whereas mipomersen achieves approximately a 25% reduction in LDL-C levels. Despite their promise in treating FH, the potential side effects of these agents, particularly their propensity to cause hepatic steatosis, have limited their clinical use [13,14].
Regarding their pleiotropic effects, data on mipomersen are limited. In contrast, recent experimental studies have explored the potential pleiotropic effects of lomitapide, including its anti-inflammatory and anti-cancer properties. Preliminary evidence suggests that lomitapide administration may enhance vascular function in obese mice by alleviating body weight gain and restoring lipid profiles, with favorable effects on inflammation and oxidative stress, respectively [179]. Additionally, lomitapide has demonstrated anti-cancer properties by facilitating autophagic cell death via mTOR inhibition and by promoting anti-proliferative effects in preclinical pancreatic cancer models [180]. Moreover, lomitapide may offer benefits when combined with immune checkpoint-blocking antibodies, as this combination has been shown to reduce tumor growth in murine preclinical syngeneic tumor models [181]. Lomitapide’s effects may also extend to hematological malignancies, with recent evidence indicating that targeting PARP14 with lomitapide attenuates drug resistance via DRP1-induced mitophagy activation in multiple myeloma [182]. PARP14, overexpressed in multiple myeloma, functions as a transcriptional co-activator for STAT6, facilitating the Th2 immune response, promoting metabolic changes to an anaerobic state, and activating cell survival pathways through JNK2 and the PGI/AMF complex [183].
4.2. ANGPTL3 Inhibitors
Recently, the FDA sanctioned a pioneering category of cholesterol-lowering medications termed angiopoietin-like 3 protein (ANGPTL3) inhibitors. These compounds operate by obstructing ANGPTL3, thereby amplifying the functions of LPL and EL. Consequently, LPL and EL aid in the elimination of remnants of VLDL via liver receptors, culminating in a decrease in LDL-C levels irrespective of LDL-R activity. ANGPTL3 inhibitors find their primary application in refractory homozygous FH [184]. Presently, evinacumab and vupanorsen stand as the principal ANGPTL3 inhibitors in clinical application, furnishing LDL-C reductions spanning 18–25% [185,186]. Recent studies have illuminated the potential positive impacts of ANGPTL3 inhibition extending beyond lipid metabolism, underscoring their prospective influence, particularly on inflammation and malignancy.
4.2.1. Inflammation
Evidence from experiments with cultured THP-1-derived macrophages reveals that human recombinant ANGPTL3 elevates the levels of proinflammatory cytokines IL-1β, IL-6, and TNF-α, underscoring the direct involvement of ANGPTL3 in inflammation. Both in vivo and in vitro data have indicated that knocking out Angptl3 may provide advantages in instances of renal dysfunction. This is achieved by promoting the conversion of M1 macrophages to M2 macrophages and reducing the activation of the NLRP3 inflammasome [187]. Beyond its secreted form, the intracellular overexpression of ANGPTL3 also significantly impacts inflammatory responses. Recently, Zhang et al. have shown that elevated ANGPTL3 levels inhibit IL-1β-induced NF-κB activation and the transcription of inflammatory genes in the liver, whereas this inhibitory effect is reversed when ANGPTL3 expression is knocked down. ANGPTL3 accomplishes this by interacting with IL1R1 and IL1RAP through its intracellular C-terminal FLD, thereby disrupting the assembly of the IL1R1-associated complex, which is critical for IL-1β-induced signaling. These insights highlight a new role for ANGPTL3 in inflammation, where it interferes with the normal interaction between IL1R1 and IL1RAP, thereby maintaining immune tolerance and homeostasis in the liver [188].
4.2.2. Vascular Health
ANGPTL3 plays a significant role in inflammation by modulating macrophage activity within atheromatous lesions through its C-terminal fibrinogen-like domain (FLD). Unlike FLDs from other angiopoietins that bind to endothelial cells via Tie receptors, the ANGPTL3 FLD specifically targets integrin αVβ3, a key component in the formation of atherosclerotic plaques [189]. This binding promotes foam cell formation by decreasing the expression of scavenger receptor A (SRA) and CD36 while simultaneously enhancing pro-angiogenic activities. Furthermore, the interaction of ANGPTL3 with αvβ3 integrin in endothelial cells facilitates endothelial cell adhesion and migration. This interaction also activates intracellular signaling pathways, such as mitogen-activated protein kinase and Akt phosphorylation, which can lead to direct vascular injury [190]. In patients undergoing maintenance hemodialysis, where endothelial dysfunction is prevalent, ANGPTL3 levels have been found to negatively correlate with the vascular reactivity index (VRI), an indicator of endothelial function [191].
4.2.3. Glucose Metabolism
While the role of ANGPTL3 in lipid metabolism is well-established, its impact on glucose metabolism remains unclear. Preliminary evidence using CRISPR-Cas9 to create ANGPTL3 knockout mice suggests that ANGPTL3 loss-of-function (LOF) may improve insulin sensitivity. This improvement might be due to increased LPL activity, which benefits insulin sensitivity and lowers free fatty acid levels in the bloodstream. This reduction in free FAs may result from impaired mobilization from adipose tissue [192]. Conversely, ANGPTL3 has been shown to enhance endogenous adipogenesis, which can reduce insulin sensitivity [193]. Thus, ANGPTL3 inhibition may potentially offer benefits related to glucose metabolism in individuals with T2DM.
4.2.4. Malignancy
Recent evidence has underscored the involvement of ANGPTL3 in the occurrence, development, and survival rates of various malignancies. Particularly, ANGPTL3 has been found to be elevated in gastrointestinal cancers, including esophageal and hepatocellular carcinoma [194,195]. Moreover, recent data highlight its involvement in colorectal cancer (CRC), as individuals with CRC exhibit upregulated ANGPTL3 expression, leading to worse survival rates. ANGPTL3 overexpression appears to facilitate the proliferation and migration of CRC cells partially via mitogen-activated protein kinase 14 (MAPK14). Interestingly, these findings may be reversed by ANGPTL3 inhibition, while ANGPTL3 downregulation may suppress tumor growth and liver metastasis. Therefore, ANGPTL3 inhibition may represent a novel treatment strategy for individuals with CRC [196].
On the other hand, data regarding the role of ANGPTL3 in ovarian cancer are conflicting. Some data suggest that increased ANGPTL3 expression is associated with shorter survival in individuals with high-grade serous ovarian cancer (HGSC) [197]. However, recently, Wu et al. have shown that ANGPTL3 inhibition may have a negative impact on individuals with ovarian cancer, as ANGPTL3 is reduced in ovarian cancer tissues and cells, predicting a favorable prognosis. Specifically, an increase in ANGPTL3 mitigates the proliferation of ovarian cancer cells in vitro and the metastatic potential of these cells. Furthermore, ANGPTL3 promotes natural killer (NK) cell killing of ovarian cancer cells while also modulating the JAK/STAT3 pathway to influence metastasis and immune resistance [198].
4.3. Lipoprotein Apheresis
Lipoprotein apheresis is a therapeutic modality designed to selectively eliminate apoB-containing lipoproteins from circulation [199]. While LA has been shown to reduce LDL-C and Lp(a) concentrations significantly, its clinical impact on CVD risk mitigation is still conflicting [200]. LA is typically recommended for individuals with FH, particularly those with the homozygous form, who fail to achieve therapeutic goals despite optimal tolerated lipid-lowering treatments [201]. Particularly in Germany, LA is indicated for patients with progressive CVD and Lp(a) concentrations exceeding 60 mg/dL, regardless of LDL-C levels [202]. Several methods are available for performing LA, including adsorption, precipitation, and filtration techniques [203]. Beyond its central role in reducing lipoprotein levels, LA has been found to offer various pleiotropic benefits that may contribute to its therapeutic effects. These benefits include reductions in inflammation and enhancements in vascular health, including improvements in endothelial function and blood viscosity [204].
Research indicates that in individuals with severe hypercholesterolemia, lipoprotein apheresis may possess anti-inflammatory effects, interfering with different pathways of the inflammatory process. LA appears to influence the complement system differently depending on the LDL apheresis system used. Plasma separation columns tend to promote the formation of proinflammatory complement factors C3a and C5a, whereas LDL apheresis columns adsorb these anaphylatoxins to different extents [205]. LA may also mitigate inflammation by reducing the mRNA expression of pro-inflammatory molecules such as IL-1α, IL-6, and TNF-α [8]. Additionally, LA has shown benefits in reducing CVD-related inflammatory biomarkers such as CRP and fibrinogen, as evidenced by several studies [206,207,208].
However, evidence regarding the effect of LA on myeloperoxidase (MPO) levels in hypercholesterolemic patients is conflicting. Some studies have reported an increase in MPO post-LDL apheresis, while others have observed a reduction, often linked to changes in total cholesterol levels [209]. MPO is a heme-binding protein associated with proinflammatory properties implicated in atherosclerotic plaque development, though it’s also suggested to play a role in ending the inflammatory process [210]. Notably, while variations exist among LDL apheresis columns and patient demographics across studies, most agree that pro-inflammatory cytokines are partially removed by the LDL apheresis columns, with some studies noting an increase in anti-inflammatory cytokines during the procedure [211,212].
Additionally, LA might affect the immune system, potentially influencing certain T cell and NK cell subsets expressing CD69 [213]. Multiple LA sessions have been linked to a decrease in these CD69-expressing cell subsets. T cells are pivotal in early-stage atherosclerosis and are prevalent in advanced atheromatous plaques [214]. Conversely, CD69, an immune activation marker, plays a multifaceted role in inflammation; its presence can promote inflammation, whereas its absence is associated with susceptibility to inflammatory or autoimmune disorders. This hints at a CD69 immunoregulatory function, potentially amplifying the suppressive role of regulatory T cells (Tregs). Hence, the modulation of CD69 expression by LA may contribute to immune regulation, impacting inflammatory processes such as atherosclerosis [215].
The oxidative modification of LDL-C is a crucial factor in the early stages of atherosclerosis, and lipid peroxidation is notably higher in patients with FH. Lipoprotein apheresis has been shown to reduce oxidative stress, suggesting that reductions in lipid peroxidation parallel to decreases in LDL-C concentrations make LA an effective method for preventing cardiovascular disease in FH patients [216]. In a recent study involving eleven patients with FH and hyperlipoproteinemia(a) treated with regular LA using the direct adsorption of lipoproteins(DALI) or membrane filtration optimized novel extracorporeal treatment (MONET) techniques, a single LA session resulted in similar reductions in lipid-related oxidative stress markers. Specifically, concentrations of 8-iso-prostaglandin F2α (8-isoPGF2) and thiobarbituric acid reactive substances (TBARS) were reduced by approximately 60% and 30%, respectively [217]. Similarly, a clinical study conducted in China with 31 FH patients reported significant reductions in free oxygen radicals test (FORT) values and significant increases in free oxygen radicals defense (FORD) values immediately after LA treatment compared with pre-treatment levels [218].
Years ago, evidence indicated that repeated LA may lead to hemodynamic alterations in individuals with FH [219]. Although most studies involve a small number of patients, likely due to the rare indication of LA use, a considerable number of studies have reported that LA may reduce blood viscosity, typically assessed using the Rheolog capillary viscometer [220]. Importantly, this reduction persists even one week after treatment [221].
The aforementioned beneficial effects of repeated LA have been demonstrated in a 6-month follow-up period, wherein LA resulted in improvements in multiple CVD-related parameters, including circulating endothelial progenitor and mature cells, flow-mediated vasodilatation, left ventricular ejection fraction, homocysteine levels, and microalbuminuria [199]. Interestingly, recent data demonstrate that in subjects with increased serum Lp(a) levels, LA may also eliminate extracellular vesicles (EVs) [222]. EVs are cell-derived particles that mediate intercellular communication by transporting various molecules, including proteins, lipids, and cytokines [223]. These particles are highly involved in maintaining homeostasis, inflammation, neo-angiogenesis, and thrombosis, all of which are pivotal underlying substrates of ASCVD [224]. Thus, EVs may serve as potential CVD biomarkers, useful for assessing the severity and progression of ASCVD [225].
Overall, therapies tailored for individuals with FH demonstrate a variety of pleiotropic effects extending beyond their primary mechanisms. Lomitapide, for instance, exhibits anti-inflammatory and anti-cancer properties, while ANGPTL3 inhibitors offer versatility by impacting inflammation, vascular health, glucose metabolism, and malignancy. Lipoprotein apheresis similarly provides significant pleiotropic benefits, primarily through its anti-inflammatory actions and improvements in vascular health. Understanding and leveraging these diverse effects is critical for optimizing therapeutic outcomes and broadening the clinical applications of these treatments beyond lipid modulation.
5. The Pleiotropic Effects of HDL-C Enhancement: Exploring Old and Novel Agents
5.1. Niacin
Niacin, also known as vitamin B3, comprises nicotinic acid and nicotinamide, which play pivotal roles in numerous biochemical reactions within the human body [226]. When administered at doses of 1–2 g/day, niacin can modestly improve HDL-C levels, typically increasing HDL-C by up to 20–25% while also reducing TGs and LDL-C [227]. Despite these beneficial effects on lipid profiles, niacin use has not been linked to significant reductions in total or cause-specific mortality or recurrent cardiovascular events in individuals with or at risk of ASCVD [228]. Nevertheless, several studies have underscored niacin’s potential anti-inflammatory properties, improvements in vascular health, and possible anti-cancer effects.
5.1.1. Inflammation
Niacin possesses both direct and indirect anti-inflammatory properties, primarily through the activation of the hydroxycarboxylic acid receptor 2 (HCA2) receptor in various tissues. It inhibits the expression of adhesive molecules in blood vessels and suppresses pro-inflammatory cytokines in adipose tissue. In monocytes, it reduces the secretion of inflammatory markers such as TNF-α and IL-6. Research involving animals and human cells demonstrates that niacin can mitigate inflammation by downregulating the NF-κB pathway and reducing the expression of inflammatory molecules. These effects occur independently of changes in lipid levels [229]. Additionally, niacin exhibits anti-oxidative properties, which may be due to its ability to enhance HDL-C levels or activate Nrf2, a transcription factor crucial for regulating mechanisms affecting oxidative stress and subsequent inflammation [230,231].
The anti-inflammatory benefits of niacin have also been demonstrated in experimental settings of CKD, where its administration has been shown to reduce oxidative stress and inflammation. Notably, niacin alleviates MCP-1, PAI-1, TGF-β, and NF-κB activation, leading to improvements in hypertension, proteinuria, glomerulosclerosis, and tubulointerstitial injury [232]. A recent meta-analysis by Rad et al. highlighted that niacin usage is associated with significant reductions in CRP and TNF-α levels, further supporting its potential anti-inflammatory effects. Moreover, niacin positively affects adipokines, increasing levels of adiponectin and leptin, thereby debilitating metabolic dysregulation [233].
5.1.2. Vascular Health
Years ago, niacin was reported to enhance endothelial function, likely due to its ability to raise HDL-C levels. The INEF study, a large-scale randomized, double-blind, placebo-controlled trial, found that extended-release niacin at 1000 mg daily might reduce endothelial dysfunction in subjects with CAD and low HDL-C but not in those with normal HDL-C [234]. Subsequent studies have explored the beneficial impact of niacin on endothelial function. Kaplon et al. demonstrated that higher dietary niacin intake is linked to enhanced vascular endothelial function and reduced systemic and vascular oxidative stress among healthy middle-aged and older subjects [235]. Recent research further confirms niacin’s potential in enhancing endothelial function and reducing vascular aging, showing that niacin activates Sirt1, a key regulator of vascular endothelial NO production [236].
Moreover, niacin at low concentrations (10 μM) appears to enhance human microvascular endothelial cell (HMVEC) angiogenic function under lipotoxic and hypoxic conditions. This effect is likely independent of lipid correction and is mediated via the activation of the niacin receptor GPR109A, which is expressed in endothelial cells [237]. Additionally, niacin promotes revascularization and functional recovery of the hind limb following ischemic injury in diet-induced obese mice with hyperlipidemia, highlighting its potential in treating peripheral ischemic vascular disease associated with metabolic syndrome [238].
Notably, niacin has shown promise in cases of ischemic stroke, potentially reducing thrombotic risk. The main mechanism proposed is niacin’s ability to reduce platelet aggregation and support blood clot breakdown. Niacin also decreases TGF-β-induced increases in PAI-1 and fibrinogen levels [239]. The positive effects of niacin on endothelial function were confirmed in a meta-analysis by Sahebkar. This analysis included seven RCTs with a total of 441 participants and concluded that niacin supplementation may improve endothelial function, indicated by a weighted mean increase in FMD of 1.98% [240]. Nevertheless, despite the plethora of evidence supporting niacin’s favorable role in vascular health, recent findings from Ferrell et al. contradict these effects. They have shown that the terminal breakdown products of excess niacin, 2PY and 4PY facilitate vascular inflammation, an association that may translate to the presence of residual CVD risk [241].
5.1.3. Glucose Metabolism
Niacin is known for its tendency to promote mild increases in blood glucose levels in both diabetic and non-diabetic individuals, highlighting the importance of monitoring glucose levels during its administration. Among 17 non-diabetic postmenopausal women, niacin has been reported to significantly impact mean glucose, insulin, and C-peptide levels, leading to increases of 10.6%, 61.8%, and 46.1%, respectively [242]. Additionally, long-term treatment with niacin has been proposed to negatively impact glucose homeostasis, although the underlying mechanisms remain unclear. Niacin’s hyperglycemic effect is presumably associated with its ability to impair pancreatic islet function by reducing glucose-stimulated insulin secretion partially through the upregulation of GPR109A and PPARγ2 [243,244]. Nevertheless, experimental evidence supports the antidiabetic potential of niacin supplementation in diabetic rats, demonstrating that its administration re-results in hyperglycemia control in a dose-dependent manner, with notable reductions in fasting glucose levels. Niacin is also suggested to alleviate oxidative stress, which is essential in the onset and progression of T2DM, further diminishing DNA damage and tissue injury caused by ROS production [245].
5.1.4. Malignancy
Mounting evidence highlights niacin’s potential beneficial impact on malignancy, including reduced mortality rates, as demonstrated by the NHANES retrospective cohort study involving 3504 individuals [246]. Niacin exerts a significant influence on DNA repair and genomic stability, crucially affecting cancer risk through its derivative nicotinamide adenine dinucleotide (NAD+). NAD is pivotal in ADP-ribosylation processes, including the activity of poly(ADP-ribose) polymerase-1 (PARP-1), essential for DNA damage response, repair, and stress signaling. Moreover, NAD+ plays a critical role in calcium signaling pathways; disruptions can lead to genomic instability and heightened cancer susceptibility, evident in both solid tumors and hematological malignancies such as skin cancer and leukemia [247].
In combating colon cancer, niacin has shown promise by activating autophagic flux in human colon cancer cells, protecting them from mitochondrial dysfunction and TRAIL-induced apoptosis [248]. Additionally, recent data illuminate niacin’s anti-cancer properties and its potential role in mitigating chemotherapy side effects. Notably, niacin appears effective in ameliorating cancer-related cachexia, a condition exacerbated by both cancer and chemotherapy. Experimental and clinical evidence reveals that severe cachexia correlates with depleted NAD+ levels and reduced Nrk2 activity, an enzyme involved in NAD+ biosynthesis. Niacin supplementation restores tissue NAD+ levels, enhances mitochondrial metabolism, and alleviates cachexia induced by cancer and chemotherapy [249]. However, certain malignancies, particularly those exhibiting BRCAness, exploit niacin by upregulating NAPRT, accelerating niacin conversion to NAD+, and enhancing DNA repair, thereby promoting tumor progression [250]. Additionally, Tosti et al. suggest that restricting niacin, in combination with inhibiting NAMPT, an enzyme in niacin metabolism, leads to synthetic lethality in neuroendocrine carcinomas of the lung and prostate [251]. Furthermore, data from a meta-analysis in both immunocompetent and immunosuppressed patients indicate insufficient evidence that niacin treatment significantly diminishes keratinocyte cancers [252].
5.2. CETP Inhibitors
Cholesteryl ester transfer protein (CETP) facilitates the movement of cholesteryl esters from HDL-C particles to lipoproteins containing ApoB. Blocking CETP leads to a marked rise in HDL-C levels and a simultaneous decrease in both LDL-C and Lp(a) levels [253]. Although CETP inhibitors have shown impressive lipid-altering capabilities, they have not yielded notable cardiovascular benefits. Nevertheless, novel research indicates that CETP inhibitors may positively impact various other pathological conditions, highlighting their potential advantages beyond reducing CVD risk [254].
5.2.1. Inflammation
Given that CETP inhibitors promote increases in HDL-C and apolipoprotein A1 (ApoA1) levels, it is reasonable to consider that these agents might offer anti-inflammatory benefits, as both HDL-C and ApoA1 are known for their anti-inflammatory and antioxidant properties. HDL-C has been shown to reduce foam cell formation, prevent LDL-C oxidation, and decrease tissue neutrophil infiltration [255,256]. Similarly, ApoA1 can attenuate the activation of macrophages by T-cells, thereby reducing the production of inflammatory cytokines and chemokines [257]. However, some data indicate that CETP inhibitors might actually enhance inflammation by increasing serum levels of hsCRP [258]. Additionally, early evidence suggests that despite improvements in HDL-C functionality, CETP inhibition may impair the anti-inflammatory capacity of HDL-C, potentially leading to fatty liver and insulin resistance, particularly in the context of obesity [259].
Conversely, data from Mendelian randomization studies have shown a causal relationship between HDL-C and IBD, indicating that genetically proxied CETP inhibition may reduce the risk of Crohn’s disease [260]. Emerging research also underscores the potential of CETP inhibitors in treating various conditions where inflammation is critical, including neurodegenerative diseases such as Alzheimer’s disease, age-related macular degeneration (AMD), and severe inflammatory states such as sepsis [254].
CETP inhibitors may hold significant promise for AD therapy. Preclinical studies and epidemiological evidence suggest that higher HDL-C levels are associated with reduced AD risk, implying that CETP inhibition could represent a novel treatment option for AD. These inhibitors reduce overall brain cholesterol content and apolipoprotein E4 (ApoE4) concentration while elevating apoA1/pre-beta HDL levels in the choroid plexus and bloodstream, thus improving cholesterol metabolism in the neurovascular unit [261]. Individuals with loss-of-function mutations in the CETP gene are shielded from the AD risk associated with carrying an ApoE4 mutation [262]. Conversely, AD patients harboring an ApoE4 allele typically exhibit lower HDL-C and ApoA1 levels [263]. This holds significance as apoA1 overexpression mitigates neuroinflammation, safeguards against cerebral amyloid angiopathy, and helps prevent cognitive dysfunction [264,265].
Enhancing HDL-C through CETP inhibition may also increase the bioavailability of xanthophylls, potentially offering protection against AMD. AMD develops when the macular surface, rich in photoreceptors, is compromised by the accumulation of lipoprotein-rich deposits known as drusen, and inflammation is highly involved in AMD pathogenesis [266]. Both preclinical and clinical findings suggest that delivering lipophilic antioxidants to the macula can provide protective benefits against AMD [267,268]. Since HDL-C particles are the primary carriers of xanthophylls, it is hypothesized that boosting HDL-C levels could improve the circulation and availability of these beneficial antioxidants [269]. By enhancing the transport of xanthophylls, which include lutein and zeaxanthin, HDL-C may help mitigate oxidative stress and inflammation in the macula, thereby reducing the risk or progression of AMD [270,271].
Mounting evidence suggests that inhibiting CETP may also be beneficial in severe inflammatory states, such as sepsis. Sepsis often leads to reduced levels of HDL-C and its associated apolipoproteins, ApoA1, and apolipoprotein C1 (ApoC1) [272,273]. Preclinical evidence has shown that administering HDL-C or genetically increasing apoA1 expression can alleviate inflammation and improve survival rates in intra-abdominal sepsis. For instance, in mice with polymicrobial sepsis, CETP inhibition helped maintain plasma HDL-C and apoA1 levels, resulting in higher survival rates compared with the placebo [274]. Genetic data have corroborated these findings, as a gain-of-function variant, rs1800777-A, has been linked to a significant decline in HDL-C levels during sepsis, increasing the risk of organ failure and mortality [275].
Regarding the underlying mechanism, it is hypothesized that HDL-related apolipoproteins can bind to and facilitate the removal of lipopolysaccharide (LPS), a component of Gram-negative bacteria, from the bloodstream. In cases of Gram-negative-induced sepsis, the release of LPS endotoxin activates Kupffer cells, which exert antibacterial effects by releasing pro-inflammatory cytokines and reducing CETP expression, thereby increasing HDL-C levels in the bloodstream. Consequently, HDL-C helps neutralize systemic endotoxemia by binding to LPS and promoting a proinflammatory reaction in macrophages, aiding in bacterial clearance [276,277].
5.2.2. Vascular Health
The effects of CETP inhibitors on endothelial function are still debated. Recent findings suggest that CETP presence in endothelial cells contributes to vascular oxidative stress and endothelial dysfunction. In cultured human aortic endothelial cells, CETP inhibition reduced oxidative stress from major ROS sources, including mitochondria and NOX2. Additionally, markers of endoplasmic reticulum stress and inflammatory molecules involved in atherosclerosis, such as TNF-α, ICAM-1, and VCAM-1, were alleviated [278]. However, experimental data show mixed results for specific CETP inhibitors. For instance, evacetrapib did not improve endothelial function, and anacetrapib might have adverse effects on the endothelium [279].
The influence of CETP on endothelial function also appears to be sex-dependent. In male mice, expression of the human CETP gene impaired endothelium-mediated vascular relaxation, correlating with oxidative stress. In contrast, increased CETP expression in female mice improved endothelial function via estrogen receptor-α and the endothelial nitric oxide synthase pathway, leading to reduced ROS production and increased eNOS-derived NO, which promotes anticontractile effects [280]. These varying effects of CETP inhibition on vascular function may partly explain the failure of CETP inhibitors to reduce cardiovascular risk despite significant increases in HDL-C levels observed after their administration.
5.2.3. Glucose Metabolism
CETP inhibition has been shown to reduce the risk of new-onset T2DM in major clinical trials, primarily due to its ability to increase HDL-C, which has glucose-lowering properties. In pancreatic islet β-cells, HDL-C promotes euglycemia by reducing β-cell apoptosis via ATP binding cassette transporters, ABCA1 and ABCG1, and by enhancing insulin secretion through apoA1 and ABCA1/ABCG1 pathways [281]. Additionally, high HDL-C levels improve muscle insulin sensitivity and stimulate insulin-dependent glucose uptake via AMPK and ApoA1 [282]. Preliminary data suggest that specific CETP inhibitors such as anacetrapib, dalcetrapib, and torcetrapib may promote insulin secretion and β-cell survival by stimulating reverse cholesterol transport in β-cells [283,284].
The beneficial effects of HDL-C are diminished when HDL-C levels are low or its metabolic actions are dysregulated, leading to glucose metabolism disruptions and elevated serum glucose levels. Genetic studies on the role of CETP in T2DM prevention have yielded conflicting results [285,286]. However, a recent larger study indicated that CETP inhibition could be beneficial for preventing T2DM by analyzing genetic associations with LDL-C, HDL-C, and TG as proxies for lower CETP concentration or activity [287].
5.2.4. Malignancy
About eight years ago, CETP was identified as a potential molecular target for inducing cell death in estrogen-positive breast cancer cells. Esau et al. have demonstrated that MCF-7 CETP knockout breast cancer cells were less resistant to cytotoxic compounds such as tamoxifen and more prone to intrinsic apoptosis [288]. Since then, numerous experimental studies have expanded on this topic. Recently, Gu et al. confirmed the role of CETP in breast cancer growth and aggressiveness, suggesting that CETP inhibition might reduce drug resistance by decreasing cholesterol accumulation in breast cancer cells [289]. Similar benefits have been reported for other types of cancers with the use of specific CETP inhibitors. For instance, Evacetrapib has shown favorable effects against colorectal cancer by inhibiting the Wnt/β-Catenin Signaling Pathway and activating the JNK Signaling Pathway [290]. Table 1 presents both preliminary and clinical studies that have highlighted the pleiotropic benefits of CETP inhibition beyond CVD risk assessment. Subsequently, Figure 2 depicts proposed anti-cancer mechanisms of action of CETP inhibitors, PCSK9 inhibitors, and bempedoic acid, as reported in experimental studies in recent years.

Table 1.
Potential Benefits of CETP Inhibition: Insights from Recent Research Beyond Cardiovascular Disease.
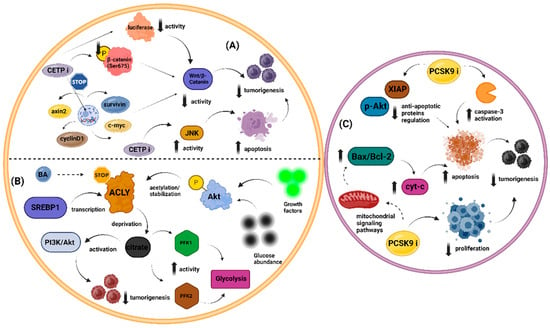
Figure 2.
Emerging evidence regarding the anti-cancer features of novel lipid-lowering treatments, including CETP inhibitors, bempedoic acid, and PCSK9 inhibitors. (A). CETP inhibitors may confer anti-cancer effects by inhibiting the Wnt/β-Catenin signaling pathway and activating the JNK signaling pathway. The activation of JNK by CETP inhibition exhibits apoptotic activity, resulting in diminished tumorigenesis. Additionally, CETP inhibition may reduce tumorigenesis by dampening the activation of Wnt/β-Catenin. Specifically, CETP silencing has been associated with decreased activation of luciferase, reduced levels of the phosphorylated form of β-catenin (p-β-catenin (Ser675)), and lower levels of target genes involved in Wnt signaling, including axin2, cyclin D1, c-myc, and survivin. (B). Bempedoic acid has been suggested to possess anti-malignant properties through its ability to inhibit ACLY. SREBP1 promotes ACLY transcription, while the phosphorylation of Akt—mediated by both growth factors and glucose abundance—facilitates the enzyme’s acetylation and stabilization. ACLY activation leads to citrate deprivation, resulting in reduced tumorigenesis by enhancing the activation of the PI3K/Akt pathway. Interestingly, citrate deprivation is also linked to increased activity of regulatory enzymes in glycolysis, specifically PFK1 and PFK2. (C). PCSK9 silencing may exhibit anti-cancer properties by either reducing tumor cell proliferation or enhancing cancer cell apoptosis. PCSK9 inhibitors can facilitate apoptosis by increasing the activation of caspase-3 and downregulating anti-apoptotic proteins such as p-Akt and XIAP. Furthermore, PCSK9 inhibition may promote tumor cell apoptosis by interfering with mitochondrial signaling pathways. This interference is associated with an increased Bax/Bcl-2 ratio, leading to the elevated release of cytochrome-c. Abbreviations: ACLY, ATP citrate lyase; Akt, protein kinase B; BA, bempedoic acid; Bax, Bcl-2-associated X-protein; Bcl-2, B cell lymphoma-2; CETPi, cholesteryl ester transfer protein inhibitors; c-myc, cellular myc transcription factor; cyt-c, cytochrome-c; JNK, Jun N-terminal kinase; p-Akt, phosphorylated-Akt; PCSK9 i, proprotein convertase subtilisin/kexin type 9 inhibitor; PFK, phosphofructokinase; PI3K, phosphatidylinositol-3 kinase; SREBP1, sterol regulatory element-binding protein-1; Wnt, wingless-type MMTV integration site family; XIAP, X-linked inhibitor of apoptosis protein. Created with www.BioRender.com. (assessed on 14 July 2024).
5.3. Recombinant HDL-C Particles
Recombinant high-density lipoprotein (rHDL) represents a novel therapeutic approach designed to emulate the beneficial properties of natural HDL-C. Utilizing synthetic or modified apolipoproteins and phospholipids, rHDL effectively promotes reverse cholesterol transport, enhances lipid metabolism, and mitigates atherosclerosis. This innovative technology shows promise in treating cardiovascular diseases by targeting cholesterol efflux pathways. Moreover, rHDL has demonstrated compelling pleiotropic effects, including anti-inflammatory and potential anti-cancer properties [291].
5.3.1. Inflammation
Recent research has increasingly focused on exploring the potential anti-inflammatory benefits of HDL-C, particularly through engineered HDL analogs such as CER001. This synthetic HDL-C particle has shown significant promise in both animal models and clinical settings. In pigs, CER001 demonstrated enhanced median survival rates and substantial reductions in inflammation, complement activation, and endothelial impairment. These findings were further corroborated in humans, where administration of CER-001 led to improvements in ApoA1 levels, enhanced clearance of LPS, and modulation of immune responses. Notably, patients treated with CER-001 exhibited a decreased incidence of severe acute kidney injury (AKI), necessitating less organ support and shorter stays in the intensive care unit [292]. Similarly, the infusion of rHDL particles has shown promise in severe SARS-CoV-2 infections, which often deplete HDL-cholesterol levels and impair HDL-C function. Following rHDL administration, plasma levels of ApoA1 increased while HDL-C levels decreased, indicating efficient cholesterol transport. Proteomic analysis revealed elevated levels of ApoA1 and demonstrated a favorable impact on inflammation, with reductions observed in pro-inflammatory proteins, markers of inflammation, and cytokines [293]. Furthermore, despite not meeting its primary endpoint in the AEGIS-II trial, CSL-112, a formulation of human ApoA1, has shown potential atheroprotective effects through its observed anti-inflammatory properties in animal models and ex vivo studies [294,295].
5.3.2. Vascular Health
Research indicates that rHDL can significantly ameliorate endothelial function through various mechanisms. For instance, reconstituted HDL has been found to restore endothelial function in hypercholesterolemic men by enhancing eNOS activity and increasing NO production. Additionally, these HDL-C particles support endothelial repair by promoting the mobilization and functionality of endothelial progenitor cells, which is crucial for maintaining the integrity of the endothelial layer [296]. rHDL particles also exhibit antioxidative properties, reducing the oxidative modification of LDL and thereby protecting endothelial cells from oxidative stress [297]. Notably, rHDL particles may also positively impact thrombosis as they have been shown to inhibit microvascular and arterial thrombosis by preventing the self-association of von Willebrand factor (VWF), which is crucial for platelet adhesion and aggregation under high shear stress conditions [298].
5.3.3. Glucose Metabolism
rHDL particles have demonstrated potential in mitigating insulin resistance by influencing glucose metabolism through various mechanisms. These include enhancing insulin sensitivity and promoting glucose uptake in tissues independently of insulin. Research has shown that rHDL infusion improves insulin sensitivity in skeletal muscle, a key tissue affected by insulin resistance. Furthermore, HDL particles play a protective role for pancreatic beta cells, shielding them from stress and apoptosis, thereby preserving their function and insulin secretion [299].
5.3.4. Malignancy
In recent years, rHDL particles have gained attention in cancer therapy because of their unique properties and mechanisms that can be harnessed for drug delivery and other therapeutic effects. One notable approach involves synthetic HDL-like nanoparticles (NPs), which show potential for targeting cancer cells and delivering various therapeutic substances. These nanoparticles can be customized to transport small molecules, photothermal agents, and nucleic acids. Additionally, they can function independently as therapeutic agents and deliver imaging substances [300]. Interestingly, rHDL nanoparticles have the unique ability to encapsulate hydrophobic drugs within their core, significantly enhancing the solubility and stability of these drugs in the bloodstream. This targeted delivery mechanism is facilitated through the binding of ApoA1 to scavenger receptor class B type 1 (SR-B1) receptors on cells [301,302]. Tumors frequently exhibit elevated expression of SR-B1 to meet the increased cholesterol demands required for rapid cell proliferation. By exploiting this overexpression, rHDL nanoparticles can efficiently deliver anti-cancer drugs directly to cancer cells, thereby maximizing therapeutic efficacy [302]. Notably, HDL-mediated cholesterol efflux from cancer cells has been shown to inhibit the growth of pancreatic ductal adenocarcinoma (PDAC) cells and induce apoptosis, highlighting the potential of rHDL-based therapies in oncology [303].
In conclusion, while enhancing HDL-C levels has thus far yielded disappointing results in mitigating CVD risk, both niacin and novel agents such as CETP inhibitors and rHDL particles may still hold promise for improving other aspects of human health, such as inflammatory conditions, metabolic syndrome, and cancer. Further research is necessary to fully elucidate these potential benefits, as well as to assess the cost-effectiveness of administering these drugs.
6. Pleiotropic Benefits of Pharmaceutical Management of Hypertriglyceridemia
6.1. Fibrates
Fibrates are a class of amphipathic carboxylic acids primarily indicated for treating severe hypertriglyceridemia. Their main mechanism of action involves activating the PPAR-α. This activation leads to a substantial reduction in serum triglyceride levels by approximately 50%, a moderate reduction in LDL-C by up to 25%, and an increase in HDL-C by 5–15% [304]. Fenofibrate is the most commonly used fibrate, while bezafibrate and pemafibrate are less frequently prescribed. Beyond their lipid-modifying effects, fibrates exhibit notable pleiotropic properties, with anti-inflammatory features being particularly prominent. These characteristics contribute to their wide-ranging therapeutic benefits across various health conditions.
6.1.1. Inflammation
Fibrates are well-known for their anti-inflammatory properties, which are largely attributed to their lipid-lowering effects, regulation of intracellular lipid metabolism via PPAR-α activation [305], and modulation of specific intracellular signaling pathways [306]. These drugs exert indirect anti-inflammatory effects primarily through their impact on lipid metabolism, which includes lowering LDL-C and increasing HDL-C. This lipid modulation helps reduce cholesterol accumulation in tissues and slows the progression of atherosclerosis, thereby providing anti-inflammatory benefits [307]. Beyond these indirect effects, fibrates also demonstrate direct anti-inflammatory actions independent of their lipid-lowering capabilities [23]. The activation of PPAR-α plays a crucial role in this process by suppressing various inflammation-related intracellular pathways and downregulating genes associated with inflammation [308]. Additionally, fibrates improve mitochondrial function and promote the differentiation of T helper 17 (Th17) cells, contributing further to their anti-inflammatory features [309].
Fenofibrate is particularly noted for its anti-inflammatory potential. It has shown protective effects against osteoarthritis (OA) by preventing senescence, defective autophagy, and inflammation in human OA and aging primary chondrocytes [310]. These findings suggest that fenofibrate may support joint health and cartilage maintenance. However, the presence of diabetes mellitus can alter these effects. Studies on diabetic mice have revealed that fenofibrate may impair bone quality by reducing osteoblast activity, which leads to decreased expression of collagen I and osteocalcin due to downregulated Runx2 expression, a critical factor in bone formation [311].
Similarly, bezafibrate has also been reported to possess anti-inflammatory features in both acute and subacute inflammatory experimental models. Pemafibrate, another fibrate, has shown promise in the treatment of non-alcoholic steatohepatitis (NASH). Evidence suggests that pemafibrate can reduce the expression of the cell adhesion molecule VCAM-1 and favorably modulate inflammation- and fibrosis-related genes in a mouse model of NASH (STAM), highlighting its potential protective role against inflammation and fibrosis in this context [312].
6.1.2. Vascular Health
The impact of hypertriglyceridemia on endothelial function remains a subject of ongoing discussion [313]. While numerous investigations have established a correlation between elevated serum triglyceride levels and endothelial dysfunction, particularly in individuals with conditions such as coronary artery disease and metabolic syndrome [314,315], the precise relationship continues to be debated. Fenofibrate is believed to have benefits for endothelial function primarily through mechanisms involving a reduction in oxidative stress and an enhancement in endothelial nitric oxide synthase [316]. This notion is supported by similar findings regarding bezafibrate, which has been shown to increase NO production and enhance the transcription level and stability of endothelial nitric oxide synthase mRNA. These effects are attributed to both PPARα-dependent pathways and non-genomic effects mediated by MAPK and PI3K pathways [317].
Additionally, fibrates may offer protection against microvascular dysfunction by decreasing inflammation and apoptosis through AMPK activation, thus extending their benefits beyond lipid-lowering effects [318]. However, despite the potential vascular health benefits associated with fibrates, including overall antithrombotic properties, several studies have raised concerns about their potential to promote venous thromboembolism. The underlying mechanism behind this phenomenon remains unclear. The current explanation centers on the observed increase in serum homocysteine levels, although this alone is considered insufficient to fully account for the thrombotic predisposition associated with fibrates [319].
Mounting evidence indicates that fenofibrate may possess uric acid-lowering properties, potentially decreasing uric acid levels by up to 23% in individuals with gout who are also receiving urate-lowering therapy [320]. Uric acid absorption into endothelial cells via uric acid transporters can promote inflammation and oxidative stress, predisposing to endothelial dysfunction by reducing the bioavailability of endothelial NO. Therefore, the positive impact of fenofibrate on uric acid concentrations may indirectly ameliorate endothelial function [321].
The beneficial uric-lowering effects of fenofibrate have primarily been observed in patients with diabetes mellitus or hypertriglyceridemia. In a post-hoc analysis of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial, 9795 participants were randomly randomized to receive either fenofibrate or a placebo. The findings revealed that fenofibrate administration resulted in a 20% reduction in uric acid levels and reduced the incidence of first-on-study gout events by approximately 50% over a five-year period [322]. The uric acid-lowering effects of fenofibrate have also been demonstrated in non-diabetic subjects. Among 863 individuals with gout, fenofibrate reduced serum uric acid levels, with a more pronounced effect observed in those receiving glucocorticoids compared with other therapies. Importantly, there were no significant differences in serum blood urea nitrogen, creatinine, and aminotransferase levels between patients treated with and without fenofibrate [323].
6.1.3. Glucose Metabolism
While some studies report no significant impact of fibrates on insulin sensitivity, mounting evidence supports their potential benefits in improving glucose metabolism and insulin resistance, particularly through anti-inflammatory mechanisms. Increasing evidence suggests the potential benefits of fenofibrate in managing insulin resistance. Experimental studies indicate that fenofibrate may help maintain insulin sensitivity by reducing insulin clearance and enhancing insulin secretion [324]. Recent research by Lee et al. demonstrated the beneficial effects of fibrates on glucose metabolism in a model of high-fat diet (HFD)-fed obese ovariectomized mice, an experimental analog for obese postmenopausal women. This study found that fenofibrate administration improved mild hyperglycemia, severe hyperinsulinemia, and glucose tolerance. It also reduced CD68-positive macrophages in both the pancreas and visceral adipose tissue, alongside reductions in serum TNF-α levels [325].
A comprehensive meta-analysis comprising 22 randomized placebo-controlled clinical trials encompassing 11,402 subjects underscores the favorable impact of fibrates on glucose homeostasis. Notably, fibrates significantly reduce fasting serum glucose levels, insulin levels, and insulin resistance. However, their effects on HbA1c values appear to be non-significant [326]. Emerging evidence suggests that the activation of PPAR-α holds promise in mitigating the inflammatory cascade associated with conditions such as diabetes mellitus. By inhibiting TNF-α-induced IL-6 promoter activity and dampening the expression of NF-κB-responsive genes, PPAR-α activation exhibits potential therapeutic effects, even in pre-diabetic individuals [327]. Moreover, fenofibrate demonstrates multifaceted benefits in combating oxidative stress and neuroinflammation. It achieves this partly through its ability to modulate the expression of Nrf2 and attenuate the activation of the NLRP3 inflammasome, particularly in subjects with diabetic retinopathy [328].
6.1.4. Malignancy
Numerous studies have underscored fenofibrate’s potential in demonstrating anticancer effects across various malignancies through different cancer-related pathways. These effects may vary in their dependence on PPAR-α agonism, even within the same type of cancer [329]. Research indicates that fenofibrate can induce ROS generation, with preliminary data suggesting that fenofibrate might reduce the cytotoxic effects of cisplatin in lung cancer patients by boosting the antioxidant defense system, thus leveraging its antioxidant properties in combination therapy to lessen cisplatin’s harmful effects [330].
Moreover, fenofibrate appears to promote apoptosis through various molecular mechanisms, such as the inhibition of the TNF-α/NF-κB pathway and increased nuclear accumulation of forkhead box protein O3a (FOXO3a) [331,332]. FoxO3A is a critical transcription factor involved in essential functions such as cell cycle progression, proliferation, DNA damage response, and apoptosis [333]. Additionally, fenofibrate may reduce semaphorin 6B protein expression, which typically promotes tumor invasion and metastasis in breast cancer patients [334]. Studies also show that fenofibrate can decrease the development and metastatic potential of tumor cells by modulating key intracellular signaling pathways, such as the p38 MAPK and Akt pathways [335,336].
Recent experimental findings suggest that fenofibrate, along with bezafibrate, may enhance the efficacy of CD8+ T cells, thereby hindering tumor progression [337,338]. Bezafibrate has been demonstrated to inhibit tumor growth by inducing G1 cell cycle arrest through the regulation of CDK2 expression or activity [339]. In a recent study involving 753 subjects with CAD and cancer, monitored over a median period of 22.5 years, the cumulative incidence of malignancy was lower in the bezafibrate group compared with the placebo group (23.9% vs. 27.2%). The benefits of bezafibrate were seen across all types of malignancies except non-small cell lung cancer and were associated with a decrease in fibrinogen levels [340]. Figure 3 presents a schematic overview of the primary pleiotropic effects of fibrates, as evidenced by various preclinical and clinical studies.
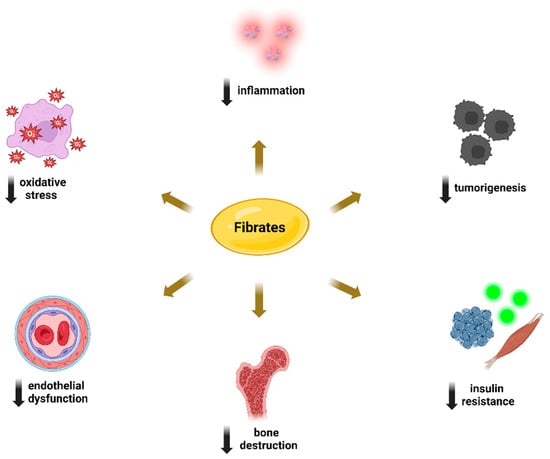
Figure 3.
Schematic presentation of the potential pleiotropic benefits associated with fibrate prescription. Created with www.BioRender.com. (assessed on 14 July 2024).
6.2. Omega-3 Fatty Acids
Omega-3 polyunsaturated fatty acids (PUFAs), typically administered at doses of 2 to 4 g daily, are well-known for their ability to lower triglyceride levels by 25–40%, with additional cardiovascular benefits beyond this effect [341]. These essential fatty acids include α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). In recent years, there has been substantial debate regarding the cardiovascular effects of omega-3 fatty acid supplementation [342]. Studies such as Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial (REDUCE-IT) and Study to Assess Statin Residual Risk With Epanova in High Cardiovascular Risk Patients With Hypertriglyceridemia (STRENGTH) have contributed significantly to this discussion with divergent findings. REDUCE-IT, utilizing 4 g/day of icosapent ethyl (IPE), a high-purity form of EPA ethyl ester, demonstrated a significant 25% reduction in primary cardiovascular events compared with placebo in high-risk patients already receiving statin therapy [343]. In contrast, the STRENGTH trial, which administered 4 g/day of a carboxylic acid formulation containing EPA and DHA, did not show a reduction in major adverse cardiovascular events among statin-treated individuals at high cardiovascular risk, prompting early termination of the trial due to a lack of efficacy [344]. Nevertheless, accumulating evidence underscores the importance of both EPA and DHA in reducing inflammation, resolving inflammatory processes, regulating glucose metabolism, and managing insulin secretion. These properties suggest potential therapeutic benefits for conditions such as inflammation-related disorders, diabetes, and possibly even certain cancers [345].
6.2.1. Inflammation
The anti-inflammatory mechanisms of omega-3 PUFAs encompass several key processes, including alterations in cell membrane phospholipid fatty acid composition, disruption of lipid rafts, improvements in mitochondrial dysfunction [346], and inhibition of the pro-inflammatory transcription factor NF-κB. This inhibition reduces the expression of inflammatory genes and helps regulate NLPR3 activity [347]. Additionally, omega-3 PUFAs activate the anti-inflammatory transcription factor NR1C3 and bind to the G protein-coupled receptor GPR120 [348]. In addition to their anti-inflammatory effects, omega-3 fatty acids demonstrate antioxidant properties, which are particularly beneficial during the third trimester of pregnancy. Evidence indicates that supplementation during this period lowers concentrations of 8-iso-PGF2α and its metabolite, reflecting reduced oxidative stress [349]. Notably, a meta-analysis conducted by Heshmati et al. underscored omega-3 fatty acids’ role in enhancing antioxidant defenses against ROS. Their findings revealed significant improvements in malondialdehyde (MDA) levels, total antioxidant capacity (TAC), and GPx activity [350].
Omega-3 PUFAs have demonstrated substantial potential in preventing and managing chronic inflammatory disorders due to their notable anti-inflammatory properties. These features make omega-3 PUFAs a viable, effective, and safe option for managing such conditions. In migraine prevention, omega-3 PUFAs exhibit promise owing to their anti-inflammatory, anti-nociceptive, antioxidant, and neuromodulatory effects. They are crucial in regulating neurobiological pathways involved in migraine pathophysiology, such as oxidative stress, mitochondrial dysfunction, and NLRP3 inflammasome activation. Notably, a higher intake of omega-3 fatty acids can help reduce oxidative stress and neuroinflammation by facilitating the production of resolvins, neuroprotectins, and maresins. Despite mixed clinical trial outcomes on their efficacy in migraine management, their safety profile and potential benefits make omega-3 PUFAs a compelling candidate for migraine prophylaxis [351].
Additionally, omega-3 PUFAs have shown a significant impact on osteoarthritis management and progression, likely because of their capacity to reduce pro-inflammatory cytokine cascades, alleviate cartilage destruction, and produce oxylipins that promote anti-inflammatory pathways [352]. Furthermore, omega-3 PUFAs may offer therapeutic advantages for IBD subjects by mediating anti-inflammatory effects, impacting the NLRP3 inflammasome, and positively influencing the gut microbiota. This includes promoting the growth of short-chain fatty acid-producing bacteria and enhancing the mucosal gut barrier, which aids in maintaining gut homeostasis and overall health [353].
6.2.2. Vascular Health
Several studies have demonstrated the benefits of omega-3 PUFA prescription on vascular health. Omega-3 PUFAs positively impact endothelial function through several mechanisms. Specifically, they enhance the production of NO by increasing the activity and expression of eNOS. Omega-3 PUFAs also reduce oxidative stress by decreasing the production of ROS and upregulating antioxidant defenses, thereby protecting NO bioavailability. Furthermore, they inhibit the expression of pro-inflammatory cytokines and adhesion molecules, reducing endothelial inflammation and improving vascular health [354]. Notably, this beneficial impact on endothelial function has been demonstrated in individuals with diabetes mellitus, obesity, and metabolic syndrome [355,356]. A recent meta-analysis by Arabi et al. confirmed the favorable effect of omega-3 PUFAs on endothelial function, as estimated by flow-mediated dilatation of the brachial artery [357].
Mounting evidence highlights the anti-thrombotic properties of omega-3 fatty FAs in both venous and arterial systems. Concerning their potential anti-thrombotic effects on venous thromboembolism (VTE), omega-3 FAs appear to reduce the risk of pulmonary embolism and symptomatic deep vein thrombosis, especially post-surgery in elderly patients with proximal femoral fractures [358]. Furthermore, they may produce favorable outcomes in VTE recurrence, as demonstrated in a multicenter study involving 826 elderly individuals with a history of VTE, where higher levels of omega-3 FAs were associated with a lower risk of recurrent VTE and overall mortality [359]. Notably, in both studies, the bleeding risk following omega-3 FA prescription was not elevated. Additionally, omega-3 PUFAs are essential components of the platelet phospholipid membrane, playing a pivotal role in regulating platelet function. Omega-3 FA supplementation may alter platelet membrane phospholipid composition and affect platelet function, potentially influencing the progression and thrombotic complications of cardiovascular disease [360]. Although evidence suggests that omega-3 FAs may predispose individuals to atrial fibrillation, recent data indicate that their prescription can reduce the risk of ischemic stroke in individuals with atrial fibrillation, likely due to their anti-thrombotic effects [361].
6.2.3. Glucose Metabolism
Omega-3 PUFAs are noted for their beneficial effects in combating insulin resistance, primarily by enhancing mitochondrial function and reducing endoplasmic reticulum (ER) stress, which collectively protect against its onset [362]. EPA activates AMPK and increases mitochondrial carnitine palmitoyltransferase 1 (CPT-1) expression, promoting fatty acid oxidation and preventing lipid accumulation and subsequent lipotoxicity, critical contributors to insulin resistance [363]. Omega-3 PUFAs also influence glucose metabolism potentially through modulation of the NLRP3 inflammasome, mitigating ROS production and mitochondrial impairment, thereby regulating insulin resistance progression via Ca2+ fluxes and ER stress [364]. A high EPA/arachidonic acid (AA) ratio correlates with improved glycemic control and reduced inflammation. Conversely, linoleic acid may also modestly decrease the incidence of T2DM, although whether this effect is due to reduced AA production or linoleic acid’s intrinsic properties remains unclear [365].
6.2.4. Malignancy
Extensive research has explored the potential anti-cancer benefits of omega-3 PUFAs. Evidence indicates that omega-3 PUFAs can inhibit cancer cell growth, promote apoptosis, and reduce inflammation, a key factor in cancer progression [366]. Epidemiological and clinical studies have suggested a correlation between higher omega-3 PUFA intake and a decreased risk of cancers such as breast, colorectal, and prostate cancer [367,368,369]. At the molecular level, omega-3 PUFAs are thought to modify the lipid composition of cell membranes, thereby influencing signaling pathways and gene expression involved in cancer development. For instance, they may impact the function of nuclear receptors such as PPARs and decrease the expression of inflammatory cytokines [370]. Interestingly, both preclinical and clinical evidence supports the idea that omega-3 PUFAs could benefit cancer-related complications, including anorexia-cachexia syndrome and chemotherapy-induced pain [371]. Overall, while the precise mechanisms are still under investigation, and some studies present conflicting results, the cumulative evidence supports the beneficial role of omega-3 PUFAs in both cancer prevention and therapy.
6.3. Volanesorsen
Volanesorsen, an antisense oligonucleotide targeting APOCIII mRNA, has recently emerged as a promising treatment for refractory or severe hypertriglyceridemia, including patients with familial chylomicronemia syndrome. ApoCIII inhibits LPL and hepatic lipase, thereby reducing the uptake of triglyceride-rich particles by the liver. One of the primary side effects of volanesorsen is thrombocytopenia [372]. ApoCIII is widely recognized as a direct inhibitor of LPL. However, the efficacy of volanesorsen in patients with severe LPL impairment suggests the existence of additional LPL-independent mechanisms [373]. Beyond its role in lipid metabolism, ApoCIII is deeply involved in the atherogenic process. It influences various steps, such as monocyte adhesion to endothelial cells and the proliferation of smooth muscle cells, which contributes to oxidative stress and vascular inflammation [372].
Recent evidence has highlighted the anti-inflammatory potential of reducing ApoCIII levels. Statin therapy, which lowers ApoCIII, has been shown to decrease vascular adhesiveness by downregulating VCAM-1, suggesting a therapeutic angle for managing vascular inflammation through ApoCIII modulation. This effect is particularly significant as it offers pathways to combat atherosclerosis beyond traditional lipid-lowering strategies [374]. Volanesorsen may also indirectly reduce inflammation by lowering apolipoprotein B48 (ApoB48) levels, markers of triglyceride-rich lipoprotein (TRL) remnants. These remnants can penetrate the arterial wall, be internalized by macrophages, and foster foam cell formation, thereby facilitating inflammation [375].
The role of ApoCIII in inflammation extends to CKD, where elevated ApoCIII levels are common. In CKD, ApoCIII exacerbates tissue damage by increasing monocyte infiltration in the kidneys and activating the NLRP3 inflammasome in human monocytes. This inflammasome activation involves caspase-8 and the dimerization of Toll-like receptors 2 and 4, illustrating a complex interplay between lipid metabolism and immune responses in CKD [376,377]. Notably, the ability of ApoCIII to activate CD14+-monocytes to express TF, the primary initiator of the blood coagulation cascade, has been suggested as a potential underlying mechanism of the antithrombotic properties of ApoCIII inhibitors [378]. Additionally, volanesorsen has shown promise in improving glucose profiles in individuals with T2DM, as it has been reported to enhance glucose disposal, insulin resistance, and hepatic steatosis in these subjects [379,380]. Although evidence regarding the potential anti-cancer role of volanesorsen is limited, Mendelian randomization data reveal that ApoCIII silencing affects lung cancer risk via sphingolipid metabolism [381]. In the coming years, research is expected to illuminate the potential pleiotropic features of two novel ApoCIII inhibitors, namely olezarsen and ARO-ApoCIII [382].
The multifaceted pharmacological actions of agents used to manage hypertriglyceridemia highlight their broad utility in treating various health conditions beyond lipid disorders. They serve as valuable components of therapeutic strategies aimed at addressing complex metabolic and inflammatory disorders.
7. Conclusions
Cardiovascular disease remains a leading cause of morbidity and mortality worldwide, with dyslipidemia recognized as a significant risk factor for its development. Over the years, a wide range of hypolipidemic drugs has emerged, offering substantial benefits in both the primary and secondary prevention of CVD. The positive impact of lipid-lowering therapy on reducing cardiovascular burden is primarily due to its ability to alter patients’ lipid profiles favorably. However, extensive preclinical and clinical data reveal that hypolipidemic therapy can also exhibit pleiotropic properties, which may have important implications for reducing cardiovascular risk and potentially extending beyond it. Notably, the anti-inflammatory effects of lipid-lowering drugs are well-documented, with numerous studies highlighting their beneficial impact on various chronic inflammatory disorders. Additionally, lipid-lowering therapy has shown promise in the treatment of neoplastic diseases.
Interestingly, the pleiotropic actions of hypolipidemic drugs extend to agents traditionally limited by their side effects, such as lomitapide, and interventions whose effects on CVD risk remain controversial, such as CETP inhibitors and lipoprotein apheresis. Conversely, existing data on the impact of lipid-lowering drugs on vascular health and glucose metabolism are somewhat conflicting. Some agents have shown promise in enhancing insulin sensitivity, while others have demonstrated potentially harmful effects. Despite these complexities, lipid-modifying therapy has revolutionized the treatment of CVD, significantly contributing to increased life expectancy. The established and potential pleiotropic actions of these drugs may offer additional health benefits. Future research is expected to elucidate these pleiotropic effects further, providing new perspectives on their therapeutic use.
Author Contributions
D.K. and N.G.V. conceived the idea of the review, organized its plan, and reviewed the study; D.K. wrote major parts of the manuscript; N.G.V., I.K., T.S. and E.R. wrote minor parts of the manuscript; M.K., V.S. and E.T. performed the literature search and prepared the tables; D.K., I.K. and E.R. made the figures and prepared all references; N.T., I.M. and M.D. reviewed, edited, and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
AA, arachidonic acid; Aβ, amyloid-beta; ABCA1, ATP binding cassette transporter A1; ABCG1, ATP binding cassette transporter G1; ACLY, ATP citrate lyase; AD, Alzheimer’s disease; AKI, acute kidney injury; Akt, protein kinase B; ALA, α-linolenic acid; AMD, age-related macular degeneration; AMPK, Adenosine monophosphate-activated protein kinase; ANGPTL3, angiopoietin-like 3 protein; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB48, apolipoprotein B48; ApoB100, apolipoprotein B100; ApoC1, apolipoprotein C1; ApoCIII, apolipoprotein CIII; ApoE4, apolipoprotein E4; ASCVD, atherosclerotic cardiovascular disease; AS, ankylosing spondylitis;AT-1R, Ang II receptor 1; ATOR, atorvastatin; BA, bempedoic acid; Bax, Bcl-2-associated X-protein; Bcl-2, B cell lymphoma-2; Bcl-xl, B-cell lymphoma-extra-large; CAD, coronary artery disease; CAP1, cyclase associated actin cytoskeleton regulatory protein 1; CD, Crohn’s disease; CETP, cholesteryl ester transfer protein; CKD, chronic kidney disease; c-myc, cellular myc transcription factor; CoQ10, coenzyme Q10; CPT-1, carnitine palmitoyltransferase 1; CRC, colorectal cancer; CRP, c-reactive protein; CVD, cardiovascular disease; cyt-c, cytochrome-c; DALI, direct adsorption of lipoproteins; DHA, docosahexaenoic acid; DRP-1, dynamin-related protein 1; EL, endothelial lipase; eNOS, endothelial nitric oxide synthase; EPA, eicosapentaenoic acid; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; EV, extracellular vesicles; EVA, evacetrapib; FAs, fatty acids; FDA, Food and Drug Administration; FH, familial hypercholesterolemia; FIELD, Event Lowering in Diabetes; FLD, fibrinogen-like domain; FMD, flow-mediated dilation; FORD, free oxygen radicals defense; FORT, free oxygen radicals test; FOXO3a, forkhead box protein O3a; GGPP, geranylgeranyl pyrophosphate; GLUT4, glucose transporter type 4; G6Pase, glucose-6-phosphatase; GPx, glutathione peroxidase; HCA2, hydroxycarboxylic acid receptor 2; HCT116, human colorectal carcinoma cell line; HDL-C, high-density lipoprotein cholesterol; HMVEC, human microvascular endothelial cell; HGSC, high-grade serous ovarian cancer; HFD, high-fat diet; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; HNSCC, head and neck squamous cell carcinoma; HoFH, homozygous familial hypercholesterolemia; HO-1, heme oxygenase-1; HOMA-IR, Homeostasis Model Assessment-Insulin Resistance; hs-CRP, high sensitivity- C-reactive protein; IBD, inflammatory bowel disease; ICAM-1, intercellular adhesion molecule-1; IHD, ischemic heart disease; IL, interleukin; IPE, icosapent ethyl; JNK, Jun N-terminal kinase; LA, lipoprotein apheresis; LDL-C, low-density lipoprotein cholesterol; LDL-R, low-density lipoprotein cholesterol receptor; LncRNA, long noncoding RNA; LOF, loss-of-function; Lp(a), lipoprotein(a); LPL, lipoprotein lipase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; MONET, membrane filtration optimized novel extracorporeal treatment; MPO, myeloperoxidase; mTOR, mammalian target of rapamycin; mTORC2, mammalian target of rapamycin complex 2; MTP, microsomal triglyceride transfer protein; NAD+, nicotinamide adenine dinucleotide; NADPH, Nicotinamide Adenine Dinucleotide Phosphate; NASH, non-alcoholic steatohepatitis; NEXN, nexilin F-actin binding protein; NEXN-AS1, nexilin F-actin binding protein antisense 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer; NLRP3, NLR family pyrin domain containing 3; NO, nitric oxide; NODM, new-onset diabetes mellitus; NOX2, NADPH oxidase 2; NPC1L1, Niemann-Pick C1-like protein 1; Nrf2, nuclear factor erythroid 2–related factor 2; NPs, nanoparticles; OA, osteoarthritis; OR, odds ratio; OxLDL, Oxidized LDL; PAI-1, plasminogen activator inhibitor-1; p-Akt, phosphorylated-Akt; PARP, poly-ADP ribose polymerase; p-β-catenin, phosphorylated β-catenin; PCSK9, proprotein convertase subtilisin/kexin type 9; PCSK9 i, proprotein convertase subtilisin/kexin type 9 inhibitor; PCSK9-mAbs, PCSK9 monoclonal antibodies; PD, Parkinson’s disease; PDAC, pancreatic ductal adenocarcinoma; PEPCK, phosphoenolpyruvate carboxykinase; PFK, phosphofructokinase; PI3K/Akt, phosphatidylinositol 3-kinase/protein kinase B; PIGF, placental growth factor; p-JNK, phosphorylated Jun N-terminal kinase; PPARa, peroxisome proliferator-activated receptor alpha; p-STAT3, phospho-STAT3; PUFAs, omega-3 polyunsaturated fatty acids; PXR, pregnane X receptor; RCT, randomized controlled trial; REDUCE-IT, Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial; rHDL, recombinant HDL; ROS, reactive oxygen species; SD, standard deviation; sEVs, small extracellular vesicles; SiRNA, small interfering RNA; SOD, superoxide dismutase; SP600125, anthrapyrazolone inhibitor of Jun N-terminal kinase; SR-B1, scavenger receptor class B type 1 receptor; SREBP1, sterol regulatory element-binding protein-1; STAM, mouse model of NASH; STAT6, signal transducer and activator of transcription 6; STRENGTH, Study to Assess Statin Residual Risk With Epanova in High Cardiovascular Risk Patients With Hypertriglyceridemia; TAC, total antioxidant capacity; T2DM, type 2 diabetes mellitus; TF, tissue factor; TG, triglyceride; Th, T helpers; THP-1, human leukemia monocytic cell line; TLR, toll-like receptor; TNF-α, tumor necrosis factor alpha; TOR, torcetrapib; Tregs, regulatory T cells; TRL, triglyceride-rich lipoprotein; TXNIP, Thioredoxin-interacting protein; UC, ulcerative colitis; VEGF, vascular endothelial growth factor; VLDL, very low-density lipoprotein cholesterol; VRI, vascular reactivity index; Wnt, wingless-type MMTV integration site family; VTE, venous thromboembolism; XIAP, X-linked inhibitor of apoptosis protein.
References
- Liu, T.; Zhao, D.; Qi, Y. Global Trends in the Epidemiology and Management of Dyslipidemia. J. Clin. Med. 2022, 11, 6377. [Google Scholar] [CrossRef] [PubMed]
- Jaam, M.; Al-Naimi, H.N.; Haddad, M.M.; Abushanab, D.; Al-Badriyeh, D. Comparative efficacy and safety among high-intensity statins. Systematic Review and Meta-Analysis. J. Comp. Eff. Res. 2023, 12, e220163. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Kotani, K.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Jones, S.R.; Ray, K.K.; Blaha, M.J.; Rysz, J.; Toth, P.P.; et al. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Statin therapy reduces plasma endothelin-1 concentrations: A meta-analysis of 15 randomized controlled trials. Atherosclerosis 2015, 241, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Phan, B.A.; Dayspring, T.D.; Toth, P.P. Ezetimibe therapy: Mechanism of action and clinical update. Vasc. Health Risk Manag. 2012, 8, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.H. Expanding the therapeutic landscape: Ezetimibe as non-statin therapy for dyslipidemia. Korean J. Intern. Med. 2023, 38, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Fernandes Silva, L. Statins and risk of type 2 diabetes: Mechanism and clinical implications. Front. Endocrinol. 2023, 14, 1239335. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, N.; Li, H.F.; Zhang, C.J.; Gong, Y.Z.; Liao, D.F.; Qin, L. Ezetimibe and Cancer: Is There a Connection? Front. Pharmacol. 2022, 13, 831657. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, Q.; Chi, J.; Hong, Z.; Liao, Y.; Zhang, S.; et al. PCSK9 Inhibition: From Current Advances to Evolving Future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef]
- Ruscica, M.; Sirtori, C.R.; Carugo, S.; Banach, M.; Corsini, A. Bempedoic Acid: For Whom and When. Curr. Atheroscler. Rep. 2022, 24, 791–801. [Google Scholar] [CrossRef]
- Goldman, A.; Raschi, E.; Cukierman-Yaffe, T.; Dankner, R.; Shouval, R.; Shechter, M.; Ben-Zvi, I.; Gerstein, H.C.; Maor, E. Hyperglycaemic disorders associated with PCSK9 inhibitors: A real-world, pharmacovigilance study. Eur. J. Prev. Cardiol. 2022, 29, 1334–1342. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Z.; Xie, J.; Xiao, S.; Li, W.; Liu, N. Efficacy and safety of PCSK9 inhibitors in patients with diabetes: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A.; Banach, M.; Catapano, A.L.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Mancini, G.B.J.; Ray, K.K.; Hanselman, J.C.; Ye, Z.; et al. Bempedoic acid in patients with type 2 diabetes mellitus, prediabetes, and normoglycaemia: A post hoc analysis of efficacy and glycaemic control using pooled data from phase 3 clinical trials. Diabetes Obes. Metab. 2022, 24, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Chambergo-Michilot, D.; Alur, A.; Kulkarni, S.; Agarwala, A. Mipomersen in Familial Hypercholesterolemia: An Update on Health-Related Quality of Life and Patient-Reported Outcomes. Vasc. Health Risk Manag. 2022, 18, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Larrey, D.; D’Erasmo, L.; O’Brien, S.; Arca, M. Italian Working Group on Lomitapide. Long-term hepatic safety of lomitapide in homozygous familial hypercholesterolaemia. Liver. Int. 2023, 43, 413–423. [Google Scholar] [CrossRef]
- Waksman, R.; Merdler, I.; Case, B.C.; Waksman, O.; Porto, I. Targeting inflammation in atherosclerosis: Overview, strategy and directions. EuroIntervention 2024, 20, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.C.; Mintah, I.J.; Alexa-Braun, C.A.; Shihanian, L.M.; Lee, J.S.; Banerjee, P.; Hamon, S.C.; Kim, H.I.; Cohen, J.C.; Hobbs, H.H.; et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J. Lipid Res. 2020, 61, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Piro, G.; Merz, V.; Simionato, F.; Santoro, R.; Zecchetto, C.; Tortora, G.; Melisi, D. Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer. Int. J. Mol. Sci. 2018, 19, 431. [Google Scholar] [CrossRef] [PubMed]
- Taylan, C.; Weber, L.T. An update on lipid apheresis for familial hypercholesterolemia. Pediatr. Nephrol. 2023, 38, 371–382. [Google Scholar] [CrossRef]
- Víšek, J.; Bláha, M.; Bláha, V.; Lášticová, M.; Lánska, M.; Andrýs, C.; Tebbens, J.D.; Igreja, E.; Sá, I.C.; Tripská, K.; et al. Monitoring of up to 15 years effects of lipoprotein apheresis on lipids, biomarkers of inflammation, and soluble endoglin in familial hypercholesterolemia patients. Orphanet. J. Rare Dis. 2021, 16, 110. [Google Scholar] [CrossRef]
- Rehman, W.U.; Yarkoni, M.; Ilyas, M.A.; Athar, F.; Javaid, M.; Ehsan, M.; Khalid, M.T.; Pasha, A.; Selma, A.B.; Yarkoni, A.; et al. Cholesteryl Ester Transfer Protein Inhibitors and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2024, 11, 152. [Google Scholar] [CrossRef]
- Cho, K.H. Synthesis of reconstituted high density lipoprotein (rHDL) containing apoA-I and apoC-III: The functional role of apoC-III in rHDL. Mol. Cells 2009, 27, 291–297. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Hey, S.P.; Ramirez, C.L.; Kesselheim, A.S. Assessment of the Role of Niacin in Managing Cardiovascular Disease Outcomes: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e192224. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Hua, H.; Ji, Y.; Jia, Z.; Peng, M.; Huang, S. Anti-inflammatory role of fenofibrate in treating diseases. Biomol. Biomed. 2023, 23, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, W.; Wang, Y.; Li, T.; Chen, Y.; Long, C.; Wen, Q.; Wu, Y.; Chen, Q. The effect of omega-3 fatty acids and its combination with statins on lipid profile in patients with hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 1039056. [Google Scholar] [CrossRef] [PubMed]
- Esan, O.; Wierzbicki, A.S. Volanesorsen in the Treatment of Familial Chylomicronemia Syndrome or Hypertriglyceridaemia: Design, Development and Place in Therapy. Drug Des. Devel. Ther. 2020, 14, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, N.; Hong, Z. Potential Benefits and Risks Associated with the Use of Statins. Pharmaceutics 2024, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; Escobar, C. Rosuvastatin-Based Lipid-Lowering Therapy for the Control of LDL Cholesterol in Patients at High Vascular Risk. J. Clin. Med. 2024, 13, 1894. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, C.; Brugnoni, R.; Bonanno, S.; Andreetta, F.; Salerno, F.; Canioni, E.; Vattemi, G.N.A.; Tonin, P.; Mantegazza, R.; Maggi, L. Toll-like receptors and IL-7 as potential biomarkers for immune-mediated necrotizing myopathies. Eur. J. Immunol. 2023, 53, e2250326. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Fereidouni, M.; Barreto, G.; Sahebkar, A. NLRP3 inflammasome as a treatment target in atherosclerosis: A focus on statin therapy. Int. Immunopharmacol. 2019, 73, 146–155. [Google Scholar] [CrossRef]
- Kureishi, Y.; Luo, Z.; Shiojima, I.; Bialik, A.; Fulton, D.; Lefer, D.J.; Sessa, W.C.; Walsh, K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000, 6, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Mangoni, A.A. A Systematic Review and Meta-Analysis of the Effect of Statins on Glutathione Peroxidase, Superoxide Dismutase, and Catalase. Antioxidants 2021, 10, 1841. [Google Scholar] [CrossRef] [PubMed]
- Gelosa, P.; Cimino, M.; Pignieri, A.; Tremoli, E.; Guerrini, U.; Sironi, L. The role of HMG-CoA reductase inhibition in endothelial dysfunction and inflammation. Vasc. Health Risk Manag. 2007, 3, 567–577. [Google Scholar] [PubMed]
- Alkakhan, W.; Farrar, N.; Sikora, V.; Emecen-Huja, P.; Huja, S.S.; Yilmaz, Ö.; Pandruvada, S.N. Statins Modulate Microenvironmental Cues Driving Macrophage Polarization in Simulated Periodontal Inflammation. Cells 2023, 12, 1961. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Y.; Peng, L.; Rong, X.; Liu, Y.; Li, J.; Luo, J. Ferroptosis in cardiovascular diseases: Role and mechanism. Cell Biosci. 2023, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Wu, S.G.; Chen, F.; Wu, Q.; Wu, C.M.; Kang, C.M.; He, X.; Zhang, R.Y.; Lu, Z.F.; Li, X.H.; et al. Atorvastatin inhibits pyroptosis through the lncRNA NEXN-AS1/NEXN pathway in human vascular endothelial cells. Atherosclerosis 2020, 293, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Asher, J.; Houston, M. Statins and C-reactive protein levels. J. Clin. Hypertens. 2007, 9, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Kandelouei, T.; Abbasifard, M.; Imani, D.; Aslani, S.; Razi, B.; Fasihi, M.; Shafiekhani, S.; Mohammadi, K.; Jamialahmadi, T.; Reiner, Ž.; et al. Effect of Statins on Serum level of hs-CRP and CRP in Patients with Cardiovascular Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mediat. Inflamm. 2022, 2022, 8732360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Tian, W.; Wang, T.; Jia, J.; Lai, R.; Wang, T.; Zhang, Z.; Song, L.; Ju, J.; et al. The effect of various types and doses of statins on C-reactive protein levels in patients with dyslipidemia or coronary heart disease: A systematic review and network meta-analysis. Front. Cardiovasc. Med. 2022, 9, 936817. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, X.; Wang, R.; Han, L.; Li, H.; Zhao, W. Mechanisms of 3-Hydroxyl 3-Methylglutaryl CoA Reductase in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 25, 170. [Google Scholar] [CrossRef]
- Lu, D.; Shen, L.; Mai, H.; Zang, J.; Liu, Y.; Tsang, C.K.; Li, K.; Xu, A. HMG-CoA Reductase Inhibitors Attenuate Neuronal Damage by Suppressing Oxygen Glucose Deprivation-Induced Activated Microglial Cells. Neural Plast. 2019, 2019, 7675496. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Lin, C.L.; Huang, C.N. Neuroprotective effects of statins against amyloid β-induced neurotoxicity. Neural Regen. Res. 2018, 13, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Papadakis, M.; Alsayegh, A.A.; Almohmadi, N.H.; Saad, H.M.; Batiha, G.E. Pros and cons for statins use and risk of Parkinson’s disease: An updated perspective. Pharmacol. Res. Perspect. 2023, 11, e01063. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Qiu, Y.; Wu, L.; Wang, H.; Wu, L.; Zhao, L.; Xie, D. Statins Have an Anti-Inflammation in CKD Patients: A Meta-Analysis of Randomized Trials. BioMed Res. Int. 2022, 2022, 4842699. [Google Scholar] [CrossRef] [PubMed]
- Peppas, S.; Piovani, D.; Peyrin-Biroulet, L.; Danese, S.; Bonovas, S. Statins and inflammatory bowel disease: Where do we stand? Eur. J. Intern. Med. 2020, 75, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.; Khalili, H.; Sachs, M.C.; Chan, A.T.; Olén, O.; Ludvigsson, J.F. Association Between Statin Use and Inflammatory Bowel Diseases: Results from a Swedish, Nationwide, Population-based Case-control Study. J. Crohns Colitis 2021, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, X.; Lei, T.; Zhang, K.; Lai, B.; Zhang, Z.; Guan, Y.; Mao, G.; Xiao, L.; Wang, N. Statins Attenuate Activation of the NLRP3 Inflammasome by Oxidized LDL or TNFα in Vascular Endothelial Cells through a PXR-Dependent Mechanism. Mol. Pharmacol. 2017, 92, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, P.O.; Chen, T. PXR: More Than Just a Master Xenobiotic Receptor. Mol. Pharmacol. 2018, 93, 119–127. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-Induced Nitric Oxide Signaling: Mechanisms and Therapeutic Implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef]
- Margaritis, M.; Channon, K.M.; Antoniades, C. Statins as regulators of redox state in the vascular endothelium: Beyond lipid lowering. Antioxid. Redox Signal. 2014, 20, 1198–1215. [Google Scholar] [CrossRef]
- Rossoni, L.V.; Wareing, M.; Wenceslau, C.F.; Al-Abri, M.; Cobb, C.; Austin, C. Acute simvastatin increases endothelial nitric oxide synthase phosphorylation via AMP-activated protein kinase and reduces contractility of isolated rat mesenteric resistance arteries. Clin. Sci. 2011, 121, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.H.; Köhler, T.; Rückschloss, U.; Just, I.; Hecker, M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Hamdulay, S.S.; Kinderlerer, A.R.; Boyle, J.J.; Lidington, E.A.; Yamaguchi, T.; Soares, M.P.; Haskard, D.O.; Randi, A.M.; Mason, J.C. Statin-mediated cytoprotection of human vascular endothelial cells: A role for Kruppel-like factor 2-dependent induction of heme oxygenase-1. J. Thromb. Haemost. 2007, 5, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Calvieri, C.; Ferro, D.; Pignatelli, P. Statins as antithrombotic drugs. Circulation 2013, 127, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Kozai, T.; Cosentino, F.; Joch, H.; Lüscher, T.F. Statin prevents tissue factor expression in human endothelial cells: Role of Rho/Rho-kinase and Akt pathways. Circulation 2002, 105, 1756–1759. [Google Scholar] [CrossRef] [PubMed]
- Hölschermann, H.; Schuster, D.; Parviz, B.; Haberbosch, W.; Tillmanns, H.; Muth, H. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 2006, 185, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Seidu, S.; Khunti, K. Statins and primary prevention of venous thromboembolism: A systematic review and meta-analysis. Lancet Haematol. 2017, 4, e83–e93. [Google Scholar] [CrossRef]
- Liu, C.; Shen, M.; Tan, W.L.W.; Chen, I.Y.; Liu, Y.; Yu, X.; Yang, H.; Zhang, A.; Liu, Y.; Zhao, M.T.; et al. Statins improve endothelial function via suppression of epigenetic-driven EndMT. Nat. Cardiovasc. Res. 2023, 2, 467–485. [Google Scholar] [CrossRef]
- Paseban, M.; Butler, A.E.; Sahebkar, A. Mechanisms of statin-induced new-onset diabetes. J. Cell. Physiol. 2019, 234, 12551–12561. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Jebari, S.; Larrea-Sebal, A.; Uribe, K.B.; Siddiqi, H.; Ostolaza, H.; Benito-Vicente, A.; Martín, C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 4725. [Google Scholar] [CrossRef]
- Sanvee, G.M.; Panajatovic, M.; Bouitbir, D.J.; Krähenbühl, D. Statins and Insulin Resistance. Eur. Cardiol. 2020, 15, e44. [Google Scholar] [CrossRef]
- Katsiki, N.; Mantzoros, C.S. Statins in relation to adiponectin: A significant association with clinical implications. Atherosclerosis 2016, 253, 270–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henriksbo, B.D.; Tamrakar, A.K.; Phulka, J.S.; Barra, N.G.; Schertzer, J.D. Statins activate the NLRP3 inflammasome and impair insulin signaling via p38 and mTOR. Am. J. Physiol. Endocrinol. Metab. 2020, 319, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef]
- Craig, E.L.; Stopsack, K.H.; Evergren, E.; Penn, L.Z.; Freedland, S.J.; Hamilton, R.J.; Allott, E.H. Statins and prostate cancer-hype or hope? The epidemiological perspective. Prostate Cancer Prostatic Dis. 2022, 25, 641–649. [Google Scholar] [CrossRef]
- Okita, Y.; Sobue, T.; Zha, L.; Kitamura, T.; Iwasaki, M.; Inoue, M.; Yamaji, T.; Tsugane, S.; Sawada, N. Long-term use of anti-cholesterol drugs and cancer risks in a Japanese population. Sci. Rep. 2024, 14, 2896. [Google Scholar] [CrossRef]
- Haukka, J.; Sankila, R.; Klaukka, T.; Lonnqvist, J.; Niskanen, L.; Tanskanen, A.; Wahlbeck, K.; Tiihonen, J. Incidence of cancer and statin usage--record linkage study. Int. J. Cancer 2010, 126, 279–284. [Google Scholar] [CrossRef]
- Mei, Z.; Liang, M.; Li, L.; Zhang, Y.; Wang, Q.; Yang, W. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. Cancer 2017, 140, 1068–1081. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Emberson, J.R.; Kearney, P.M.; Blackwell, L.; Newman, C.; Reith, C.; Bhala, N.; Holland, L.; Peto, R.; Keech, A.; et al. Lack of effect of lowering LDL cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 2012, 7, e29849. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Hu, B.; Shi, J.; Chen, Y.; Huang, K. New insights into the therapeutic potentials of statins in cancer. Front. Pharmacol. 2023, 14, 1188926. [Google Scholar] [CrossRef]
- Zaky, M.Y.; Fan, C.; Zhang, H.; Sun, X.F. Unraveling the Anticancer Potential of Statins: Mechanisms and Clinical Significance. Cancers 2023, 15, 4787. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, B.O.; Boroń, D.; Morawiec, E.; Michalski, P.; Palazzo-Michalska, V.; Pach, Ł.; Dziuk, B.; Świder, M.; Zmarzły, N. Crosstalk between Statins and Cancer Prevention and Therapy: An Update. Pharmaceuticals 2021, 14, 1220. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Li, Y.; Li, W.; Chen, Y. Simvastatin prevents proliferation and bone metastases of lung adenocarcinoma in vitro and in vivo. Neoplasma 2013, 60, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Y.; Fard, J.K.; Ghafoor, D.; Eid, A.H.; Sahebkar, A. Paradoxical effects of statins on endothelial and cancer cells: The impact of concentrations. Cancer Cell Int. 2023, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mukthavaram, R.; Chao, Y.; Nomura, N.; Bharati, I.S.; Fogal, V.; Pastorino, S.; Teng, D.; Cong, X.; Pingle, S.C.; et al. In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. Br. J. Cancer 2014, 111, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Bocci, G.; Fioravanti, A.; Orlandi, P.; Bernardini, N.; Collecchi, P.; Del Tacca, M.; Danesi, R. Fluvastatin synergistically enhances the antiproliferative effect of gemcitabine in human pancreatic cancer MIAPaCa-2 cells. Br. J. Cancer 2005, 93, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Tulk, A.; Watson, R.; Erdrich, J. The Influence of Statin Use on Chemotherapeutic Efficacy in Studies of Mouse Models: A Systematic Review. Anticancer Res. 2023, 43, 4263–4275. [Google Scholar] [CrossRef]
- Davis, H.R.; Veltri, E.P. Zetia: Inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J. Atheroscler. Thromb. 2007, 14, 99–108. [Google Scholar] [CrossRef]
- Lee, J.; Egolum, U.; Parihar, H.; Cooley, M.; Ling, H. Effect of Ezetimibe Added to High-Intensity Statin Therapy on Low-Density Lipoprotein Cholesterol Levels: A Meta-Analysis. Cardiol. Res. 2021, 12, 98–108. [Google Scholar] [CrossRef]
- Pearson, T.A.; Ballantyne, C.M.; Veltri, E.; Shah, A.; Bird, S.; Lin, J.; Rosenberg, E.; Tershakovec, A.M. Pooled analyses of effects on C-reactive protein and low density lipoprotein cholesterol in placebo-controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am. J. Cardiol. 2009, 103, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.; Ballantyne, C.; Sisk, C.; Shah, A.; Veltri, E.; Maccubbin, D. Comparison of effects of ezetimibe/simvastatin versus simvastatin versus atorvastatin in reducing C-reactive protein and low-density lipoprotein cholesterol levels. Am. J. Cardiol. 2007, 99, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Min, Y.J.; Kwon, J.E.; Cho, E.J.; Kim, J.E.; Lee, W.S.; Lee, K.J.; Kim, S.W.; Kim, T.H.; Kim, C.J.; et al. Effects of ezetimibe added to ongoing statin therapy on C-reactive protein levels in hypercholesterolemic patients. Korean Circ. J. 2011, 41, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Dolezelova, E.; Stein, E.; Derosa, G.; Maffioli, P.; Nachtigal, P.; Sahebkar, A. Effect of ezetimibe on plasma adipokines: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2017, 83, 1380–1396. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Galimberti, F.; Olmastroni, E.; Luscher, T.F.; Carugo, S.; Catapano, A.L.; Casula, M. META-LIPID Group. Effect of lipid-lowering therapies on C-reactive protein levels: A comprehensive meta-analysis of randomized controlled trials. Cardiovasc. Res. 2024, 120, 333–344. [Google Scholar] [CrossRef]
- Qin, L.; Yang, Y.B.; Yang, Y.X.; Zhu, N.; Li, S.X.; Liao, D.F.; Zheng, X.L. Anti-inflammatory activity of ezetimibe by regulating NF-κB/MAPK pathway in THP-1 macrophages. Pharmacology 2014, 93, 69–75. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.N.; Matei, N.; Li, X.; Pang, J.W.; Mo, J.; Chen, S.P.; Tang, J.P.; Yan, M.; Zhang, J.H. Ezetimibe Attenuates Oxidative Stress and Neuroinflammation via the AMPK/Nrf2/TXNIP Pathway after MCAO in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 4717258. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, G.; Han, D.H.; Lee, M.; Kim, I.; Kim, B.; Kim, K.H.; Song, Y.M.; Yoo, J.E.; Wang, H.J.; et al. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy 2017, 13, 1767–1781. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.Y.; Na, H.S.; Lee, A.R.; Cho, K.H.; Choi, J.W.; Park, S.H.; Cho, M.L. Ezetimibe ameliorates clinical symptoms in a mouse model of ankylosing spondylitis associated with suppression of Th17 differentiation. Front. Immunol. 2022, 13, 922531. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; Schmidt-Lucke, C.; Bojunga, S.; Rössig, L.; Heeschen, C.; Dimmeler, S.; Zeiher, A.M. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: Clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur. Heart J. 2006, 27, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Efrati, S.; Averbukh, M.; Dishy, V.; Faygenzo, M.; Friedensohn, L.; Golik, A. The effect of simvastatin, ezetimibe and their combination on the lipid profile, arterial stiffness and inflammatory markers. Eur. J. Clin. Pharmacol. 2007, 63, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Liu, Y.W.; Lin, L.J.; Chen, J.H.; Liao, J.K. Evidence for statin pleiotropy in humans: Differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation 2009, 119, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kuhlencordt, P.J.; Padmapriya, P.; Rützel, S.; Schödel, J.; Hu, K.; Schäfer, A.; Huang, P.L.; Ertl, G.; Bauersachs, J. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2009, 202, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Hussein, O.; Minasian, L.; Itzkovich, Y.; Shestatski, K.; Solomon, L.; Zidan, J. Ezetimibe’s effect on platelet aggregation and LDL tendency to peroxidation in hypercholesterolaemia as monotherapy or in addition to simvastatin. Br. J. Clin. Pharmacol. 2008, 65, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Schulze, T.J.; Schmitt, M.; Trinkmann, F.; El-Battrawy, I.; Akin, I.; Kälsch, T.; Borggrefe, M.; Stach, K. Ezetimibe inhibits platelet activation and uPAR expression on endothelial cells. Int. J. Cardiol. 2017, 227, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.; Hinderliter, A.L.; Lee, C.R. The impact of ezetimibe on endothelial function and other markers of cardiovascular risk. Ann. Pharmacother. 2009, 43, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyoshi, T.; Yunoki, K.; Ito, H. Postprandial hyperlipidemia as a potential residual risk factor. J. Cardiol. 2016, 67, 335–339. [Google Scholar] [CrossRef]
- Nakamura, A.; Sato, K.; Kanazawa, M.; Kondo, M.; Endo, H.; Takahashi, T.; Nozaki, E. Impact of decreased insulin resistance by ezetimibe on postprandial lipid profiles and endothelial functions in obese, non-diabetic-metabolic syndrome patients with coronary artery disease. Heart Vessel. 2019, 34, 916–925. [Google Scholar] [CrossRef]
- Takase, S.; Matoba, T.; Nakashiro, S.; Mukai, Y.; Inoue, S.; Oi, K.; Higo, T.; Katsuki, S.; Takemoto, M.; Suematsu, N.; et al. Ezetimibe in Combination With Statins Ameliorates Endothelial Dysfunction in Coronary Arteries After Stenting: The CuVIC Trial (Effect of Cholesterol Absorption Inhibitor Usage on Target Vessel Dysfunction After Coronary Stenting), a Multicenter Randomized Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 350–358. [Google Scholar] [CrossRef]
- Ikeda, S.; Maemura, K. Ezetimibe and vascular endothelial function. Curr. Vasc. Pharmacol. 2011, 9, 87–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olijhoek, J.K.; Hajer, G.R.; van der Graaf, Y.; Dallinga-Thie, G.M.; Visseren, F.L. The effects of low-dose simvastatin and ezetimibe compared to high-dose simvastatin alone on post-fat load endothelial function in patients with metabolic syndrome: A randomized double-blind crossover trial. J. Cardiovasc. Pharmacol. 2008, 52, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Settergren, M.; Böhm, F.; Rydén, L.; Pernow, J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur. Heart J. 2008, 29, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Lally, S.; Tan, C.Y.; Owens, D.; Tomkin, G.H. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: The role of Niemann-Pick C1-like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia 2006, 49, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, R.H.; Park, H.; Wang, H.J.; Lee, H.; Kang, E.S. Effect of Ezetimibe on Glucose Metabolism and Inflammatory Markers in Adipose Tissue. Biomedicines 2020, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Roh, E. Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2024, 48, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shang, H.; Wu, J. Effect of ezetimibe on glycemic control: A systematic review and meta-analysis of randomized controlled trials. Endocrine 2018, 60, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Joung, K.H.; Lee, J.C.; Kim, O.S.; Choung, S.; Kim, J.M.; Kang, Y.E.; Yi, H.S.; Lee, J.H.; Ku, B.J.; et al. Comparative Efficacy of Rosuvastatin Monotherapy and Rosuvastatin/Ezetimibe Combination Therapy on Insulin Sensitivity and Vascular Inflammatory Response in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2024, 48, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Pelton, K.; Coticchia, C.M.; Curatolo, A.S.; Schaffner, C.P.; Zurakowski, D.; Solomon, K.R.; Moses, M.A. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am. J. Pathol. 2014, 184, 2099–2110. [Google Scholar] [CrossRef]
- Wang, Y.; You, S.; Su, S.; Yeon, A.; Lo, E.M.; Kim, S.; Mohler, J.L.; Freeman, M.R.; Kim, H.L. Cholesterol-Lowering Intervention Decreases mTOR Complex 2 Signaling and Enhances Antitumor Immunity. Clin. Cancer Res. 2022, 28, 414–424. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Ohnishi, H.; Morimoto, N.; Minami, S.; Ishioka, M.; Watanabe, S.; Tsukui, M.; Takaoka, Y.; Nomoto, H.; Isoda, N.; et al. Ezetimibe suppresses development of liver tumors by inhibiting angiogenesis in mice fed a high-fat diet. Cancer Sci. 2019, 110, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. PCSK 9 Inhibitors: A Short History and a New Era of Lipid-lowering Therapy. Heart Views 2019, 20, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.D.; Peng, Z.S.; Gu, H.M.; Wang, M.; Wang, G.Q.; Zhang, D.W. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 764038. [Google Scholar] [CrossRef]
- Beltran, R.A.; Zemeir, K.J.; Kimberling, C.R.; Kneer, M.S.; Mifflin, M.D.; Broderick, T.L. Is a PCSK9 Inhibitor Right for Your Patient? A Review of Treatment Data for Individualized Therapy. Int. J. Environ. Res. Public Health 2022, 19, 16899. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Leiter, L.A.; Verma, S.; Park, J.G.; Sever, P.S.; Lira Pineda, A.; Honarpour, N.; Wang, H.; Murphy, S.A.; et al. Inflammatory and Cholesterol Risk in the FOURIER Trial. Circulation 2018, 138, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.X.; Li, S.; Liu, H.H.; Li, J.J. Impact of PCSK9 monoclonal antibodies on circulating hs-CRP levels: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2018, 8, e022348. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Paolisso, P.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; Ferraraccio, F.; Panarese, I.; et al. Evidence of an anti-inflammatory effect of PCSK9 inhibitors within the human atherosclerotic plaque. Atherosclerosis 2023, 378, 117180. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jiang, L.; Peng, J.; Ren, Z.; Wei, D.; Wu, C.; Pan, L.; Jiang, Z.; Liu, L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived macrophages. Int. J. Mol. Med. 2012, 30, 931–938. [Google Scholar] [CrossRef]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, D.; Yang, Q.; You, J.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Interactions between PCSK9 and NLRP3 inflammasome signaling in atherosclerosis. Front. Immunol. 2023, 14, 1126823. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, X.; Liu, S.; Zhou, S.; Kore, R.A.; Mu, S.; Deng, X.; Fan, Y.; Mehta, J.L. NLRP3 inflammasome via IL-1β regulates PCSK9 secretion. Theranostics 2020, 10, 7100–7110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Liu, S.; Brickell, A.N.; Zhang, J.; Wu, Z.; Zhou, S.; Ding, Z. PCSK9 regulates pyroptosis via mtDNA damage in chronic myocardial ischemia. Basic Res. Cardiol. 2020, 115, 66. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kim, S.; Lee, H.; Lee, H.C.; Lee, J.; Park, H.W.; Fukai, M.; Choi, E.; Choi, S.; Koo, B.J.; et al. PCSK9 stimulates Syk, PKCδ, and NF-κB, leading to atherosclerosis progression independently of LDL receptor. Nat. Commun. 2024, 15, 2789. [Google Scholar] [CrossRef] [PubMed]
- Ugovšek, S.; Šebeštjen, M. Non-Lipid Effects of PCSK9 Monoclonal Antibodies on Vessel Wall. J. Clin. Med. 2022, 11, 3625. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shores, K.L.; Breithaupt, J.J.; Lee, C.S.; Fodera, D.M.; Kwon, J.B.; Ettyreddy, A.R.; Myers, K.M.; Evison, B.J.; Suchowerska, A.K.; et al. PCSK9 activation promotes early atherosclerosis in a vascular microphysiological system. APL Bioeng. 2023, 7, 046103. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Domingo, E.; Rubio, A.; Martinez-Hervás, S.; Ascaso, J.F.; Piqueras, L.; Real, J.T.; Sanz, M.J. Beneficial effects of PCSK9 inhibition with alirocumab in familial hypercholesterolemia involve modulation of new immune players. Biomed. Pharmacother. 2022, 145, 112460. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Marfella, R.; Sardu, C.; Martino, E.; Scisciola, L.; Marfella, L.; Grotta, R.; Frigé, C.; Paolisso, G.; et al. SIRT3 mediates the effects of PCSK9 inhibitors on inflammation, autophagy, and oxidative stress in endothelial cells. Theranostics 2023, 13, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Cheng, Z.; Lv, Z.; Luo, S.; Xia, Y. PCSK9 Promotes Endothelial Dysfunction During Sepsis via the TLR4/MyD88/NF-κB and NLRP3 Pathways. Inflammation 2023, 46, 115–128. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; Wang, Y.; Xu, Z.; Li, G. Investigation of highly expressed PCSK9 in atherosclerotic plaques and ox-LDL-induced endothelial cell apoptosis. Mol. Med. Rep. 2017, 16, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Maulucci, G.; Cipriani, F.; Russo, D.; Casavecchia, G.; Di Staso, C.; Di Martino, L.; Ruggiero, A.; Di Biase, M.; Brunetti, N.D. Improved endothelial function after short-term therapy with evolocumab. J. Clin. Lipidol. 2018, 12, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Péč, M.J.; Benko, J.; Jurica, J.; Péčová, M.; Samec, M.; Hurtová, T.; Bolek, T.; Galajda, P.; Péč, M.; Samoš, M.; et al. The Anti-Thrombotic Effects of PCSK9 Inhibitors. Pharmaceuticals 2023, 16, 1197. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L. Type 2 scavenger receptor CD36 in platelet activation: The role of hyperlipemia and oxidative stress. Clin. Lipidol. 2009, 4, 767. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Cammisotto, V.; Baratta, F.; Simeone, P.G.; Barale, C.; Lupia, E.; Galardo, G.; Santilli, F.; Russo, I.; Pignatelli, P. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Beyond Lipids: The Role in Oxidative Stress and Thrombosis. Antioxidants 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Marston, N.; AGurmu, Y.; Melloni, G.E.M.; Bonaca, M.; Gencer, B.; Sever, P.S.; Pedersen, T.R.; Keech, A.C.; Roselli, C.; Lubitz, S.A.; et al. The Effect of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibition on the Risk of Venous Thromboembolism. Circulation 2020, 141, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Kereiakes, D.J.; McKenney, J.M.; Roth, E.M.; Hanotin, C.; Gipe, D.; Du, Y.; Ferrand, A.C.; Ginsberg, H.N.; Stein, E.A. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am. J. Cardiol. 2014, 114, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Basiak, M.; Hachula, M.; Kosowski, M.; Okopien, B. Effect of PCSK9 Inhibitors on Hemostasis in Patients with Isolated Hypercholesterolemia. J. Clin. Med. 2022, 11, 2542. [Google Scholar] [CrossRef]
- Scalise, V.; Sanguinetti, C.; Neri, T.; Cianchetti, S.; Lai, M.; Carnicelli, V.; Celi, A.; Pedrinelli, R. PCSK9 Induces Tissue Factor Expression by Activation of TLR4/NFkB Signaling. Int. J. Mol. Sci. 2021, 22, 12640. [Google Scholar] [CrossRef]
- Leiter, L.A.; Cariou, B.; Müller-Wieland, D.; Colhoun, H.M.; Del Prato, S.; Tinahones, F.J.; Ray, K.K.; Bujas-Bobanovic, M.; Domenger, C.; Mandel, J.; et al. Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: The ODYSSEY DM-INSULIN randomized trial. Diabetes Obes. Metab. 2017, 19, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Leiter, L.A.; Wiviott, S.D.; Giugliano, R.P.; Deedwania, P.; De Ferrari, G.M.; Murphy, S.A.; Kuder, J.F.; Gouni-Berthold, I.; Lewis, B.S.; et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Rahman, H.; Okunrintemi, V.; Riaz, H.; Khan, M.S.; Sattur, S.; Kaluski, E.; Lincoff, A.M.; Martin, S.S.; Blaha, M.J. Association of Lowering Low-Density Lipoprotein Cholesterol With Contemporary Lipid-Lowering Therapies and Risk of Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e011581. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M.; Sirois, F.; Mayne, J.; Wang, G.S.; Chen, A.; Dewpura, T.; Prat, A.; Seidah, N.G.; Chretien, M.; Scott, F.W. PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett. 2010, 584, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Carugo, S.; Sirtori, C.R.; Corsini, A.; Tokgozoglu, L.; Ruscica, M. PCSK9 Inhibition and Risk of Diabetes: Should We Worry? Curr. Atheroscler. Rep. 2022, 24, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Oza, P.P.; Kashfi, K. The evolving landscape of PCSK9 inhibition in cancer. Eur. J. Pharmacol. 2023, 949, 175721. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Bisceglia, I.; Berretta, M.; Iovine, M.; Canale, M.L.; Maurea, C.; Giordano, V.; Paccone, A.; Inno, A.; Maurea, N. PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers 2023, 15, 1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Zhu, X.D.; Feng, L.H.; Li, X.L.; Liu, X.F.; Sun, H.C.; Tang, Z.Y. PCSK9 promotes tumor growth by inhibiting tumor cell apoptosis in hepatocellular carcinoma. Exp. Hematol. Oncol. 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Luo, H.; Lu, Q.; Yu, S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 303. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Zhang, D.; Mi, Y.; Zhang, J.; He, G. The Causal Relationship between PCSK9 Inhibitors and Malignant Tumors: A Mendelian Randomization Study Based on Drug Targeting. Genes 2024, 15, 132. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Y.; Cao, L.; Zhang, Y.; Yin, Y.; Hu, X. PCSK9 regulates apoptosis in human lung adenocarcinoma A549 cells via endoplasmic reticulum stress and mitochondrial signaling pathways. Exp. Ther. Med. 2017, 13, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Żak, J.; Malinowski, B.; Popek, G.; Grześk, G. PCSK9 signaling pathways and their potential importance in clinical practice. EPMA J. 2017, 8, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.; Luo, H.H.; Yu, S.W.; Wang, L. Pro-protein convertase subtilisin/kexin type 9 promotes intestinal tumor development by activating Janus kinase 2/signal transducer and activator of transcription 3/SOCS3 signaling in ApcMin/+ mice. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211038345. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Essalmani, R.; Day, R.; Khatib, A.M.; Seidah, N.G.; Prat, A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia 2012, 14, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C.Y. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020, 588, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cai, T.; Zheng, X.; Ren, Y.; Qi, J.; Lu, X.; Chen, H.; Lin, H.; Chen, Z.; Liu, M.; et al. Potentiating CD8+ T cell antitumor activity by inhibiting PCSK9 to promote LDLR-mediated TCR recycling and signaling. Protein Cell 2021, 12, 240–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef] [PubMed]
- Egelston, C.A.; Guo, W.; Tan, J.; Avalos, C.; Simons, D.L.; Lim, M.H.; Huang, Y.J.; Nelson, M.S.; Chowdhury, A.; Schmolze, D.B.; et al. Tumor-infiltrating exhausted CD8+ T cells dictate reduced survival in premenopausal estrogenreceptor-positive breast cancer. JCI Insight 2022, 7, e153963. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Nik, M.E.; Jaafari, M.R.; Banach, M.; Sahebkar, A. Effects of immunisation against PCSK9 in mice bearing melanoma. Arch. Med. Sci. 2019, 16, 189–199. [Google Scholar] [CrossRef]
- Gan, S.S.; Ye, J.Q.; Wang, L.; Qu, F.J.; Chu, C.M.; Tian, Y.J.; Yang, W.; Cui, X.G. Inhibition of PCSK9 protects against radiation-induced damage of prostate cancer cells. Onco. Targets Ther. 2017, 10, 2139–2146. [Google Scholar] [CrossRef]
- Safaeian, L.; Vaseghi, G.; Jabari, H.; Dana, N. Evolocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, promotes angiogenesis in vitro. Can. J. Physiol. Pharmacol. 2019, 97, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhandari, M.; Vishwakarma, P.; Singh, A.; Perrone, M.A.; Sethi, R. Bempedoic Acid: An Emerging Therapy for Uncontrolled Low-Density Lipoprotein (LDL) Cholesterol. J. Cardiovasc. Dev. Dis. 2023, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Ruscica, M.; Santos, R.D.; Corsini, A. Fixed Combination for the Treatment of Dyslipidaemia. Curr. Atheroscler. Rep. 2023, 25, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Yarrarapu, S.N.S.; Goyal, A.; Venkata, V.S.; Panchal, V.; Sivasubramanian, B.P.; Du, D.T.; Jakulla, R.S.; Pamulapati, H.; Afaq, M.A.; Owens, S.; et al. Comprehensive review of statin-intolerance and the practical application of Bempedoic Acid. J. Cardiol. 2024, 84, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pinkosky, S.L.; Filippov, S.; Srivastava, R.A.; Hanselman, J.C.; Bradshaw, C.D.; Hurley, T.R.; Cramer, C.T.; Spahr, M.A.; Brant, A.F.; Houghton, J.L.; et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J. Lipid Res. 2013, 54, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Filippov, S.; Pinkosky, S.L.; Lister, R.J.; Pawloski, C.; Hanselman, J.C.; Cramer, C.T.; Srivastava, R.A.K.; Hurley, T.R.; Bradshaw, C.D.; Spahr, M.A.; et al. ETC-1002 regulates immune response, leukocyte homing, and adipose tissue inflammation via LKB1-dependent activation of macrophage AMPK. J. Lipid Res. 2013, 54, 2095–2108. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Lei, L.; Ray, K.K.; Ballantyne, C.M.; Bradwin, G.; Rifai, N. Effects of bempedoic acid on CRP, IL-6, fibrinogen and lipoprotein(a) in patients with residual inflammatory risk: A secondary analysis of the CLEAR harmony trial. J. Clin. Lipidol. 2023, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Leiter, L.A.; Mancini, G.B.J.; Ray, K.K.; Flaim, J.; Ye, Z.; Catapano, A.L. Association of Bempedoic Acid Administration With Atherogenic Lipid Levels in Phase 3 Randomized Clinical Trials of Patients With Hypercholesterolemia. JAMA Cardiol. 2020, 5, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Verberk, S.G.S.; Kuiper, K.L.; Lauterbach, M.A.; Latz, E.; Van den Bossche, J. The multifaceted therapeutic value of targeting ATP-citrate lyase in atherosclerosis. Trends Mol. Med. 2021, 27, 1095–1105. [Google Scholar] [CrossRef]
- Govindaraju, A.; Sabarathinam, S. Bempedoic acid: A nonstatin drug for the management of hypercholesterolemia. Health Sci. Rep. 2021, 4, e431. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Mohamed, S.K.; Nofal, S.; El Morsy, E.M.; Ahmed, A.A.E. Effect of bempedoic acid on angiotensin-II induced hypertension and vascular tissue remodelling in renal hypertensive rats through AMPK multiple signalling pathways modulation. Life Sci. 2023, 320, 121573. [Google Scholar] [CrossRef] [PubMed]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis inhibitors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2022, 21, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Vinci, P.; Mangogna, A.; Landolfo, M.; Schincariol, P.; Fiotti, N.; Mearelli, F.; Di Girolamo, F.G. Mechanism of action and therapeutic use of bempedoic acid in atherosclerosis and metabolic syndrome. Front. Cardiovasc. Med. 2022, 9, 1028355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019, 5, 30–45. [Google Scholar] [CrossRef]
- Icard, P.; Wu, Z.; Fournel, L.; Coquerel, A.; Lincet, H.; Alifano, M. ATP citrate lyase: A central metabolic enzyme in cancer. Cancer Lett. 2020, 471, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cheng, A.; Li, B.; Tian, X.; Han, Z.; Feng, Z. The small extracellular vesicle-mediated intercellular transformation of CXCR1Low to CXCR1High tumour cells promotes the progression of head and neck squamous cell carcinoma. J. Extracell. Vesicles 2024, 13, e12427. [Google Scholar] [CrossRef] [PubMed]
- Velez, B.C.; Petrella, C.P.; DiSalvo, K.H.; Cheng, K.; Kravtsov, R.; Krasniqi, D.; Krucher, N.A. Combined inhibition of ACLY and CDK4/6 reduces cancer cell growth and invasion. Oncol. Rep. 2023, 49, 32. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Bachmann, H.S. Regulation of protein prenylation. Biomed. Pharmacother. 2023, 164, 114915. [Google Scholar] [CrossRef]
- Munkhsaikhan, U.; Kwon, Y.; Sahyoun, A.M.; Ait-Aissa, K.; Kassan, A.; Kassan, M. The microsomal triglyceride transfer protein inhibitor lomitapide improves vascular function in mice with obesity. Obesity 2022, 30, 893–901. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; He, H.; Luo, H.; Xia, Y.; Jiang, Y.; Jiang, J.; Sun, L. Repositioning Lomitapide to block ZDHHC5-dependant palmitoylation on SSTR5 leads to anti-proliferation effect in preclinical pancreatic cancer models. Cell Death Discov. 2023, 9, 60. [Google Scholar] [CrossRef]
- Lee, B.; Park, S.J.; Lee, S.; Lee, J.; Lee, E.; Yoo, E.S.; Chung, W.S.; Sohn, J.W.; Oh, B.C.; Kim, S. Lomitapide, a cholesterol-lowering drug, is an anticancer agent that induces autophagic cell death via inhibiting mTOR. Cell Death Dis. 2022, 13, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Hu, Y.; Gao, Y.; Chen, J.; Meng, Y.; Qiu, Y.; Hu, R.; Liao, P.; Li, M.; et al. Targeting PARP14 with lomitapide suppresses drug resistance through the activation of DRP1-induced mitophagy in multiple myeloma. Cancer Lett. 2024, 588, 216802. [Google Scholar] [CrossRef] [PubMed]
- Schweiker, S.S.; Tauber, A.L.; Sherry, M.E.; Levonis, S.M. Structure, Function and Inhibition of Poly(ADP-ribose)polymerase, Member 14 (PARP14). Mini Rev. Med. Chem. 2018, 18, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Bousvarou, M.D.; Sourlas, A.; Papakonstantinou, E.J.; Peña Genao, E.; Echavarria Uceta, R.; Guzman, E. Angiopoietin-Like Protein 3 (ANGPTL3) Inhibitors in the Management of Refractory Hypercholesterolemia. Clin. Pharmacol. 2022, 14, 49–59. [Google Scholar] [CrossRef]
- Sosnowska, B.; Adach, W.; Surma, S.; Rosenson, R.S.; Banach, M. Evinacumab, an ANGPTL3 Inhibitor, in the Treatment of Dyslipidemia. J. Clin. Med. 2022, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Marston, N.A.; Bramson, C.R.; Curto, M.; Ramos, V.; Jevne, A.; Kuder, J.F.; Park, J.G.; Murphy, S.A.; Verma, S.; et al. TRANSLATE-TIMI 70 Investigators. Effect of Vupanorsen on Non-High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients With Elevated Cholesterol: TRANSLATE-TIMI 70. Circulation 2022, 145, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Y.; Xu, H.; Du, N. The influence of angiopoietin-like protein 3 on macrophages polarization and its effect on the podocyte EMT in diabetic nephropathy. Front. Immunol. 2023, 14, 1228399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.T.; Wan, S.Y.; Yang, J.; Wei, Y.J.; Chen, H.J.; Zhou, W.Z.; Song, Q.Y.; Niu, S.X.; Zheng, L.; et al. ANGPTL3 negatively regulates IL-1β-induced NF-κB activation by inhibiting the IL1R1-associated signaling complex assembly. J. Mol. Cell Biol. 2024, 15, mjad053. [Google Scholar] [CrossRef]
- Lv, Q.; Han, X.; Ni, J.; Ma, Q.; Dai, R.; Liu, J.; Liu, J.; Zhai, Y.; Shen, Q.; Sun, L.; et al. Anti-ANGPTL3-FLD monoclonal antibody treatment ameliorates podocyte lesions through attenuating mitochondrial damage. Cell Death Dis. 2022, 13, 867. [Google Scholar] [CrossRef]
- Camenisch, G.; Pisabarro, M.T.; Sherman, D.; Kowalski, J.; Nagel, M.; Hass, P.; Xie, M.H.; Gurney, A.; Bodary, S.; Liang, X.H.; et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J. Biol. Chem. 2002, 277, 17281–17290. [Google Scholar] [CrossRef]
- Wu, T.C.; Hsu, B.G.; Kuo, C.H.; Wang, C.H.; Tsai, J.P. Serum Angiopoietin-like Protein 3 Levels Are Associated with Endothelial Function in Patients with Maintenance Hemodialysis. Life 2023, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Robciuc, M.R.; Maranghi, M.; Lahikainen, A.; Rader, D.; Bensadoun, A.; Öörni, K.; Metso, J.; Minicocci, I.; Ciociola, E.; Ceci, F.; et al. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, M.; Dang Thanh, C.; Huynh Quang, T. The Correlation Between Angiopoietin-Like 3 and Metabolic Markers of Some Lipid and Glucose in Type 2 Diabetes Mellitus Patients at the First Diagnosis. Diabetes Metab. Syndr. Obes. 2022, 15, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Tang, L.; He, X. Angiopoietin-like 3 (ANGPTL3) drives cell proliferation, migration and angiogenesis in cervical cancer via binding to integrin alpha v beta 3. Bioengineered 2022, 13, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- El-Shal, A.S.; Zidan, H.E.; Rashad, N.M.; Wadea, F.M. Angiopoietin-like protein 3 and 4 expression 4 and their serum levels in hepatocellular carcinoma. Cytokine 2017, 96, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, Y.; Pan, S.; Zhang, Y.; Fu, J.; Wu, X.; Qin, X. Angiopoietin-like protein 3 promotes colorectal cancer progression and liver metastasis partly via the mitogen-activated protein kinase 14 pathway. Mol. Carcinog. 2023, 62, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Wong Chong, E.; Joncas, F.H.; Douville, P.; Bachvarov, D.; Diorio, C.; Calon, F.; Bergeron, A.C.; Blais, J.; Leung, S.O.A.; Seidah, N.G.; et al. Pre-operative levels of angiopoietin protein-like 3 (ANGPTL3) in women diagnosed with high-grade serous carcinoma of the ovary. Lipids Health Dis. 2024, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zheng, Y.; Jin, Z. ANGPTL3 affects the metastatic potential and the susceptibility of ovarian cancer cells to natural killer cell-mediated cytotoxicity. Heliyon 2023, 9, e18799. [Google Scholar] [CrossRef] [PubMed]
- Kayikcioglu, M. LDL Apheresis and Lp (a) Apheresis: A Clinician’s Perspective. Curr. Atheroscler. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Tsioulos, G.; Kounatidis, D.; Vallianou, N.G.; Poulaki, A.; Kotsi, E.; Christodoulatos, G.S.; Tsilingiris, D.; Karampela, I.; Skourtis, A.; Dalamaga, M. Lipoprotein (a) and Atherosclerotic Cardiovascular Disease: Where Do We Stand? Int. J. Mol. Sci. 2024, 25, 3537. [Google Scholar] [CrossRef]
- Mickiewicz, A.; Borowiec-Wolna, J.; Bachorski, W.; Gilis-Malinowska, N.; Gałąska, R.; Raczak, G.; Chmara, M.; Wasąg, B.; Jaguszewski, M.J.; Fijałkowski, M.; et al. Long-term lipoprotein apheresis in the treatment of severe familial hypercholesterolemia refractory to high intensity statin therapy: Three year experience at a lipoprotein apheresis centre. Cardiol. J. 2019, 26, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Schettler, V.J.J.; Neumann, C.L.; Peter, C.; Zimmermann, T.; Julius, U.; Hohenstein, B.; Roeseler, E.; Heigl, F.; Grützmacher, P.; Blume, H.; et al. Scientific Board of GLAR for the German Apheresis Working Group. Lipoprotein apheresis is an optimal therapeutic option to reduce increased Lp (a) levels. Clin. Res. Cardiol. Suppl. 2019, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Bambauer, R.; Bambauer, C.; Lehmann, B.; Latza, R.; Schiel, R. LDL-apheresis: Technical and clinical aspects. Sci. World J. 2012, 2012, 314283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kounatidis, D.; Vallianou, N.G.; Poulaki, A.; Evangelopoulos, A.; Panagopoulos, F.; Stratigou, T.; Geladari, E.; Karampela, I.; Dalamaga, M. ApoB100 and Atherosclerosis: What’s New in the 21st Century? Metabolites 2024, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Oda, O.; Nagaya, T.; Ogawa, H. Analysis of protein absorbed by LDL column (Liposorber) with special reference to complement component factor D. Clin. Chim. Acta 2004, 342, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Stefanutti, C.; Mazza, F.; Pasqualetti, D.; Di Giacomo, S.; Watts, G.F.; Massari, M.S.; de Neve, J.; Morozzi, C.; Fischer, M. Lipoprotein apheresis downregulates IL-1α, IL-6 and TNF-α mRNA expression in severe dyslipidaemia. Atheroscler. Suppl. 2017, 30, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Hovland, A.; Hardersen, R.; Sexton, J.; Mollnes, T.E.; Lappegård, K.T. Different inflammatory responses induced by three LDL-lowering apheresis columns. J. Clin. Apher. 2009, 24, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Berster, J.; Otto, B.; Parhofer, K.G. Effects of two whole blood systems (DALI and Liposorber D) for LDL apheresis on lipids and cardiovascular risk markers in severe hypercholesterolemia. J. Clin. Apher. 2007, 22, 301–305. [Google Scholar] [CrossRef]
- Kobayashi, S.; Oka, M.; Moriya, H.; Maesato, K.; Okamoto, K.; Ohtake, T. LDL-apheresis reduces P-Selectin, CRP and fibrinogen, possible important implications for improving atherosclerosis. Ther. Apher. Dial. 2006, 10, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Puntoni, M.; Sbrana, F.; Bigazzi, F.; Minichilli, F.; Ferdeghini, E.; Sampietro, T. Myeloperoxidase modulation by LDL apheresis in familial hypercholesterolemia. Lipids Health Dis. 2011, 10, 185. [Google Scholar] [CrossRef]
- Arnhold, J.; Flemmig, J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 2010, 500, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, K.; Zhao, R.; Hu, J.; Hao, Z.; Wang, F.; Lu, Y.; Liu, F.; Zhang, Y. Inflammatory biomarkers of coronary heart disease. Front. Biosci. 2018, 10, 185–196. [Google Scholar] [CrossRef]
- Walther, R.; Wehner, R.; Tunger, A.; Julius, U.; Schatz, U.; Tselmin, S.; Bornstein, S.R.; Schmitz, M.; Graessler, J. Repeated lipoprotein apheresis and immune response: Effects on different immune cell populations. Ther. Apher. Dial. 2022, 26, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Hajighasemi, S.; Kiaie, N.; Gheibi Hayat, S.M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. The pivotal role of CD69 in autoimmunity. J. Autoimmun. 2020, 114, 102548. [Google Scholar] [CrossRef] [PubMed]
- Blaha, V.; Blaha, M.; Solichová, D.; Krčmová, L.K.; Lánská, M.; Havel, E.; Vyroubal, P.; Zadák, Z.; Žák, P.; Sobotka, L. Antioxidant defense system in familial hypercholesterolemia and the effects of lipoprotein apheresis. Atheroscler. Suppl. 2017, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, A.; Kreft, E.; Kuchta, A.; Wieczorek, E.; Marlęga, J.; Ćwiklińska, A.; Paprzycka, M.; Gruchała, M.; Fijałkowski, M.; Jankowski, M. The Impact of Lipoprotein Apheresis on Oxidative Stress Biomarkers and High-Density Lipoprotein Subfractions. Oxid. Med. Cell. Longev. 2020, 2020, 9709542. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Dong, Q.; Liu, G.; Gao, Y.; Li, X.L.; Jin, J.L.; Li, J.J.; Guo, Y.L. Improvement of oxidative stress status by lipoprotein apheresis in Chinese patients with familial hypercholesterolemia. J. Clin. Lab. Anal. 2020, 34, e23161. [Google Scholar] [CrossRef] [PubMed]
- Rubba, P.; Iannuzzi, A.; Postiglione, A.; Scarpato, N.; Montefusco, S.; Gnasso, A.; Nappi, G.; Cortese, C.; Mancini, M. Hemodynamic changes in the peripheral circulation after repeat low density lipoprotein apheresis in familial hypercholesterolemia. Circulation 1990, 81, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, P.M.; Gibson, C.A.; Kensey, K.R.; Hogenauer, W. Effect of low-density lipoprotein cholesterol apheresis on blood viscosity. Am. J. Cardiol. 2004, 93, 1044–1046. [Google Scholar] [CrossRef]
- Sinzinger, H.; Steiner, S.; Derfler, K. Pleiotropic effects of regular lipoprotein-apheresis. Atheroscler. Suppl. 2017, 30, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Marlęga-Linert, J.; Gąsecka, A.; van der Pol, E.; Kuchta, A.; Filipiak, K.J.; Fijałkowski, M.; Gruchała, M.; Nieuwland, R.; Mickiewicz, A. Lipoprotein apheresis affects the concentration of extracellular vesicles in patients with elevated lipoprotein (a). Sci. Rep. 2024, 14, 2762. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.E.; D’Souza-Schorey, C. The biology of extracellular microvesicles. Traffic 2018, 19, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ventura, J.L.; Roncal, C.; Orbe, J.; Blanco-Colio, L.M. Role of Extracellular Vesicles as Potential Diagnostic and/or Therapeutic Biomarkers in Chronic Cardiovascular Diseases. Front. Cell. Dev. Biol. 2022, 10, 813885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Cheng, Q.; Bai, L.; Huang, S.; Gao, J. Extracellular Vesicles in Cardiovascular Diseases: Diagnosis and Therapy. Front. Cell Dev. Biol. 2022, 10, 875376. [Google Scholar] [CrossRef] [PubMed]
- Freese, R.; Lysne, V. Niacin—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10299. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Amar, M.J.; Jeiran, K.; Stagliano, M.; Staller, E.; Playford, M.P.; Mehta, N.N.; Vaisar, T.; Remaley, A.T. Effect of niacin monotherapy on high density lipoprotein composition and function. Lipids Health Dis. 2020, 19, 190. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sharma, A.; Krishnamoorthy, P.; Garg, J.; Virmani, D.; Sharma, T.; Stefanini, G.; Kostis, J.B.; Mukherjee, D.; Sikorskaya, E. Role of Niacin in Current Clinical Practice: A Systematic Review. Am. J. Med. 2017, 130, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Vecka, M.; Perlík, F.; Staňková, B.; Hromádka, R.; Tvrzická, E.; Širc, J.; Hrib, J.; Žák, A. Pleiotropic effects of niacin: Current possibilities for its clinical use. Acta Pharm. 2016, 66, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, 83–99. [Google Scholar] [CrossRef]
- Wu, B.J.; Chen, K.; Barter, P.J.; Rye, K.A. Niacin inhibits vascular inflammation via the induction of heme oxygenase-1. Circulation 2012, 125, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kim, H.J.; Rodriguez-Iturbe, B.; Vaziri, N.D. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am. J. Physiol. Renal. Physiol. 2009, 297, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rad, E.Y.; Saboori, S.; Tammam, J.; Thondre, P.S.; Coe, S. The effect of niacin on inflammatory markers and adipokines: A systematic review and meta-analysis of interventional studies. Eur. J. Nutr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Warnholtz, A.; Wild, P.; Ostad, M.A.; Elsner, V.; Stieber, F.; Schinzel, R.; Walter, U.; Peetz, D.; Lackner, K.; Blankenberg, S.; et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: Results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis 2009, 204, 216–221. [Google Scholar] [CrossRef]
- Kaplon, R.E.; Gano, L.B.; Seals, D.R. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J. Appl. Physiol. 1985 2014, 116, 156–163. [Google Scholar] [CrossRef]
- Ganji, S.; Kamanna, S.; Kamanna, V.S.; Kashyap, M.L. Niacin increases human aortic endothelial Sirt1 activity and nitric oxide: Effect on endothelial function and vascular aging. Am. J. Transl. Res. 2023, 15, 6771–6778. [Google Scholar] [PubMed]
- Hughes-Large, J.M.; Pang, D.K.; Robson, D.L.; Chan, P.; Toma, J.; Borradaile, N.M. Niacin receptor activation improves human microvascular endothelial cell angiogenic function during lipotoxicity. Atherosclerosis 2014, 237, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.K.; Nong, Z.; Sutherland, B.G.; Sawyez, C.G.; Robson, D.L.; Toma, J.; Pickering, J.G.; Borradaile, N.M. Niacin promotes revascularization and recovery of limb function in diet-induced obese mice with peripheral ischemia. Pharmacol. Res. Perspect. 2016, 4, e00233. [Google Scholar] [CrossRef]
- Keener, A.; Sanossian, N. Niacin for stroke prevention: Evidence and rationale. CNS Neurosci. Ther. 2008, 14, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effect of niacin on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Vasc. Med. 2014, 19, 54–66. [Google Scholar] [CrossRef]
- Ferrell, M.; Wang, Z.; Anderson, J.T.; Li, X.S.; Witkowski, M.; DiDonato, J.A.; Hilser, J.R.; Hartiala, J.A.; Haghikia, A.; Cajka, T.; et al. Publisher Correction: A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat. Med. 2024, 30, 1791. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Bidstrup, H.; Nichols, D.L. Niacin increased glucose, insulin, and C-peptide levels in sedentary nondiabetic postmenopausal women. Int. J. Womens Health 2014, 6, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, B.; Lin, P.; Liu, X.; Gao, J.; Yin, D.; Zeng, J.; Liao, B.; Kang, Z. Niacin exacerbates β cell lipotoxicity in diet-induced obesity mice through upregulation of GPR109A and PPARγ2: Inhibition by incretin drugs. Front. Endocrinol. 2022, 13, 1057905. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; So, W.Y.; Li, S.Y.; Cheng, Q.; Boucher, B.J.; Leung, P.S. Niacin-induced hyperglycemia is partially mediated via niacin receptor GPR109a in pancreatic islets. Mol. Cell. Endocrinol. 2015, 404, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.M.; Alam, M.M.; Iqbal, Z.; Naseem, I. Therapeutic effect of vitamin B3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed. Pharmacother. 2018, 105, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Gao, L.; Liao, N.; Xu, X.; Yu, W.; Hong, W. Association between niacin and mortality among patients with cancer in the NHANES retrospective cohort. BMC Cancer 2022, 22, 1173. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.B. Niacin and carcinogenesis. Nutr. Cancer 2003, 46, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, J.H.; Moon, J.H.; Nazim, U.M.; Lee, Y.J.; Seol, J.W.; Hur, J.; Eo, S.K.; Lee, J.H.; Park, S.Y. Niacin alleviates TRAIL-mediated colon cancer cell death via autophagy flux activation. Oncotarget 2016, 7, 4356–4368. [Google Scholar] [CrossRef] [PubMed]
- Beltrà, M.; Pöllänen, N.; Fornelli, C.; Tonttila, K.; Hsu, M.Y.; Zampieri, S.; Moletta, L.; Corrà, S.; Porporato, P.E.; Kivelä, R.; et al. NAD+ repletion with niacin counteracts cancer cachexia. Nat. Commun. 2023, 14, 1849. [Google Scholar] [CrossRef]
- Jabbari, P.; Yazdanpanah, O.; Benjamin, D.J.; Rezazadeh Kalebasty, A. Supplement Use and Increased Risks of Cancer: Unveiling the Other Side of the Coin. Cancers 2024, 16, 880. [Google Scholar] [CrossRef]
- Nomura, M.; Ohuchi, M.; Sakamoto, Y.; Kudo, K.; Yaku, K.; Soga, T.; Sugiura, Y.; Morita, M.; Hayashi, K.; Miyahara, S.; et al. Niacin restriction with NAMPT-inhibition is synthetic lethal to neuroendocrine carcinoma. Nat. Commun. 2023, 14, 8095. [Google Scholar] [CrossRef]
- Tosti, G.; Pepe, F.; Gnagnarella, P.; Silvestri, F.; Gaeta, A.; Queirolo, P.; Gandini, S. The Role of Nicotinamide as Chemo-Preventive Agent in NMSCs: A Systematic Review and Meta-Analysis. Nutrients 2023, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.; Holmes, M.V.; Preiss, D. Cholesteryl Ester Transfer Protein Inhibition for Preventing Cardiovascular Events: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 477–487. [Google Scholar] [CrossRef]
- Mehta, N.; Dangas, K.; Ditmarsch, M.; Rensen, P.C.N.; Dicklin, M.R.; Kastelein, J.J.P. The evolving role of cholesteryl ester transfer protein inhibition beyond cardiovascular disease. Pharmacol. Res. 2023, 197, 106972. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Dusting, G.J.; Cutri, B.; Bao, S.; Drummond, G.R.; Rye, K.A.; Barter, P.J. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 2005, 111, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Javadifar, A.; Rastgoo, S.; Banach, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Foam Cells as Therapeutic Targets in Atherosclerosis with a Focus on the Regulatory Roles of Non-Coding RNAs. Int. J. Mol. Sci. 2021, 22, 2529. [Google Scholar] [CrossRef]
- Iqbal, A.J.; Barrett, T.J.; Taylor, L.; McNeill, E.; Manmadhan, A.; Recio, C.; Carmineri, A.; Brodermann, M.H.; White, G.E.; Cooper, D.; et al. Acute exposure to apolipoprotein A1 inhibits macrophage chemotaxis in vitro and monocyte recruitment in vivo. Elife 2016, 5, e15190. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Rhainds, D.; Rhéaume, E.; Dubé, M.P. CETP: Pharmacogenomics-Based Response to the CETP Inhibitor Dalcetrapib. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 396–400. [Google Scholar] [CrossRef][Green Version]
- Zhu, L.; Luu, T.; Emfinger, C.H.; Parks, B.A.; Shi, J.; Trefts, E.; Zeng, F.; Kuklenyik, Z.; Harris, R.C.; Wasserman, D.H.; et al. CETP Inhibition Improves HDL Function but Leads to Fatty Liver and Insulin Resistance in CETP-Expressing Transgenic Mice on a High-Fat Diet. Diabetes 2018, 67, 2494–2506. [Google Scholar] [CrossRef]
- Tao, H.; Yu, Z.; Dong, Y.; Liu, L.; Peng, L.; Chen, X. Lipids, lipid-lowering agents, and inflammatory bowel disease: A Mendelian randomization study. Front. Immunol. 2023, 14, 1160312. [Google Scholar] [CrossRef]
- Husain, M.A.; Laurent, B.; Plourde, M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef] [PubMed]
- Phénix, J.; Côté, J.; Dieme, D.; Recinto, S.J.; Oestereich, F.; Efrem, S.; Haddad, S.; Bouchard, M.; Munter, L.M. CETP inhibitor evacetrapib enters mouse brain tissue. Front. Pharmacol. 2023, 14, 1171937. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.V.; Zheng, J.; Agus, J.K.; Tang, X.; Lebrilla, C.B.; Jin, L.W.; Maezawa, I.; Erickson, K.; Harvey, D.J.; DeCarli, C.S.; et al. High-Density Lipoprotein Changes in Alzheimer’s Disease Are APOE Genotype-Specific. Biomedicines 2022, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, R.; Simonelli, S.; Cox, A.; Formicola, B.; Corti, R.; Cassina, V.; Nardo, L.; Mantegazza, F.; Salerno, D.; Grasso, G.; et al. The Extent of Human Apolipoprotein A-I Lipidation Strongly Affects the β-Amyloid Efflux Across the Blood-Brain Barrier in vitro. Front. Neurosci. 2019, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Borràs, C.; Mercer, A.; Sirisi, S.; Alcolea, D.; Escolà-Gil, J.C.; Blanco-Vaca, F.; Tondo, M. HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2022, 23, 9356. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Nam, H.Y.; Lew, S.Y.; Naidu, M.; David, P.; Kamalden, T.A.; Hadie, S.N.H.; Lim, L.W. Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review. Pharmaceuticals 2022, 15, 101. [Google Scholar] [CrossRef]
- Thomas, S.E.; Harrison, E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J. Lipid Res. 2016, 57, 1865–1878. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Z.T.; Su, K.Y.; Lian, Y.; Lu, L.; Hu, A.D. Apolipoprotein A1 suppresses the hypoxia-induced angiogenesis of human retinal endothelial cells by targeting PlGF. Int. J. Ophthalmol. 2023, 16, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Couret, D.; Tran-Dinh, A.; Duranteau, J.; Montravers, P.; Schwendeman, A.; Meilhac, O. High-density lipoproteins during sepsis: From bench to bedside. Crit. Care 2020, 24, 134. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Mishra, M. Impact of High-Density Lipoproteins on Sepsis. Int. J. Mol. Sci. 2022, 23, 12965. [Google Scholar] [CrossRef] [PubMed]
- Trinder, M.; Wang, Y.; Madsen, C.M.; Ponomarev, T.; Bohunek, L.; Daisely, B.A.; Julia Kong, H.; Blauw, L.L.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; et al. Inhibition of Cholesteryl Ester Transfer Protein Preserves High-Density Lipoprotein Cholesterol and Improves Survival in Sepsis. Circulation 2021, 143, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Trinder, M.; Genga, K.R.; Kong, H.J.; Blauw, L.L.; Lo, C.; Li, X.; Cirstea, M.; Wang, Y.; Rensen, P.C.N.; Russell, J.A.; et al. Cholesteryl Ester Transfer Protein Influences High-Density Lipoprotein Levels and Survival in Sepsis. Am. J. Respir. Crit. Care Med. 2019, 199, 854–862. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, E.P.C.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.A.; Tas, S.W.; Wolfs, I.M.J.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-κB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Blauw, L.L.; Wang, Y.; Willems van Dijk, K.; Rensen, P.C.N. A Novel Role for CETP as Immunological Gatekeeper: Raising HDL to Cure Sepsis? Trends Endocrinol. Metab. 2020, 31, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Wanschel, A.C.B.A.; Guizoni, D.M.; Lorza-Gil, E.; Salerno, A.G.; Paiva, A.A.; Dorighello, G.G.; Davel, A.P.; Balkan, W.; Hare, J.M.; Oliveira, H.C.F. The Presence of Cholesteryl Ester Transfer Protein (CETP) in Endothelial Cells Generates Vascular Oxidative Stress and Endothelial Dysfunction. Biomolecules 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Simic, B.; Mocharla, P.; Crucet, M.; Osto, E.; Kratzer, A.; Stivala, S.; Kühnast, S.; Speer, T.; Doycheva, P.; Princen, H.M.; et al. Anacetrapib, but not evacetrapib, impairs endothelial function in CETP-transgenic mice in spite of marked HDL-C increase. Atherosclerosis 2017, 257, 186–194. [Google Scholar] [CrossRef]
- Lazaro, C.M.; Victorio, J.A.; Davel, A.P.; Oliveira, H.C.F. CETP expression ameliorates endothelial function in female mice through estrogen receptor-α and endothelial nitric oxide synthase pathway. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, 592–600. [Google Scholar] [CrossRef]
- Siebel, A.L.; Heywood, S.E.; Kingwell, B.A. HDL and glucose metabolism: Current evidence and therapeutic potential. Front. Pharmacol. 2015, 6, 258. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, A.M.; Domingo-Espín, J.; Lundsgaard, A.M.; Kleinert, M.; Israelsen, I.; Carl, C.S.; Nicolaisen, T.S.; Kjøbsted, R.; Jeppesen, J.F.; Wojtaszewski, J.F.P.; et al. ApoA-1 improves glucose tolerance by increasing glucose uptake into heart and skeletal muscle independently of AMPKα2. Mol. Metab. 2020, 35, 100949. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wu, B.J.; Guiney, L.; Barter, P.J.; Rye, K.A. Cholesteryl ester transfer protein and its inhibitors. J. Lipid Res. 2018, 59, 772–783. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A.; Tardif, J.C.; Waters, D.D.; Boekholdt, S.M.; Breazna, A.; Kastelein, J.J. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation 2011, 124, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mambiya, M.; Shang, M.; Wang, Y.; Li, Q.; Liu, S.; Yang, L.; Zhang, Q.; Zhang, K.; Liu, M.; Nie, F.; et al. The Play of Genes and Non-genetic Factors on Type 2 Diabetes. Front. Public Health 2019, 7, 349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dangas, K.; Navar, A.M.; Kastelein, J.J.P. The effect of CETP inhibitors on new-onset diabetes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 622–632. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Hunt, N.B.; Gordillo-Marañón, M.; Charoen, P.; Drenos, F.; Kivimaki, M.; Lawlor, D.A.; Giambartolomei, C.; Papacosta, O.; Chaturvedi, N.; et al. Cholesteryl ester transfer protein (CETP) as a drug target for cardiovascular disease. Nat. Commun. 2021, 12, 5640. [Google Scholar] [CrossRef] [PubMed]
- Esau, L.; Sagar, S.; Bangarusamy, D.; Kaur, M. Identification of CETP as a molecular target for estrogen positive breast cancer cell death by cholesterol depleting agents. Genes Cancer 2016, 7, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Pillay, R.P.; Aronson, R.; Kaur, M. Cholesteryl ester transfer protein knock-down in conjunction with a cholesterol-depleting agent decreases tamoxifen resistance in breast cancer cells. IUBMB Life 2024. [Google Scholar] [CrossRef]
- Hu, L.; Dong, H.; He, L.; Shi, M.; Xiang, N.; Su, Y.; Wang, C.; Tian, Y.; Hu, Y.; Wang, H.; et al. Evacetrapib Elicits Antitumor Effects on Colorectal Cancer by Inhibiting the Wnt/β-Catenin Signaling Pathway and Activating the JNK Signaling Pathway. Biol. Pharm. Bull. 2022, 45, 1238–1245. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Huang, L.H.; Huang, H. Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery. Int. J. Mol. Sci. 2020, 21, 739. [Google Scholar] [CrossRef] [PubMed]
- Stasi, A.; Fiorentino, M.; Franzin, R.; Staffieri, F.; Carparelli, S.; Losapio, R.; Crovace, A.; Lacitignola, L.; Cimmarusti, M.T.; Murgolo, F.; et al. Beneficial effects of recombinant CER-001 high-density lipoprotein infusion in sepsis: Results from a bench to bedside translational research project. BMC Med. 2023, 21, 392. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Begue, F.; Veeren, B.; Tran-Dinh, A.; Robert, T.; Tashk, P.; Lortat-Jacob, B.; Faille, D.; de Chaisemartin, L.; Zappella, N.; et al. First Recombinant High-Density Lipoprotein Particles Administration in a Severe ICU COVID-19 Patient, a Multi-Omics Exploratory Investigation. Biomedicines 2022, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Giordano, S.; Capodanno, D.; Mehran, R.; Gibson, C.M.; Angiolillo, D.J. Clinical Pharmacokinetics and Pharmacodynamics of CSL112. Clin. Pharmacokinet. 2023, 62, 541–558. [Google Scholar] [CrossRef]
- Gibson, C.M.; Duffy, D.; Korjian, S.; Bahit, M.C.; Chi, G.; Alexander, J.H.; Lincoff, A.M.; Heise, M.; Tricoci, P.; Deckelbaum, L.I.; et al. AEGIS-II Committees and Investigators. Apolipoprotein A1 Infusions and Cardiovascular Outcomes after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1560–1571. [Google Scholar] [CrossRef]
- Vucic, E.; Rosenson, R.S. Recombinant high-density lipoprotein formulations. Curr. Atheroscler. Rep. 2011, 13, 81–87. [Google Scholar] [CrossRef]
- Riwanto, M.; Rohrer, L.; von Eckardstein, A.; Landmesser, U. Dysfunctional HDL: From structure-function-relationships to biomarkers. Handb. Exp. Pharmacol. 2015, 224, 337–366. [Google Scholar] [CrossRef]
- Chung, D.W.; Platten, K.; Ozawa, K.; Adili, R.; Pamir, N.; Nussdorfer, F.; St John, A.; Ling, M.; Le, J.; Harris, J.; et al. Low-density lipoprotein promotes microvascular thrombosis by enhancing von Willebrand factor self-association. Blood 2023, 142, 1156–1166. [Google Scholar] [CrossRef]
- Szili-Torok, T.; Annema, W.; Anderson, J.L.C.; Bakker, S.J.L.; Tietge, U.J.F. HDL Cholesterol Efflux Predicts Incident New-Onset Diabetes After Transplantation (NODAT) in Renal Transplant Recipients Independent of HDL Cholesterol Levels. Diabetes 2019, 68, 1915–1923. [Google Scholar] [CrossRef]
- McMahon, K.M.; Foit, L.; Angeloni, N.L.; Giles, F.J.; Gordon, L.I.; Thaxton, C.S. Synthetic high-density lipoprotein-like nanoparticles as cancer therapy. Cancer Treat. Res. 2015, 166, 129–150. [Google Scholar] [CrossRef]
- Raut, S.; Mooberry, L.; Sabnis, N.; Garud, A.; Dossou, A.S.; Lacko, A. Reconstituted HDL: Drug Delivery Platform for Overcoming Biological Barriers to Cancer Therapy. Front. Pharmacol. 2018, 9, 1154. [Google Scholar] [CrossRef]
- Mooberry, L.K.; Sabnis, N.A.; Panchoo, M.; Nagarajan, B.; Lacko, A.G. Targeting the SR-B1 Receptor as a Gateway for Cancer Therapy and Imaging. Front. Pharmacol. 2016, 7, 466. [Google Scholar] [CrossRef]
- Oberle, R.; Kührer, K.; Österreicher, T.; Weber, F.; Steinbauer, S.; Udonta, F.; Wroblewski, M.; Ben-Batalla, I.; Hassl, I.; Körbelin, J.; et al. The HDL particle composition determines its antitumor activity in pancreatic cancer. Life Sci. Alliance 2022, 5, e202101317. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Hetherington, I.; Totary-Jain, H. Anti-atherosclerotic therapies: Milestones, challenges, and emerging innovations. Mol. Ther. 2022, 30, 3106–3117. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Cheng, H.; Xi, Y.; Chi, X.; Wu, Y.; Liu, G. Fenofibrate treatment of rats with experimental autoimmune myocarditis by alleviating Treg/Th17 disorder. Cent. Eur. J. Immunol. 2016, 41, 64–70. [Google Scholar] [CrossRef]
- Nogueira-Recalde, U.; Lorenzo-Gómez, I.; Blanco, F.J.; Loza, M.I.; Grassi, D.; Shirinsky, V.; Shirinsky, I.; Lotz, M.; Robbins, P.D.; Domínguez, E.; et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 2019, 45, 588–605. [Google Scholar] [CrossRef]
- Shi, T.; Lu, K.; Shen, S.; Tang, Q.; Zhang, K.; Zhu, X.; Shi, Y.; Liu, X.; Teng, H.; Li, C.; et al. Fenofibrate decreases the bone quality by down regulating Runx2 in high-fat-diet induced Type 2 diabetes mellitus mouse model. Lipids Health Dis. 2017, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Asahiyama, M.; Tanaka, T.; Yamamoto, S.; Murakami, K.; Kamiya, W.; Matsumura, Y.; Osawa, T.; Anai, M.; Fruchart, J.C.; et al. Pemafibrate, a selective PPARα modulator, prevents non-alcoholic steatohepatitis development without reducing the hepatic triglyceride content. Sci. Rep. 2020, 10, 7818. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Nakamura, K.; Miyoshi, T.; Enko, K.; Kubo, M.; Murakami, M.; Hata, Y.; Kohno, K.; Morita, H.; Kusano, K.F.; et al. Impact of hypertriglyceridemia on endothelial dysfunction during statin ± ezetimibe therapy in patients with coronary heart disease. Am. J. Cardiol. 2011, 108, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Rinkūnienė, E.; Dženkevičiūtė, V.; Petrulionienė, Ž.; Majauskienė, E.; Ryliškytė, L.; Puronaitė, R.; Badarienė, J.; Navickas, R.; Laucevičius, A. Hypertriglyceridemia impact on arterial parameters in patients with metabolic syndrome. BMC Cardiovasc. Disord. 2021, 21, 393. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.E.; Kaplon, R.E.; Lucking, S.M.; Russell-Nowlan, M.J.; Eckel, R.H.; Seals, D.R. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension 2012, 60, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Ravingerová, T.; Carnická, S.; Nemčeková, M.; Ledvényiová, V.; Adameová, A.; Kelly, T.; Barlaka, E.; Galatou, E.; Khandelwal, V.K.; Lazou, A. PPAR-alpha activation as a preconditioning-like intervention in rats in vivo confers myocardial protection against acute ischaemia-reperfusion injury: Involvement of PI3K-Akt. Can. J. Physiol. Pharmacol. 2012, 90, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, A.; Hattori, Y.; Inoue, T.; Hattori, S.; Kasai, K. Fenofibrate suppresses microvascular inflammation and apoptosis through adenosine monophosphate-activated protein kinase activation. Metabolism 2011, 60, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Humbert, X.; Faucon, M.; Tournilhac, C.; Sassier, M.; Fedrizzi, S.; Milliez, P.; Lelong-Boulouard, V.; Coquerel, A.; Puddu, P.E.; et al. Association between venous thromboembolism events and fibrates: A comparative study. Therapie 2019, 74, 421–430. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, C.H.; Lee, J. Effect of fenofibrate in combination with urate lowering agents in patients with gout. Korean J. Intern. Med. 2006, 21, 89–93. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Giordano, M. Hyperuricemia and Endothelial Function: Is It a Simple Association or Do Gender Differences Play a Role in This Binomial? Biomedicines 2022, 10, 3067. [Google Scholar] [CrossRef] [PubMed]
- Waldman, B.; Ansquer, J.C.; Sullivan, D.R.; Jenkins, A.J.; McGill, N.; Buizen, L.; Davis, T.M.E.; Best, J.D.; Li, L.; Feher, M.D.; et al. FIELD investigators. Effect of fenofibrate on uric acid and gout in type 2 diabetes: A post-hoc analysis of the randomised, controlled FIELD study. Lancet Diabetes Endocrinol. 2018, 6, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Choi, Y.; Suh, C.H.; Yoon, D.; Kim, H.A. Effect of fenofibrate on uric acid level in patients with gout. Sci. Rep. 2018, 8, 16767. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.K.; Russo, L.; Ghanem, S.S.; Patel, P.R.; Oyarce, A.M.; Heinrich, G.; Najjar, S.M. Fenofibrate Decreases Insulin Clearance and Insulin Secretion to Maintain Insulin Sensitivity. J. Biol. Chem. 2016, 291, 23915–23924. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, S.; Lee, M.; Yoon, M. Fenofibrate alleviates insulin resistance by reducing tissue inflammation in obese ovariectomized mice. Nutr. Diabetes 2023, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sánchez-García, A.; Banach, M.; Atkin, S.L.; Gotto, A.M., Jr.; Sahebkar, A. Effect of fibrates on glycemic parameters: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol. Res. 2018, 132, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Gdula-Dymek, A.; Bachowski, R.; Okopien, B. Pleiotropic effects of atorvastatin and fenofibrate in metabolic syndrome and different types of pre-diabetes. Diabetes Care 2010, 33, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, F.; Zhang, X.; Cheng, R.; Ma, J.X.; Yi, J.; Li, J. Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol. Cell. Biochem. 2018, 445, 105–115. [Google Scholar] [CrossRef]
- Lian, X.; Wang, G.; Zhou, H.; Zheng, Z.; Fu, Y.; Cai, L. Anticancer Properties of Fenofibrate: A Repurposing Use. J. Cancer 2018, 9, 1527–1537. [Google Scholar] [CrossRef]
- Kogami, M.; Abe, S.; Nakamura, H.; Aoshiba, K. Fenofibrate attenuates the cytotoxic effect of cisplatin on lung cancer cells by enhancing the antioxidant defense system in vitro. Oncol. Lett. 2023, 26, 313. [Google Scholar] [CrossRef]
- Zak, Z.; Gelebart, P.; Lai, R. Fenofibrate induces effective apoptosis in mantle cell lymphoma by inhibiting the TNFalpha/NF-kappaB signaling axis. Leukemia 2010, 24, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.; Urbanska, K.; Grabacka, M.; Mullinax, J.; Marcinkiewicz, C.; Impastato, D.; Estrada, J.J.; Reiss, K. Fenofibrate-induced nuclear translocation of FoxO3A triggers Bim-mediated apoptosis in glioblastoma cells in vitro. Cell Cycle 2012, 11, 2660–2671. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.; Collet, P.; Huin-Schohn, C.; Al-Makdissy, N.; Kerjan, G.; Chedotal, A.; Donner, M.; Devignes, M.D.; Becuwe, P.; Schohn, H.; et al. Effects of PPAR and RXR ligands in semaphorin 6B gene expression of human MCF-7 breast cancer cells. Int. J. Oncol. 2006, 28, 977–984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L.; Yang, C.H.; Chen, T.H.; Li, T.T.; Liu, L.L. Fenofibrate suppresses melanogenesis in B16-F10 melanoma cells via activation of the p38 mitogen-activated protein kinase pathway. Chem. Biol. Interact. 2013, 205, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Grabacka, M.; Plonka, P.M.; Urbanska, K.; Reiss, K. Peroxisome proliferator-activated receptor alpha activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clin. Cancer Res. 2006, 12, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Hasanpourghadi, M.; Chekaoui, A.; Kurian, S.; Kurupati, R.; Ambrose, R.; Giles-Davis, W.; Saha, A.; Xiaowei, X.; Ertl, H.C.J. Treatment with the PPARα agonist fenofibrate improves the efficacy of CD8+ T cell therapy for melanoma. Mol. Ther. Oncolytics. 2023, 31, 100744. [Google Scholar] [CrossRef]
- Wan, H.; Xu, B.; Zhu, N.; Ren, B. PGC-1α activator-induced fatty acid oxidation in tumor-infiltrating CTLs enhances effects of PD-1 blockade therapy in lung cancer. Tumori 2020, 106, 55–63. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Chen, X.; Zhang, Y.; Pan, X.; Wang, G.; Ye, Y. Expression Profiling Identifies Bezafibrate as Potential Therapeutic Drug for Lung Adenocarcinoma. J. Cancer 2015, 6, 1214–1221. [Google Scholar] [CrossRef]
- Iakobishvili, Z.; Hasin, T.; Klempfner, R.; Shlomo, N.; Goldenberg, I.; Brenner, R.; Kornowski, R.; Gerber, Y. Association of Bezafibrate Treatment With Reduced Risk of Cancer in Patients With Coronary Artery Disease. Mayo. Clin. Proc. 2019, 94, 1171–1179. [Google Scholar] [CrossRef]
- Weinberg, R.L.; Brook, R.D.; Rubenfire, M.; Eagle, K.A. Cardiovascular Impact of Nutritional Supplementation With Omega-3 Fatty Acids: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Omega-3 for Cardiovascular Diseases: Where Do We Stand After REDUCE-IT and STRENGTH? Circulation 2021, 144, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits-A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Borja-Magno, A.I.; Furuzawa-Carballeda, J.; Guevara-Cruz, M.; Arias, C.; Granados, J.; Bourges, H.; Tovar, A.R.; Sears, B.; Noriega, L.G.; Gómez, F.E. Supplementation with EPA and DHA omega-3 fatty acids improves peripheral immune cell mitochondrial dysfunction and inflammation in subjects with obesity. J. Nutr. Biochem. 2023, 120, 109415. [Google Scholar] [CrossRef]
- Lin, C.; Chao, H.; Li, Z.; Xu, X.; Liu, Y.; Bao, Z.; Hou, L.; Liu, Y.; Wang, X.; You, Y.; et al. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp. Neurol. 2017, 290, 115–122. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Sley, E.G.; Rosen, E.M.; van ‘t Erve, T.J.; Sathyanarayana, S.; Barrett, E.S.; Nguyen, R.H.N.; Bush, N.R.; Milne, G.L.; Swan, S.H.; Ferguson, K.K. Omega-3 fatty acid supplement use and oxidative stress levels in pregnancy. PLoS ONE 2020, 15, e0240244. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Chen, T.B.; Yang, C.C.; Tsai, I.J.; Yang, H.W.; Hsu, Y.C.; Chang, C.M.; Yang, C.P. Neuroimmunological effects of omega-3 fatty acids on migraine: A review. Front. Neurol. 2024, 15, 1366372. [Google Scholar] [CrossRef] [PubMed]
- Shawl, M.; Geetha, T.; Burnett, D.; Babu, J.R. Omega-3 Supplementation and Its Effects on Osteoarthritis. Nutrients 2024, 16, 1650. [Google Scholar] [CrossRef] [PubMed]
- Arjomand Fard, N.; Bording-Jorgensen, M.; Wine, E. A Potential Role for Gut Microbes in Mediating Effects of Omega-3 Fatty Acids in Inflammatory Bowel Diseases: A Comprehensive Review. Curr. Microbiol. 2023, 80, 363. [Google Scholar] [CrossRef] [PubMed]
- Felau, S.M.; Sales, L.P.; Solis, M.Y.; Hayashi, A.P.; Roschel, H.; Sá-Pinto, A.L.; Andrade, D.C.O.; Katayama, K.Y.; Irigoyen, M.C.; Consolim-Colombo, F.; et al. Omega-3 Fatty Acid Supplementation Improves Endothelial Function in Primary Antiphospholipid Syndrome: A Small-Scale Randomized Double-Blind Placebo-Controlled Trial. Front. Immunol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, M.; Sayyari, A.; Aryaeian, N.; Olang, B.; Alaei, M.; Khalili, M.; Hosseini, A.; Salehi, M. Effects of omega-3 supplementation on endothelial function, vascular structure, and metabolic parameters in adolescents with type 1 diabetes mellitus: A randomized clinical trial. Front. Nutr. 2022, 9, 962773. [Google Scholar] [CrossRef] [PubMed]
- Arabi, S.M.; Bahari, H.; Chambari, M.; Bahrami, L.S.; Mohaildeen Gubari, M.I.; Watts, G.F.; Sahebkar, A. Omega-3 fatty acids and endothelial function: A GRADE-assessed systematic review and meta-analysis. Eur. J. Clin. Investig. 2024, 54, e14109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jia, R.; Li, Y.; Liu, T.; Wang, Z. Omega-3 fatty acids reduce post-operative risk of deep vein thrombosis and pulmonary embolism after surgery for elderly patients with proximal femoral fractures: A randomized placebo-controlled, double-blind clinical trial. Int. Orthop. 2020, 44, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.F.; Stivala, S.; Limacher, A.; Bonetti, N.R.; Méan, M.; Egloff, M.; Rodondi, N.; Aujesky, D.; von Schacky, C.; Lüscher, T.F.; et al. Omega-3 fatty acids predict recurrent venous thromboembolism or total mortality in elderly patients with acute venous thromboembolism. J. Thromb. Haemost. 2017, 15, 47–56. [Google Scholar] [CrossRef]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef]
- Reiner, M.F.; Bertschi, D.A.; Werlen, L.; Wiencierz, A.; Aeschbacher, S.; Lee, P.; Rodondi, N.; Moutzouri, E.; Bonati, L.; Reichlin, T.; et al. Omega-3 Fatty Acids and Markers of Thrombosis in Patients with Atrial Fibrillation. Nutrients 2024, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Haque, M.; Lugova, H.; Kumar, S. The Effect of Omega-3 Fatty Acids on Insulin Resistance. Life 2023, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Apostolova, N.; Diaz-Rua, R.; Muntane, J.; Victor, V.M. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol. Metab. 2020, 31, 725–741. [Google Scholar] [CrossRef]
- Egalini, F.; Guardamagna, O.; Gaggero, G.; Varaldo, E.; Giannone, B.; Beccuti, G.; Benso, A.; Broglio, F. The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review. Nutrients 2023, 15, 2672. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef]
- Theinel, M.H.; Nucci, M.P.; Alves, A.H.; Dias, O.F.M.; Mamani, J.B.; Garrigós, M.M.; Oliveira, F.A.; Rego, G.N.A.; Valle, N.M.E.; Cianciarullo, G.; et al. The Effects of Omega-3 Polyunsaturated Fatty Acids on Breast Cancer as a Preventive Measure or as an Adjunct to Conventional Treatments. Nutrients 2023, 15, 1310. [Google Scholar] [CrossRef]
- Tojjari, A.; Choucair, K.; Sadeghipour, A.; Saeed, A.; Saeed, A. Anti-Inflammatory and Immune Properties of Polyunsaturated Fatty Acids (PUFAs) and Their Impact on Colorectal Cancer (CRC) Prevention and Treatment. Cancers 2023, 15, 4294. [Google Scholar] [CrossRef]
- Gu, Z.; Suburu, J.; Chen, H.; Chen, Y.Q. Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. Biomed. Res. Int. 2013, 2013, 824563. [Google Scholar] [CrossRef]
- Edwards, I.J.; O’Flaherty, J.T. Omega-3 Fatty Acids and PPARgamma in Cancer. PPAR Res. 2008, 2008, 358052. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Kolovou, G.; Kolovou, V.; Katsiki, N. Volanesorsen: A New Era in the Treatment of Severe Hypertriglyceridemia. J. Clin. Med. 2022, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Brisson, D.; Tremblay, K.; Alexander, V.J.; Singleton, W.; Hughes, S.G.; Geary, R.S.; Baker, B.F.; Graham, M.J.; Crooke, R.M.; et al. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 2014, 371, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Azcutia, V.; Aikawa, E.; Figueiredo, J.L.; Croce, K.; Sonoki, H.; Sacks, F.M.; Luscinskas, F.W.; Aikawa, M. Statins suppress apolipoprotein CIII-induced vascular endothelial cell activation and monocyte adhesion. Eur. Heart J. 2013, 34, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, I.; Lupoli, R.; Di Minno, A.; Di Minno, M.N.D. Volanesorsen to treat severe hypertriglyceridaemia: A pooled analysis of randomized controlled trials. Eur. J. Clin. Investig. 2022, 52, e13841. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. ApoC3 fires up monocytes to promote tissue damage. Nat. Rev. Nephrol. 2020, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 2020, 21, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, O.; Gasperini, S.; Calzetti, F.; Gardiman, E.; Castagna, A.; Martinelli, N.; Tamassia, N.; Cassatella, M.A. CD14+-Monocytes Exposed to Apolipoprotein CIII Express Tissue Factor. Int. J. Mol. Sci. 2023, 24, 2223. [Google Scholar] [CrossRef] [PubMed]
- Digenio, A.; Dunbar, R.L.; Alexander, V.J.; Hompesch, M.; Morrow, L.; Lee, R.G.; Graham, M.J.; Hughes, S.G.; Yu, R.; Singleton, W.; et al. Antisense-Mediated Lowering of Plasma Apolipoprotein C-III by Volanesorsen Improves Dyslipidemia and Insulin Sensitivity in Type 2 Diabetes. Diabetes Care 2016, 39, 1408–1415. [Google Scholar] [CrossRef]
- Lightbourne, M.; Startzell, M.; Bruce, K.D.; Brite, B.; Muniyappa, R.; Skarulis, M.; Shamburek, R.; Gharib, A.M.; Ouwerkerk, R.; Walter, M.; et al. Volanesorsen, an antisense oligonucleotide to apolipoprotein C-III, increases lipoprotein lipase activity and lowers triglycerides in partial lipodystrophy. J. Clin. Lipidol. 2022, 16, 850–862. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Yang, F.; Feng, X.; Fu, R.; Zhao, R.; Li, X.; Li, H. Lipid-lowering drugs affect lung cancer risk via sphingolipid metabolism: A drug-target Mendelian randomization study. Front. Genet. 2023, 14, 1269291. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, B.; Wu, Q.Y.; Zhong, Y.M.; Li, Y.H. Advances in Dyslipidaemia Treatments: Focusing on ApoC3 and ANGPTL3 Inhibitors. J. Lipid Atheroscler. 2024, 13, 2–20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).