The Impact of Weight Cycling on Health and Obesity
Abstract
1. Introduction
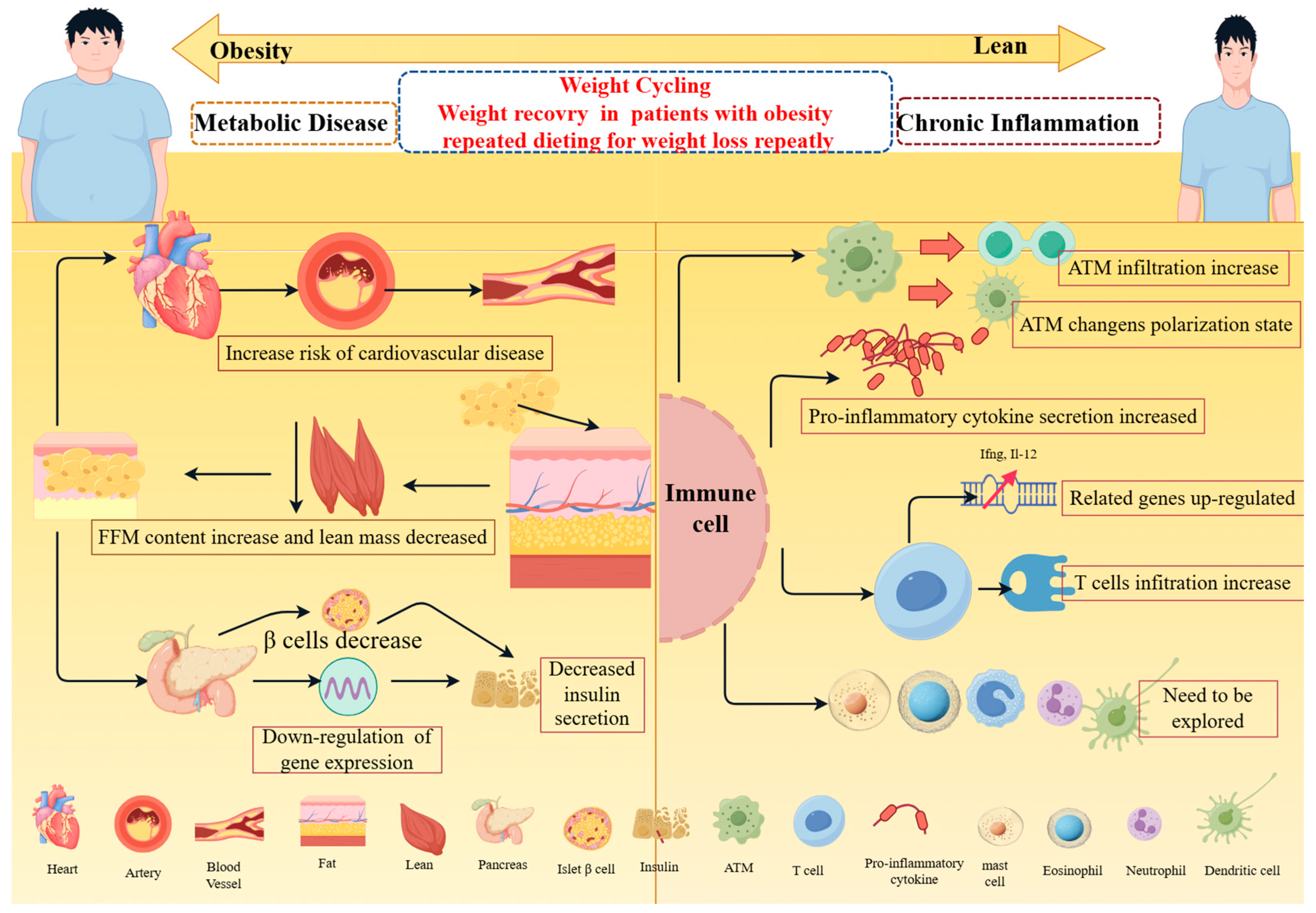
2. Effects of Weight Cycling on Tissues and Organs
2.1. Effect of Weight Cycling on Adipose Tissue
2.2. Effect of Weight Cycling on Cardiovascular Metabolism
2.3. Effect of Weight Cycling on Pancreatic Tissue
3. Effect of Weight Cycling on Chronic Inflammation
3.1. Effect of Weight Cycling on Macrophages
3.2. Effects of Body Weight Circulation on T Cells
3.3. Effects of Weight Cycling on Other Immune Cells
4. Weight Cycle Model
5. Strategy for Weight Cycling Control
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hojs, R.; Ekart, R.; Bevc, S.; Hojs, N.V. Chronic Kidney Disease and Obesity. Nephron 2023, 147, 660–664. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Montani, J.P. Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: An overview. Obes. Rev. 2015, 16 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Montani, J.P.; Schutz, Y.; Dulloo, A.G. Dieting and weight cycling as risk factors for cardiometabolic diseases: Who is really at risk? Obes. Rev. 2015, 16 (Suppl. S1), 7–18. [Google Scholar] [CrossRef]
- Petria, I.; Albuquerque, S.; Varoquaux, G.; Vie, J.-J.; Venteclef, N.; Mohammedi, K.; Roussel, R.; Camoin, M.; Perseghin, G.; Velho, G.; et al. Body-weight variability and risk of cardiovascular outcomes in patients with type 1 diabetes: A retrospective observational analysis of data from the DCCT/EDIC population. Cardiovasc. Diabetol. 2022, 21, 1–8. [Google Scholar] [CrossRef]
- Rhee, E.J. Weight Cycling and Its Cardiometabolic Impact. J. Obes. Metab.Syndr. 2017, 26, 237–242. [Google Scholar] [CrossRef]
- Delahanty, L.M.; Pan, Q.; Jablonski, K.A.; Aroda, V.R.; Watson, K.E.; Bray, G.A.; Kahn, S.E.; Florez, J.C.; Perreault, L.; Franks, P.W.; et al. Effects of Weight Loss, Weight Cycling, and Weight Loss Maintenance on Diabetes Incidence and Change in Cardiometabolic Traits in the Diabetes Prevention Program. Diabetes Care 2014, 37, 2738–2745. [Google Scholar] [CrossRef]
- Cornejo, M.A.; Ortiz, R.M. Body mass cycling and predictors of body mass regain and its impact on cardiometabolic health. Metabolism 2021, 125, 154912. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Strohacker, K. Influence of obesity physical inactivity and weight cycling on chronic inflammation. Front. Biosci. 2010, E2, 98–104. [Google Scholar] [CrossRef]
- Tannir, H.; Itani, L.; El Masri, D.; Kreidieh, D.; El Ghoch, M. Lifetime Weight Cycling and Central Fat Distribution in Females with Obesity: A Brief Report. Diseases 2020, 8, 8. [Google Scholar] [CrossRef]
- Murphy, R.A.; Patel, K.V.; Kritchevsky, S.B.; Houston, D.K.; Newman, A.B.; Koster, A.; Simonsick, E.M.; Tylvasky, F.A.; Cawthon, P.M.; Harris, T.B. Weight change, body composition, and risk of mobility disability and mortality in older adults: A population-based cohort study. J. Am. Geriatr. Soc. 2014, 62, 1476–1483. [Google Scholar] [CrossRef]
- Cereda, E.; Malavazos, A.E.; Caccialanza, R.; Rondanelli, M.; Fatati, G.; Barichella, M. Weight cycling is associated with body weight excess and abdominal fat accumulation: A cross-sectional study. Clin. Nutr. 2011, 30, 718–723. [Google Scholar] [CrossRef]
- Dandanell, S.; Ritz, C.; Verdich, E.; Dela, F.; Helge, J.W. Repeated lifestyle interventions lead to progressive weight loss: A retrospective review chart study. Scand. J. Public Health 2017, 45, 305–313. [Google Scholar] [CrossRef]
- Strychar, I.; Lavoie, M.; Messier, L.; Karelis, A.D.; Doucet, E.; Prud’Homme, D.; Fontaine, J.; Rabasa-Lhoret, R. Anthropometric, Metabolic, Psychosocial, and Dietary Characteristics of Overweight/Obese Postmenopausal Women with a History of Weight Cycling: A MONET (Montreal Ottawa New Emerging Team) Study. J. Am. Diet. Assoc. 2009, 109, 718–724. [Google Scholar] [CrossRef]
- Charlot, A.; Bringolf, A.; Debrut, L.; Mallard, J.; Charles, A.-L.; Crouchet, E.; Duteil, D.; Geny, B.; Zoll, J. Changes in Macronutrients during Dieting Lead to Weight Cycling and Metabolic Complications in Mouse Model. Nutrients 2024, 16, 646. [Google Scholar] [CrossRef]
- Dankel, S.N.; Degerud, E.M.; Borkowski, K.; Fjære, E.; Midtbø, L.K.; Haugen, C.; Solsvik, M.H.; Lavigne, A.M.; Liaset, B.; Sagen, J.V.; et al. Weight cycling promotes fat gain and altered clock gene expression in adipose tissue in C57BL/6J mice. Am. J. Physiol. Metab. 2014, 306, E210–E224. [Google Scholar] [CrossRef]
- Caslin, H.L.; Cottam, M.A.; Piñon, J.M.; Boney, L.Y.; Hasty, A.H. Weight cycling induces innate immune memory in adipose tissue macrophages. Front. Immunol. 2022, 13, 984859. [Google Scholar] [CrossRef]
- Barclay, J.L.; Husse, J.; Bode, B.; Naujokat, N.; Meyer-Kovac, J.; Schmid, S.M.; Lehnert, H.; Oster, H. Circadian Desynchrony Promotes Metabolic Disruption in a Mouse Model of Shiftwork. PLoS ONE 2012, 7, e37150. [Google Scholar] [CrossRef]
- Smith, D.L., Jr.; Yang, Y.; Mestre, L.M.; Henschel, B.; Parker, E.; Dickinson, S.; Patki, A.; Allison, D.B.; Nagy, T.R. Impact of sustained calorie restriction and weight cycling on body composition in high-fat diet-fed male and female C57BL/6J mice. Obesity 2024, 32, 959–968. [Google Scholar] [CrossRef]
- Yates, T.; Biddle GJ, H.; Henson, J.; Edwardson, C.L.; Arsenyadis, F.; Goff, L.M.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Impact of weight loss and weight gain trajectories on body composition in a population at high risk of type 2 diabetes: A prospective cohort analysis. Diabetes Obes. Metab. 2024, 26, 1008–1015. [Google Scholar] [CrossRef]
- Barbosa-Da-Silva, S.; Fraulob-Aquino, J.C.; Lopes, J.R.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Weight Cycling Enhances Adipose Tissue Inflammatory Responses in Male Mice. PLoS ONE 2012, 7, e39837. [Google Scholar] [CrossRef][Green Version]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Jeong, S.; Choi, S.; Chang, J.; Kim, K.; Kim, S.M.; Hwang, S.Y.; Son, J.S.; Lee, G.; Park, S.M. Association of weight fluctuation with cardi-ovascular disease risk among initially obese adults. Sci. Rep. 2021, 11, 10152. [Google Scholar] [CrossRef]
- Zeigler, Z.S.; Birchfield, N.; Moreno, K.; James, D.; Swan, P. Fatness and Fluctuating Body Weight: Effect on Central Vasculature. BioRes. Open Access 2018, 7, 90–100. [Google Scholar] [CrossRef]
- Byun, S.S.; Bello, N.A.; Liao, M.; Makarem, N.; Aggarwal, B. Associations of weight cycling with cardiovascular health using American Heart Association’s Life’s Simple 7 in a diverse sample of women. Prev. Med. Rep. 2019, 16, 100991. [Google Scholar] [CrossRef]
- Schofield, S.E.; Parkinson, J.R.; Henley, A.B.; Sahuri-Arisoylu, M.; Sanchez-Canon, G.J.; Bell, J.D. Metabolic dysfunction following weight cycling inmale mice. Int. J. Obes. 2017, 41, 402–411. [Google Scholar] [CrossRef]
- Ahmed, H.; Hannan, J.L.; Apolzan, J.W.; Osikoya, O.; Cushen, S.C.; Romero, S.A.; Goulopoulou, S.A. free-choice high-fat, high-sucrose diet induces hyperphagia, obesity, and cardiovascular dysfunction in female cycling and pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R472–R485. [Google Scholar] [CrossRef]
- Kakinami, L.; Knäuper, B.; Brunet, J. Weight cycling is associated with adverse cardiometabolic markers in a cross-sectional rep-resentative US sample. J. Epidemiol. Community Health 2020, 74, 662–667. [Google Scholar] [CrossRef]
- Mason, C.; Foster-Schubert, K.E.; Imayama, I.; Xiao, L.; Kong, A.; Campbell, K.L.; Duggan, C.R.; Wang, C.-Y.; Alfano, C.M.; Ulrich, C.M.; et al. History of weight cycling does not impede future weight loss or metabolic improvements in postmenopausal women. Metabolism 2013, 62, 127–136. [Google Scholar] [CrossRef]
- Carey, K.J.; Vitek, W. Weight Cycling in Women: Adaptation or Risk? Semin. Reprod. Med. 2022, 40, 277–282. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Campbell-Thompson, M.; Kusmartseva, I.; Kaestner, K.H. Organisation of the human pancreas in health and in diabetes. Diabetologia 2020, 63, 1966–1973. [Google Scholar] [CrossRef]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Weyer, C.; Hanson, R.L.; A Tataranni, P.; Bogardus, C.; Pratley, R.E. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: Evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 2000, 49, 2094–2101. [Google Scholar] [CrossRef]
- Waring, M.E.; Eaton, C.B.; Lasater, T.M.; Lapane, K.L. Incident Diabetes in Relation to Weight Patterns During Middle Age. Am. J. Epidemiol. 2010, 171, 550–556. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, J.S.; Kim, S.P.; Choi, S.H.; Yoon, H.J. The association between diabetes and hypertension with the number and extent of weight cycles determined from 6 million participants. Sci. Rep. 2022, 12, 5235. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Winn, N.C.; Cottam, M.A.; Bhanot, M.; Caslin, H.L.; Garcia, J.N.; Arrojo e Drigo, R.; Hasty, A.H. Weight Cycling Impairs Pancreatic Insulin Secretion but Does Not Perturb Whole-Body Insulin Action in Mice with Diet-Induced Obesity. Diabetes 2022, 71, 2313–2330. [Google Scholar] [CrossRef]
- Paschen, M.; Moede, T.; Valladolid-Acebes, I.; Leibiger, B.; Moruzzi, N.; Jacob, S.; García-Prieto, C.F.; Brismar, K.; Leibiger, I.B.; Berggren, P.O. Diet-induced β-cell insulin resistance results in reversible loss of functional β-cell mass. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 204–218. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose toxicity in beta-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003, 52, 581–587. [Google Scholar] [CrossRef]
- Bouche, C.; Lopez, X.; Fleischman, A.; Cypess, A.M.; O’Shea, S.; Stefanovski, D.; Bergman, R.N.; Rogatsky, E.; Stein, D.T.; Kahn, C.R.; et al. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc. Natl. Acad. Sci. USA 2010, 107, 4770–4775. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Ferrante, A.W., Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 34–38. [Google Scholar] [CrossRef]
- Caslin, H.L.; Bhanot, M.; Bolus, W.R.; Hasty, A.H. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunol. Rev. 2020, 295, 101–113. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W., Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Investig. 2010, 120, 3466–3479. [Google Scholar] [CrossRef]
- Zamarron, B.F.; Mergian, T.A.; Cho, K.W.; Martinez-Santibanez, G.; Luan, D.; Singer, K.; DelProposto, J.L.; Geletka, L.M.; Muir, L.A.; Lumeng, C.N. Macrophage Proliferation Sustains Adipose Tissue Inflammation in Formerly Obese Mice. Diabetes 2017, 66, 392–406. [Google Scholar] [CrossRef]
- Griffin, C.; Hutch, C.R.; Abrishami, S.; Stelmak, D.; Eter, L.; Li, Z.; Chang, E.; Agarwal, D.; Zamarron, B.; Varghese, M.; et al. Inflammatory responses to dietary and surgical weight loss in male and female mice. Biol. Sex Differ. 2019, 10, 16. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Bernier, M.; Wright, V.P.; Gebhardt, G.; Anandani, K.; Liu, J.; Jalilvand, A.; Bergin, S.; Wysocki, V.; Somogyi, A.; et al. Obesogenic Memory Maintains Adipose Tissue Inflammation and Insulin Resistance. Immunometabolism 2020, 2, e200023. [Google Scholar] [CrossRef]
- Anderson, E.K.; Gutierrez, D.A.; Kennedy, A.; Hasty, A.H. Weight Cycling Increases T-Cell Accumulation in Adipose Tissue and Impairs Systemic Glucose Tolerance. Diabetes 2013, 62, 3180–3188. [Google Scholar] [CrossRef]
- Van Herck, M.A.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; De Winter, B.Y.; Francque, S.M.; Vonghia, L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front. Immunol. 2019, 10, 82. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Maillard, I.; Saltiel, A.R. T-ing up inflammation in fat. Nat. Med. 2009, 15, 846–847. [Google Scholar] [CrossRef]
- Zou, J.; Lai, B.; Zheng, M.; Chen, Q.; Jiang, S.; Song, A.; Huang, Z.; Shi, P.; Tu, X.; Wang, D.; et al. CD4+ T cells memorize obesity and promote weight regain. Cell. Mol. Immunol. 2018, 15, 630–639. [Google Scholar] [CrossRef]
- Woodland, D.L.; Kohlmeier, J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009, 9, 153–161. [Google Scholar] [CrossRef]
- Cottam, M.A.; Caslin, H.L.; Winn, N.C.; Hasty, A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 2022, 13, 2950. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accu-mulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Davanzo, G.G.; Castro, G.; Moraes-Vieira, P.M.M. Immunometabolic regulation of adipose tissue resident immune cells. Curr. Opin. Pharmacol. 2021, 58, 44–51. [Google Scholar] [CrossRef]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef]
- Urb, M.; Sheppard, D.C. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef]
- Bot, I.; Shi, G.-P.; Kovanen, P.T. Mast Cells as Effectors in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2015, 35, 265–271. [Google Scholar] [CrossRef]
- Caslin, H.L.; Cottam, M.A.; Betjemann, A.M.; Mashayekhi, M.; Silver, H.J.; Hasty, A.H. Single cell RNA-sequencing suggests a novel lipid associated mast cell population following weight cycling. bioRxiv 2023. [Google Scholar] [CrossRef]
- McDonnell, M.E.; Ganley-Leal, L.M.; Mehta, A.; Bigornia, S.J.; Mott, M.; Rehman, Q.; Farb, M.G.; Hess, D.T.; Joseph, L.; Gokce, N.; et al. B Lymphocytes in Human Subcutaneous Adipose Crown-Like Structures. Obesity 2012, 20, 1372–1378. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Diaz, A.; Blomberg, B.B. Obesity accelerates age defects in B cells, and weight loss improves B cell function. Immun. Ageing 2023, 20, 35. [Google Scholar] [CrossRef]
- Cuellar-Tamez, R.X.; Villarreal-Calderon, J.R.; Rubio-Infante, N.; Castillo, E.C.; García-Garza, M.; Elizondo-Montemayor, L.; García-Rivas, G. Bariatric surgery-induced weight loss reduces B cell activating cytokines and IgG immunoglobulins related to autoim-munity. Surg. Endosc. 2021, 35, 5147–5158. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.-E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef]
- Knights, A.J.; Vohralik, E.J.; Hoehn, K.L.; Crossley, M.; Quinlan, K.G.R. Defining Eosinophil Function in Adiposity and Weight Loss. BioEssays 2018, 40, e1800098. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Dyer, K.D.; Foster, P.S. Eosinophils: Changing perspectives in health and disease. Nat. Rev. Immunol. 2013, 13, 9–22. [Google Scholar] [CrossRef]
- Barden, A.; Shinde, S.; Tsai, I.-J.; Croft, K.; Beilin, L.; Puddey, I.; Mori, T. Effect of weight loss on neutrophil resolvins in the metabolic syndrome. Prostaglandins Leukot. Essent. Fat. Acids 2019, 148, 25–29. [Google Scholar] [CrossRef]
- Sun, T.; Nguyen, A.; Gommerman, J.L. Dendritic Cell Subsets in Intestinal Immunity and Inflammation. J. Immunol. 2020, 204, 1075–1083. [Google Scholar] [CrossRef]
- Riol-Blanco, L.; Sánchez-Sánchez, N.; Torres, A.; Tejedor, A.; Narumiya, S.; Corbí, A.L.; Sánchez-Mateos, P.; Rodríguez-Fernández, J.L. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and mi-gratory speed. J. Immunol. 2005, 174, 4070–4080. [Google Scholar] [CrossRef]
- Phelan, S.; Wing, R.R.; Loria, C.M.; Kim, Y.; Lewis, C.E. Prevalence and predictors of weight-loss maintenance in a biracial cohort: Results from the coronary artery risk development in young adults study. Am. J. Prev.Med. 2010, 39, 546–554. [Google Scholar] [CrossRef]
- Vincent, H.K.; Johnson, A.J.; Sibille, K.T.; Vincent, K.R.; Cruz-Almeida, Y. Weight-cycling over 6 years is associated with pain, physical function and depression in the Osteoarthritis Initiative cohort. Sci. Rep. 2023, 13, 17045. [Google Scholar] [CrossRef]
- Rossi, A.P.; Rubele, S.; Calugi, S.; Caliari, C.; Pedelini, F.; Soave, F.; Chignola, E.; Bazzani, P.V.; Mazzali, G.; Grave, R.D.; et al. Weight Cycling as a Risk Factor for Low Muscle Mass and Strength in a Population of Males and Females with Obesity. Obesity 2019, 27, 1068–1075. [Google Scholar] [CrossRef]
- Zamarron, B.F.; Porsche, C.E.; Luan, D.; Lucas, H.R.; Mergian, T.A.; Martinez-Santibanez, G.; Cho, K.W.; DelProposto, J.L.; Geletka, L.M.; Muir, L.A.; et al. Weight Regain in Formerly Obese Mice Hastens Development of Hepatic Steatosis Due to Impaired Adipose Tissue Function. Obesity 2020, 28, 1086–1097. [Google Scholar] [CrossRef]
- Li, X.; Jiang, L.; Yang, M.; Wu, Y.W.; Sun, J.Z. Impact of weight cycling on CTRP3 expression, adipose tissue inflammation and insulin sensitivity in C57BL/6J mice. Exp. Ther. Med. 2018, 16, 2052–2059. [Google Scholar] [CrossRef]
- Wing, R.R.; Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005, 82 (Suppl. S1), 222s–225s. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metab. Clin. Exp. 2019, 92, 163–169. [Google Scholar] [CrossRef]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: An overview of 12 systematic reviews and 149 studies. Obes. Rev. 2021, 22, e13256. [Google Scholar] [CrossRef]
- Menshikova, E.V.; Ritov, V.B.; Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Coen, P.M.; Goodpaster, B.H. Calorie Re-striction-induced Weight Loss and Exercise Have Differential Effects on Skeletal Muscle Mitochondria Despite Similar Effects on Insulin Sensitivity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 73, 81–87. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef]
- De Souza, R.J.; Bray, G.A.; Carey, V.J.; Hall, K.D.; LeBoff, M.S.; Loria, C.M.; Laranjo, N.M.; Sacks, F.M.; Smith, S.R. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: Results from the POUNDS LOST trial. Am. J. Clin. Nutr. 2012, 95, 614–625. [Google Scholar] [CrossRef]
- Witjaksono, F.; Jutamulia, J.; Annisa, N.G.; Prasetya, S.I.; Nurwidya, F. Comparison of low calorie high protein and low calorie standard protein diet on waist circumference of adults with visceral obesity and weight cycling. BMC Res. Notes 2018, 11, 674. [Google Scholar] [CrossRef]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef]
- Tremblay, A.; Royer, M.-M.; Chaput, J.-P.; Doucet, É. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int. J. Obes. 2013, 37, 759–764. [Google Scholar] [CrossRef]
- Bagot, S.; Pélissier, L.; Pereira, B.; Chanséaume Bussiere, E.; Duclos, M.; Dulloo, A.; Miles-Chan, J.; Charlot, K.; Boirie, Y.; Thivel, D.; et al. Weight regain, body composition, and metabolic responses to weight loss in weight cycling athletes: A systematic review and meta-analyses. Obes.Rev. Off. J. Int. Assoc. Study Obes. 2024, 25, e13658. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Redman, L.; Heilbronn, L.K.; Martin, C.K.; Ravussin, E. Caloric restriction with or without exercise: The fitness versus fatness debate. Med. Sci. Sports Exerc. 2010, 42, 152–159. [Google Scholar] [CrossRef]
- Flore, G.; Preti, A.; Carta, M.G.; Deledda, A.; Fosci, M.; Nardi, A.E.; Loviselli, A.; Velluzzi, F. Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention. Nutrients 2022, 14, 1259. [Google Scholar] [CrossRef]
- Vibarel-Rebot, N.; Asselin, M.; Amiot, V.; Collomp, K. Short-Term Effect of Bariatric Surgery on Cardiorespiratory Response at Submaximal, Ventilatory Threshold, and Maximal Exercise in Women with Severe Obesity. Obes. Surg. 2023, 33, 1528–1535. [Google Scholar] [CrossRef]
- Lawson, W.J.; Shirey, K.B.; Spann, R.A.B.; Zamarripa, C.A.B.; Hosler, J.P.; Grayson, B.E.P. Vertical sleeve gastrectomy improves indices of metabolic disease in rodent model of surgical menopause. Menopause 2017, 24, 426–436. [Google Scholar] [CrossRef]
- Frank, A.P.; Zechner, J.F.; Clegg, D.J. Gastric Bypass Surgery but not Caloric Restriction Improves Reproductive Function in Obese Mice. Obes. Surg. 2016, 26, 467–473. [Google Scholar] [CrossRef][Green Version]
- Neumann, A.-M.; Geißler, C.; Pilorz, V.; Olejniczak, I.; Lewis, A.G.; Seeley, R.J.; Shomroni, O.; Salinas-Riester, G.; Kirchner, H.; Oster, H. Restructuring of the male mice peripheral circadian network after bariatric surgery. J. Endocrinol. 2021, 250, 67–79. [Google Scholar] [CrossRef]
- Coulter, A.A.; Greenway, F.L.; Zhang, D.; Ghosh, S.; Coulter, C.R.; James, S.L.; He, Y.; Cusimano, L.A.; Rebello, C.J. Naringenin and β-carotene convert human white adipocytes to a beige phenotype and elevate hormone- stimulated lipolysis. Front. Endocrinol. 2023, 14, 1148954. [Google Scholar] [CrossRef]
- Christoffersen, B.; Sanchez-Delgado, G.; John, L.M.; Ryan, D.H.; Raun, K.; Ravussin, E. Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity 2022, 30, 841–857. [Google Scholar] [CrossRef]
- Liu, S.; Sheng, L.; Miao, H.; Saunders, T.L.; MacDougald, O.A.; Koenig, R.J.; Xu, B. SRA Gene Knockout Protects against Diet-induced Obesity and Improves Glucose Tolerance. J. Biol. Chem. 2014, 289, 13000–13009. [Google Scholar] [CrossRef]
- Li, A.; Shi, W.; Wang, J.; Wang, X.; Zhang, Y.; Lei, Z.; Jiao, X.-Y. The gene knockout of angiotensin II type 1a receptor improves high-fat diet-induced obesity in rat via promoting adipose lipolysis. PLoS ONE 2022, 17, e0267331. [Google Scholar] [CrossRef]
- Kraus, D.; Yang, Q.; Kong, D.; Banks, A.S.; Zhang, L.; Rodgers, J.T.; Pirinen, E.; Pulinilkunnil, T.C.; Gong, F.; Wang, Y.-C.; et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 2014, 508, 258–262. [Google Scholar] [CrossRef]
- Radlinger, B.; Ress, C.; Folie, S.; Salzmann, K.; Lechuga, A.; Weiss, B.; Salvenmoser, W.; Graber, M.; Hirsch, J.; Holfeld, J.; et al. Empagliflozin protects mice against diet-induced obesity, insulin resistance and hepatic steatosis. Diabetologia 2023, 66, 754–767. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; He, W.; Yang, G.; Zhu, L.; Liu, X. The Impact of Weight Cycling on Health and Obesity. Metabolites 2024, 14, 344. https://doi.org/10.3390/metabo14060344
Wang H, He W, Yang G, Zhu L, Liu X. The Impact of Weight Cycling on Health and Obesity. Metabolites. 2024; 14(6):344. https://doi.org/10.3390/metabo14060344
Chicago/Turabian StyleWang, Huan, Wenbi He, Gaoyuan Yang, Lin Zhu, and Xiaoguang Liu. 2024. "The Impact of Weight Cycling on Health and Obesity" Metabolites 14, no. 6: 344. https://doi.org/10.3390/metabo14060344
APA StyleWang, H., He, W., Yang, G., Zhu, L., & Liu, X. (2024). The Impact of Weight Cycling on Health and Obesity. Metabolites, 14(6), 344. https://doi.org/10.3390/metabo14060344





