Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria
Abstract
1. Introduction
2. Pathogenic Algae, Cyanobacteria, and Fungi
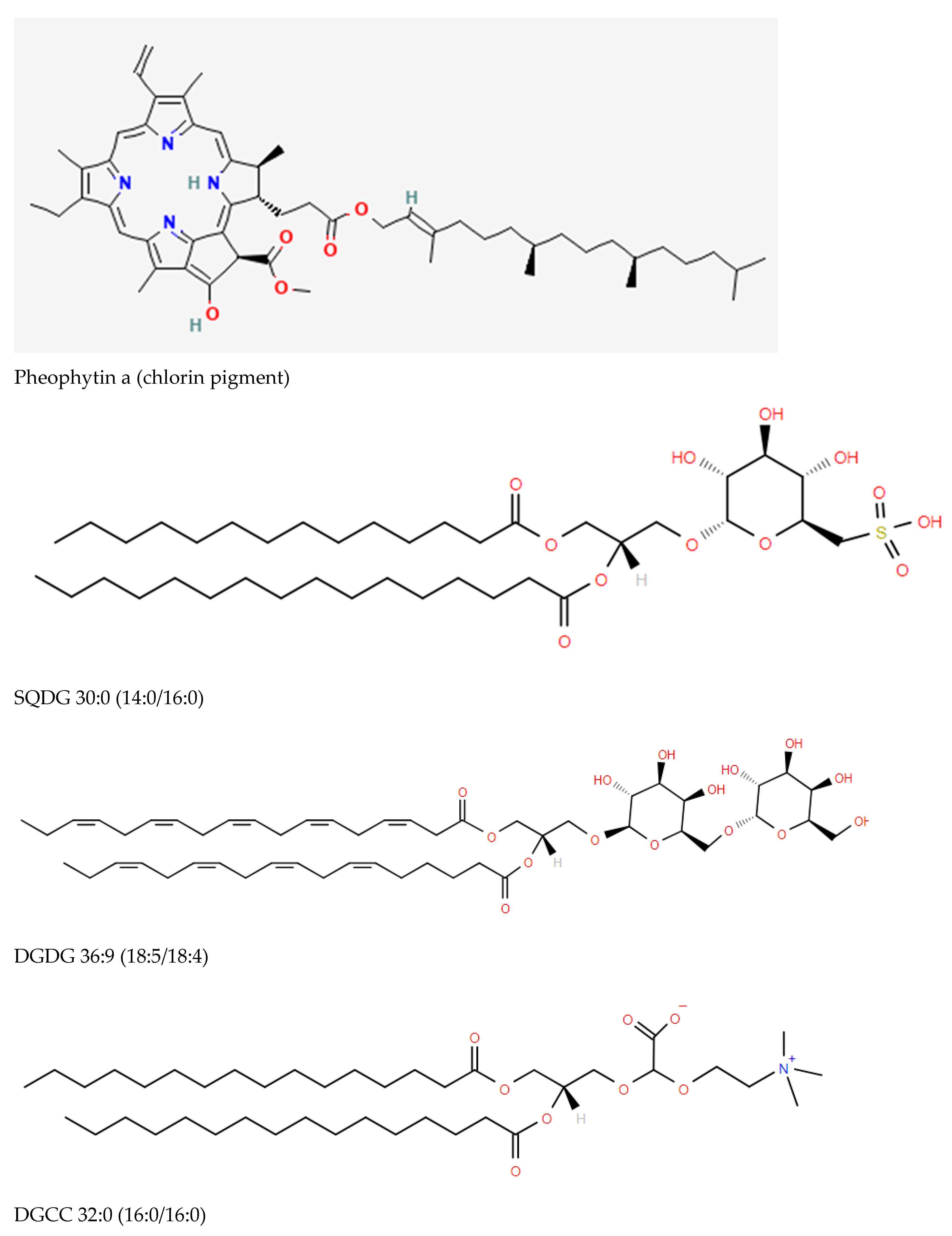
2.1. Algal and Cyanobacterial Chloroplasts
- Sulfoquinovosylmonoacylglycerols (SQMG) and sulfoquinovosyldiacylglycerols (SQDG) ([3,4,5]; Figure 1), which are sulfonolipids localized to the thylakoid membrane of chloroplasts functioning in the maintenance of photosystem II (PSII) [6,7,8]. Other possible functional roles include the regulation of DNA synthesis [9] and stimulation of glycosyltransferases involved in monohexosyldiacylglycerol (MHDG) and dihexosyldiacylglycerol (DHDG) synthesis [10].
- Monoacylglyceryl carboxyhydroxymethylcholines (MGCCs) and diacylglyceryl carboxyhydroxymethylcholines (DGCCs) possess the same zwitterionic properties as choline and ethanolamine glycerophospholipids, making them available to substitute for these membrane glycerophospholipids [11].
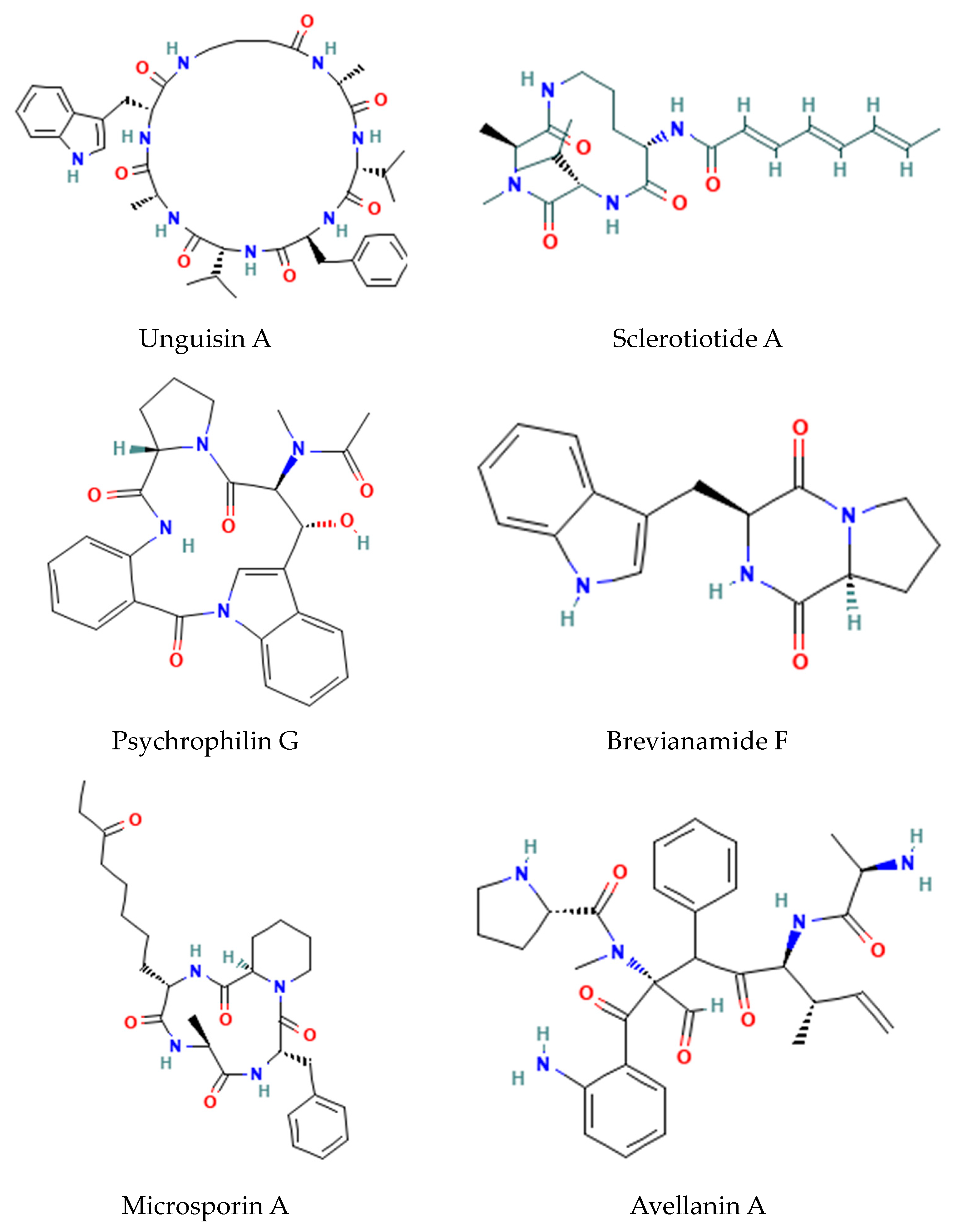
2.2. Cyanobacterial and Fungal Non-Ribosomal Peptide Synthesis (NRPS)
- Fungal GABA-containing cyclic heptapeptides: Unguisins [15];
- Fungal linear tri-peptides: Sclerotiotides, psychrophilins, and implicilliumtides [16];
- Fungal dipeptides: Brevianamides [17];
- Fungal cyclic tetrapeptide histone deacetylase inhibitors: Microsporins [16];
- Cyclic pentapeptides: Avellanins [16].
2.3. Cyanobacterial Fatty Acyl Organics
- Fatty acid esters:
- ○
- Chlorosphaerolactylates;
- ○
- Nocuolactylates;
- ○
- The actions of lactylates remain to be elucidated since they only demonstrate weak antimicrobial activity [22].
- Monoterpinoids: Malyngamides, regulators of glucose transport, via Acyl(lipoyl) transferases.
- Acylphenols: Hierridins, voltage-dependent anion-selective channel 1 blockers, via type III PKS-catalyzed chain extension.
- Glyceryl-Chloro-Dialkylresorcinols: Bartolosides involving head-to-head condensation of two fatty acid-derived precursors via dialkylresorcinol/pyrone-forming and fatty acyl AMP ligase (FAALs) enzymes. The functions of these metabolites remain to be elucidated.
2.4. Cyanobacterial and Fungal Indole Alkaloids
| Cyanobacteria Indole Family | Biological Actions |
|---|---|
| Fischerindoles |
|
| Hapalindoles |
|
| Welwitindolinones |
|
| Ambiguines |
|
| Fungal Indole Family | Biological Actions |
| Apochalasins |
|
| Fumitremorgins |
|
2.5. Carotenoids
2.6. Fungal Ergosterol
2.7. Fungal Surfactant Glycolipids
2.8. Fungal Glycosylinositol-Phosphorylceramides (GIPCs)
3. Mycobacteria
3.1. Mycolic Acids
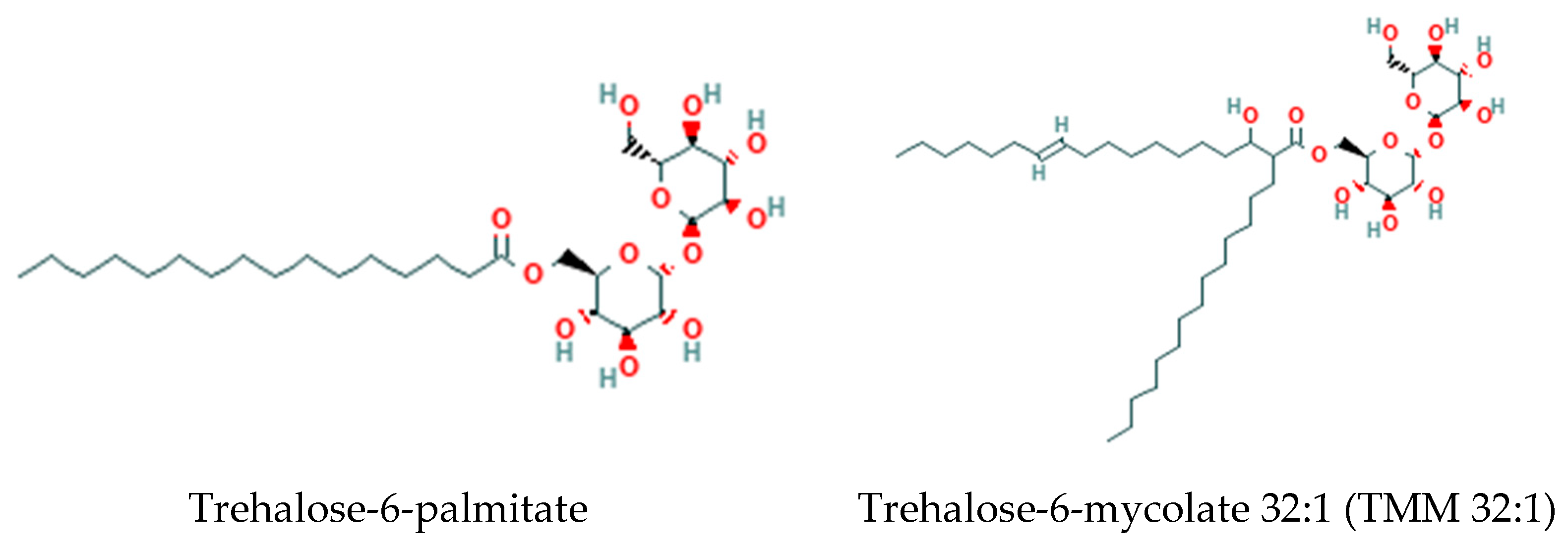
3.2. Trehalose Lipids
3.3. Glycopeptidolipids (GPLs)
4. Gram-Positive Bacteria
4.1. Lipoteichoic Acids (LTAs)
4.2. Lipopeptides
4.3. Modified Diacylglycerols
4.4. Siolipin A
4.5. Quorum Sensing (QS) Molecules: Oligopeptides
5. Gram-Negative Bacteria
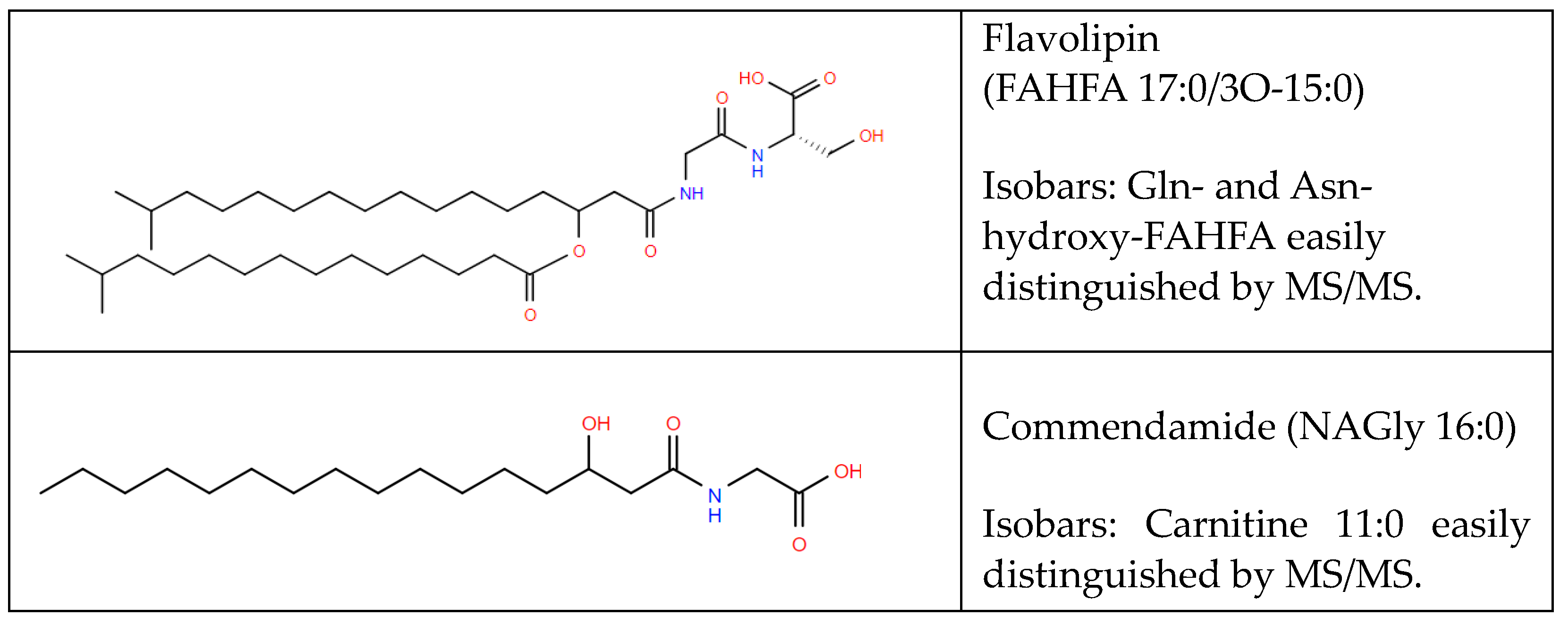
5.1. Aminoacyl Fatty Acyls of Hydroxy Fatty Acids (FAHFAs)
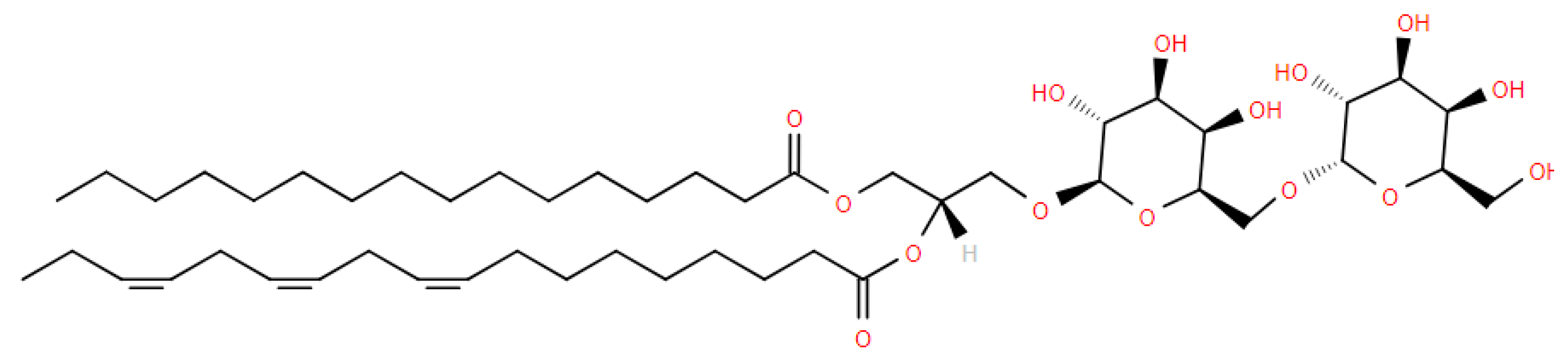
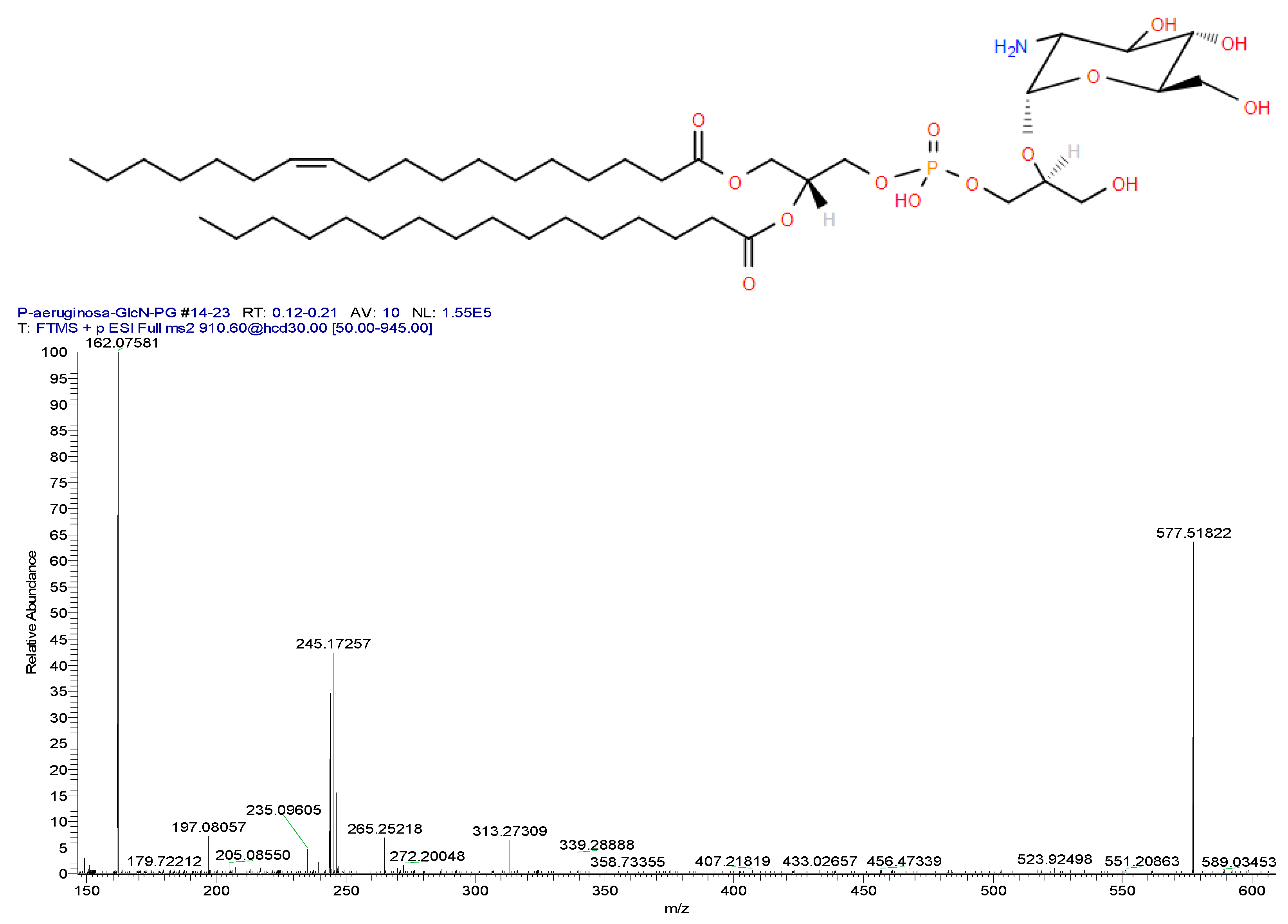
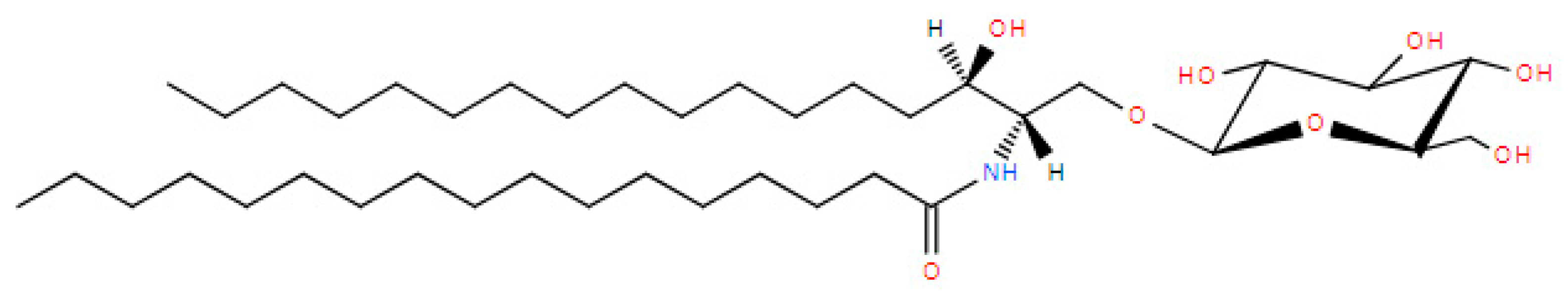
5.2. Glucosaminylphosphatidylglycerol (GlcN-PG)
5.3. Ethanolamine and Glycerol Phosphoryl Ceramides
5.4. Hexosyl Ceramides
5.5. Ceramide Sulfonates: Sulfobactins
5.6. Cholesteryl Acyl-ᾳ-Glycosides (CAGs)
5.7. Quorum Sensing (QS) Molecules: N-Acyl Homoserine Lactones (AHL)
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Janiszewska, D.; Szultka-Młyńska, M.; Pomastowski, P.; Buszewski, B. “Omic” Approaches to Bacteria and Antibiotic Resistance Identification. Int. J. Mol. Sci. 2022, 23, 9601. [Google Scholar] [CrossRef] [PubMed]
- Solntceva, V.; Kostrzewa, M.; Larrouy-Maumus, G. Detection of Species-Specific Lipids by Routine MALDI TOF Mass Spectrometry to Unlock the Challenges of Microbial Identification and Antimicrobial Susceptibility Testing. Front. Cell Infect. Microbiol. 2021, 10, 621452. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Erol, E. Construction of a Bacterial Lipidomics Analytical Platform: Pilot Validation with Bovine Paratuberculosis Serum. Metabolites 2023, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Lee, H.; Kim, M.; Hong, S.J.; Lee, H.; Kim, D.M.; Cho, B.K.; Lee, C.G.; Choi, H.K. Enhanced Production of Photosynthetic Pigments and Various Metabolites and Lipids in the Cyanobacteria Synechocystis sp. PCC 7338 Culture in the Presence of Exogenous Glucose. Biomolecules 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Treblin, M.; Sitz, T.; Rohn, S. Development of a targeted HPLC-ESI-QqQ-MS/MS method for the quantification of sulfolipids from a cyanobacterium, selected leafy vegetables, and a microalgae species. Anal. Bioanal. Chem. 2021, 413, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.S.; Lawton, L.A.; Kindt, R.; Edwards, C. Rapid analytical methods for the icroalgal and cyanobacterial biorefinery: Application on strains of industrial importance. Microbiol. Open 2021, 10, e1156. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Aoki, M.; Maru, Y.; Sonoike, K.; Minoda, A.; Tsuzuki, M. Involvement of sulfoquinovosyl diacylglycerol in the structural integrity and heat-tolerance of photosystem II. Planta 2003, 217, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Selão, T.T.; Zhang, L.; Knoppová, J.; Komenda, J.; Norling, B. Photosystem II Assembly Steps Take Place in the Thylakoid Membrane of the Cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 2016, 57, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Tsuzuki, M.; Sato, N. Involvement of sulfoquinovosyl diacylglycerol in DNA synthesis in Synechocystis sp. PCC 6803. BMC Res. Notes 2012, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Selão, T.T.; Zhang, L.; Ariöz, C.; Wieslander, Å.; Norling, B. Subcellular localization of monoglucosyldiacylglycerol synthase in Synechocystis sp. PCC6803 and its unique regulation by lipid environment. PLoS ONE 2014, 9, e88153. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Wood, M.D.; Kunigelis, S.C. Pilot Lipidomics Study of Copepods: Investigation of Potential Lipid-Based Biomarkers for the Early Detection and Quantification of the Biological Effects of Climate Change on the Oceanic Food Chain. Life 2023, 13, 2335. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yoshihara, A.; Kubota-Kawai, H. Evolutionary implications from lipids in membrane bilayers and photosynthetic complexes in cyanobacteria and chloroplasts. J. Biochem. 2023, 174, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.R.; do Amaral, S.C.; Siqueira, A.S.; Xavier, L.P.; Santos, A.V. Anabaenopeptins: What We Know So Far. Toxins 2021, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- Fewer, D.P.; Jokela, J.; Heinilä, L.; Aesoy, R.; Sivonen, K.; Galica, T.; Hrouzek, P.; Herfindal, L. Chemical diversity and cellular effects of antifungal cyclic lipopeptides from cyanobacteria. Physiol. Plant 2021, 173, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, J.; Ryager, A.; Anthoni, U.; Nielsen, P.H. Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry 2002, 60, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.A.; Remaley, A.T.; Campbell, I.M. Brevianamides A and B Are Formed Only After Conidiation Has Begun in Solid Cultures of Penicillium brevicompactum. Appl. Environ. Microbiol. 1981, 42, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.N.; Martins, T.P.; Abt, K.; Reis, J.P.A.; Figueiredo, S.; Castelo-Branco, R.; Freitas, S. Incorporation and modification of fatty acids in cyanobacterial natural products biosynthesis. Chem. Commun. 2023, 59, 4436–4446. [Google Scholar] [CrossRef] [PubMed]
- Kahn, A.; Oliveira, P.; Cuau, M.; Leão, P.N. Incorporation, fate, and turnover of free fatty acids in cyanobacteria. FEMS Microbiol. Rev. 2023, 47, fuad015. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, S.A.C.; Preto, M.; Moreira, G.; Martins, T.P.; Abt, K.; Melo, A.; Vasconcelos, V.M.; Leão, P.N. Discovery of Cyanobacterial Natural Products Containing Fatty Acid Residues. Angew. Chem. Int. Ed. Engl. 2021, 60, 10064–10072. [Google Scholar] [CrossRef]
- Szulc, J.; Ruman, T. Laser Ablation Remote-Electrospray Ionisation Mass Spectrometry (LARESI MSI) Imaging-New Method for Detection and Spatial Localization of Metabolites and Mycotoxins Produced by Moulds. Toxins 2020, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Del-Río, I.; Brugerolle de Fraissinette, N.; Castelo-Branco, R.; Oliveira, F.; Morais, J.; Redondo-Blanco, S.; Villar, C.J.; Iglesias, M.J.; Soengas, R.; Cepas, V.; et al. Chlorosphaerolactylates A-D: Natural Lactylates of Chlorinated Fatty Acids Isolated from the Cyanobacterium Sphaerospermopsis sp. LEGE 00249. J. Nat. Prod. 2020, 83, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Abt, K.; Castelo-Branco, R.; Leão, P.N. Biosynthesis of Chlorinated Lactylates in Sphaerospermopsis sp. LEGE 00249. J. Nat. Prod. 2021, 84, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Nandagopal, P.; Steven, A.N.; Chan, L.W.; Rahmat, Z.; Jamaluddin, H.; Mohd Noh, N.I. Bioactive Metabolites Produced by Cyanobacteria for Growth Adaptation and Their Pharmacological Properties. Biology 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.P.D.C.; Brancini, G.T.P.; de Castro, P.A.; Valero, C.; Ferreira Filho, J.A.; Silva, L.P.; Rocha, M.C.; Malavazi, I.; Pontes, J.G.M.; Fill, T.; et al. Aspergillus fumigatus G-Protein Coupled Receptors GprM and GprJ Are Important for the Regulation of the Cell Wall Integrity Pathway, Secondary Metabolite Production, and Virulence. mBio 2020, 11, e02458-20. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, D.G. Derivation of the multiply-branched ergot alkaloid pathway of fungi. Microb. Biotechnol. 2023, 16, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Choy, H.L.; Gaylord, E.A.; Doering, T.L. Ergosterol distribution controls surface structure formation and fungal pathogenicity. mBio 2023, 14, e0135323. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, S.; Claus, S.; Van Bogaert, I. Yeast glycolipid biosurfactants. FEBS Lett. 2018, 592, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, T. Sphingolipids from the human fungal pathogen Aspergillus fumigatus. Biochimie 2017, 141, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kalscheuer, R.; Koliwer-Brandl, H. Genetics of Mycobacterial Trehalose Metabolism. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.; Riley, L.W. Bacterial immunostat: Mycobacterium tuberculosis lipids and their role in the host immune response. Rev. Soc. Bras. Med. Trop. 2017, 50, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Pacheco, S.; Turk, J.; Purdy, G. Structural determination of glycopeptidolipids of Mycobacterium smegmatis by high-resolution multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J. Mass Spectrom. 2012, 47, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Illouz, M.; Leclercq, L.D.; Dessenne, C.; Hatfull, G.; Daher, W.; Kremer, L.; Guérardel, Y. Multiple Mycobacterium abscessus O-acetyltransferases influence glycopeptidolipid structure and colony morphotype. J. Biol. Chem. 2023, 299, 104979. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y. Alanylated lipoteichoic acid primer in Bacillus subtilis. F1000Res 2016, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Barbosa, J.; Maciel, E.; da Costa, E.; Alves, E.; Domingues, P.; Mendo, S.; Domingues, M.R.M. Lipidomic signature of Bacillus licheniformis I89 during the different growth phases unravelled by high-resolution liquid chromatography-mass spectrometry. Arch. Biochem. Biophys. 2019, 663, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Hue, N.; Serani, L.; Laprévote, O. Structural investigation of cyclic peptidolipids from Bacillus subtilis by high-energy tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kleiboeker, B.A.; Frankfater, C.; Davey, M.E.; Hsu, F.F. Lipidomic analysis of Porphyromonas gingivalis reveals novel glycerol bisphosphoceramide, phosphatidyl-, and phosphoglycerol dipeptide lipid families. J. Lipid Res. 2023, 64, 100470. [Google Scholar] [CrossRef] [PubMed]
- Frankfater, C.; Henson, W.R.; Juenger-Leif, A.; Foston, M.; Moon, T.S.; Turk, J.; Kao, J.L.; Haas, A.; Hsu, F.F. Structural Determination of a New Peptidolipid Family from Rhodococcus opacus and the Pathogen Rhodococcus equi by Multiple Stage Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2020, 31, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Goldfine, H. Lipid diversity in clostridia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158966. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Chen, L.; Gerritsen, J.; Smidt, H.; Goldfine, H. The cellular lipids of Romboutsia. Biochim. Biophys. Acta 2016, 1861 Pt A, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.C.; Aygun-Sunar, S.; Guan, Z.; Ribeiro, A.A.; Daldal, F.; Raetz, C.R.; Goldfine, H. A phosphoethanolamine-modified glycosyl diradylglycerol in the polar lipids of Clostridium tetani. J. Lipid Res. 2010, 51, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, J. Lipids of Streptomyces toyocaensis. On the structure of siolipin. Chem. Phys. Lipids 1971, 7, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, J.; Kimura, A.; Otsuka, H. Siolipin A: A new lipoamino acid ester isolated from Streptomyces sioyaensis. Biochim. Biophys. Acta 1968, 152, 808–810. [Google Scholar] [PubMed]
- Kawanami, J.; Otsuka, H. Lipids of streptomyces sioyaensis. VI. On the beta-hydroxy fatty acids in siolipin. Chem. Phys. Lipids 1969, 3, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Milly, T.A.; Renshaw, C.P.; Tal-Gan, Y. Developing multispecies quorum-sensing modulators based on the Streptococcus mitis competence-stimulating peptide. J. Biol. Chem. 2023, 299, 105448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Egea, M.C.; Akir, A.; Esteban, J. Mycobacterium biofilms. Biofilm 2023, 5, 100107. [Google Scholar] [CrossRef] [PubMed]
- Buré, C.; Le Sénéchal, C.; Macias, L.; Tokarski, C.; Vilain, S.; Brodbelt, J.S. Characterization of Isomers of Lipid A from Pseudomonas aeruginosa PAO1 by Liquid Chromatography with Tandem Mass Spectrometry with Higher-Energy Collisional Dissociation and Ultraviolet Photodissociation. Anal. Chem. 2021, 93, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Fatty Acyl Esters of Hydroxy Fatty Acid (FAHFA) Lipid Families. Metabolites 2020, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Le, A.; Palazzolo, D.L. Comparative Lipidomics of Oral Commensal and Opportunistic Bacteria. Metabolites 2024, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.J.; Kang, H.S.; Chu, J.; Huang, Y.H.; Gordon, E.A.; Reddy, B.V.; Ternei, M.A.; Craig, J.W.; Brady, S.F. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4825-34. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.K.; Brinkmann, S.; Oberpaul, M.; Patras, M.A.; Leis, B.; Marner, M.; Maitre, M.P.; Hammann, P.E.; Vilcinskas, A.; Schuler, S.M.M.; et al. Novel Glycerophospholipid, Lipo- and N-acyl Amino Acids from Bacteroidetes: Isolation, Structure Elucidation and Bioactivity. Molecules 2021, 26, 5195. [Google Scholar] [CrossRef] [PubMed]
- Nichols, F.C.; Bhuse, K.; Clark, R.B.; Provatas, A.A.; Carrington, E.; Wang, Y.H.; Zhu, Q.; Davey, M.E.; Dewhirst, F.E. Serine/Glycine Lipid Recovery in Lipid Extracts from Healthy and Diseased Dental Samples: Relationship to Chronic Periodontitis. Front. Oral Health 2021, 2, 698481. [Google Scholar] [CrossRef] [PubMed]
- Nichols, F.C.; Clark, R.B.; Maciejewski, M.W.; Provatas, A.A.; Balsbaugh, J.L.; Dewhirst, F.E.; Smith, M.B.; Rahmlow, A. A novel phosphoglycerol serine-glycine lipodipeptide of Porphyromonas gingivalis is a TLR2 ligand. J. Lipid Res. 2020, 61, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Castro, L.A.; Salgado-Morales, R.; Torres, M.; Martínez-Aguilar, L.; Lozano, L.; Vences-Guzmán, M.Á.; Guan, Z.; Dantán-González, E.; Serrano, M.; Sohlenkamp, C. Ornithine Lipids in Burkholderia spp. Pathogenicity. Front. Mol. Biosci. 2021, 7, 610932. [Google Scholar] [CrossRef] [PubMed]
- Abbes, I.; Rihouey, C.; Hardouin, J.; Jouenne, T.; De, E.; Alexandre, S. Identification by mass spectrometry of glucosaminylphosphatidylglycerol, a phosphatidylglycerol derivative, produced by Pseudomonas aeruginosa. Rapid Commun. Mass Spectrom. 2018, 32, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.A.; McDaniel, M.S.; Loughran, A.J.; Johns, J.D.; Narayanaswamy, V.; Fernandez Petty, C.; Birket, S.E.; Baker, S.M.; Barnaby, R.; Stanton, B.A.; et al. Poly (acetyl, arginyl) glucosamine disrupts Pseudomonas aeruginosa biofilms and enhances bacterial clearance in a rat lung infection model. Microbiology 2022, 168, 001121. [Google Scholar] [CrossRef] [PubMed]
- Panevska, A.; Skočaj, M.; Križaj, I.; Maček, P.; Sepčić, K. Ceramide phosphoethanolamine, an enigmatic cellular membrane sphingolipid. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Wieland Brown, L.C.; Penaranda, C.; Kashyap, P.C.; Williams, B.B.; Clardy, J.; Kronenberg, M.; Sonnenburg, J.L.; Comstock, L.E.; Bluestone, J.A.; Fischbach, M.A. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 2013, 11, e1001610. [Google Scholar] [CrossRef] [PubMed]
- Frankfater, C.F.; Sartorio, M.G.; Valguarnera, E.; Feldman, M.F.; Hsu, F.F. Lipidome of the Bacteroides Genus Containing New Peptidolipid and Sphingolipid Families Revealed by Multiple-Stage Mass Spectrometry. Biochemistry 2023, 62, 1160–1180. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Suzuki, A.; Ohira, S.; Go, S.; Ishizuka, Y.; Moriya, T.; Miyaji, Y.; Nakatsuka, T.; Hirata, K.; Nagai, A.; et al. The Urinary Bladder is Rich in Glycosphingolipids Composed of Phytoceramides. J. Lipid Res. 2022, 63, 100303. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Pfitzner, B.; Harir, M.; Schaubeck, M.; Calasan, J.; Heinzmann, S.S.; Turaev, D.; Rattei, T.; Endesfelder, D.; Castell, W.Z.; et al. Sulfonolipids as novel metabolite markers of Alistipes and Odoribacter affected by high-fat diets. Sci. Rep. 2017, 7, 11047. [Google Scholar] [CrossRef] [PubMed]
- Baronio, M.; Lattanzio, V.M.; Vaisman, N.; Oren, A.; Corcelli, A. The acylhalocapnines of halophilic bacteria: Structural details of unusual sulfonate sphingoids. J. Lipid Res. 2010, 51, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Hove, P.R.; Magunda, F.; de Mello Marques, M.A.; Islam, M.N.; Harton, M.R.; Jackson, M.; Belisle, J.T. Identification and functional analysis of a galactosyltransferase capable of cholesterol glycolipid formation in the Lyme disease spirochete Borrelia burgdorferi. PLoS ONE 2021, 16, e0252214. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Toyonaga, K.; Ishikawa, E.; Haji, S.; Okahashi, N.; Takahashi, M.; Izumi, Y.; Imamura, A.; Takato, K.; Ishida, H.; et al. Helicobacter pylori metabolites exacerbate gastritis through C-type lectin receptors. J. Exp. Med. 2021, 218, e20200815. [Google Scholar] [CrossRef] [PubMed]
- Stübs, G.; Fingerle, V.; Zähringer, U.; Schumann, R.R.; Rademann, J.; Schröder, N.W. Acylated cholesteryl galactosides are ubiquitous glycolipid antigens among Borrelia burgdorferi sensu lato. FEMS Immunol. Med. Microbiol. 2011, 63, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.M.; Ito, E.; Yamasaki, S.; Williams, S.J. Cholesteryl 6-O-acyl-α-glucosides from diverse Helicobacter spp. signal through the C-type lectin receptor Mincle. Org. Biomol. Chem. 2020, 18, 7907–7915. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Jan, H.M.; Hsieh, M.Y.; Mondal, S.; Liu, W.C.; Ko, Y.A.; Yang, W.Y.; Mong, K.T.; Chen, G.C.; Lin, C.H. Enhanced enzymatic production of cholesteryl 6’-acylglucoside impairs lysosomal degradation for the intracellular survival of Helicobacter pylori. J. Biomed. Sci. 2021, 28, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Quan, C.; Wang, X.; Zhao, P.; Fan, S. Extraction, purification and identification of bacterial signal molecules based on N-acyl homoserine lactones. Microb Biotechnol. 2011, 4, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Coquant, G.; Grill, J.P.; Seksik, P. Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity. Front. Immunol. 2020, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.A.; Ahmer, B.M. Detection of acyl-homoserine lactones by Escherichia and Salmonella. Curr. Opin. Microbiol. 2011, 14, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Borges, A.; Flament-Simon, S.C.; Simões, M. Quorum sensing architecture network in Escherichia coli virulence and pathogenesis. FEMS Microbiol. Rev. 2023, 47, fuad031. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. (Ed.) Non-targeted lipidomics utilizing constant infusion high resolution ESI mass spectrometry. In Neuromethods: Lipidomics; Springer Protocols; Humana Press: New York, NY, USA, 2017; Volume 125, pp. 13–19, ISBN 978-1-0716-0863-0, ISBN eBook 978-1-0716-0864-0. [Google Scholar]
- Wood, P.L. (Ed.) Flow injection ESI high-resolution mass spectrometry metabolomics analytical platform. In Neuromethods: Metabolomics; Springer Protocols; Humana Press: New York, NY, USA, 2021; Volume 159, pp. 1–8, ISBN 978-1-0716-0863-0, ISBN eBook 978-1-0716-0864-0. [Google Scholar]













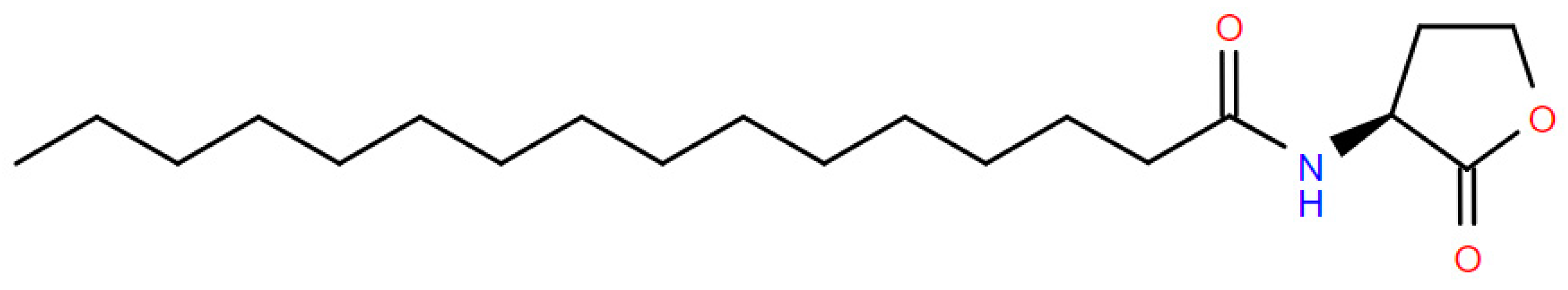

| Large Member Families | Small Member Families |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, P.L. Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria. Metabolites 2024, 14, 378. https://doi.org/10.3390/metabo14070378
Wood PL. Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria. Metabolites. 2024; 14(7):378. https://doi.org/10.3390/metabo14070378
Chicago/Turabian StyleWood, Paul L. 2024. "Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria" Metabolites 14, no. 7: 378. https://doi.org/10.3390/metabo14070378
APA StyleWood, P. L. (2024). Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria. Metabolites, 14(7), 378. https://doi.org/10.3390/metabo14070378






