Development of an LC-HRMS/MS Method for Quantifying Steroids and Thyroid Hormones in Capillary Blood: A Potential Tool for Assessing Relative Energy Deficiency in Sport (RED-S)
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals and Reagents
2.2. Stock and Working Solutions
2.3. VAMS® Sample Preparation
2.3.1. VAMS® Sample Extraction
2.3.2. Venous Blood Sample Collection and Preparation
2.4. Instrumentation and Analytical Conditions
2.5. Quantification/Statistical Evaluation of Data
2.6. Method Validation
2.6.1. Selectivity
2.6.2. Linearity and Calibration Curve
2.6.3. Imprecision
2.6.4. Accuracy
2.6.5. Limit of Quantification (LOQ)
2.6.6. Recovery
2.6.7. Matrix Effects
2.6.8. Carryover
2.6.9. Robustness
2.6.10. Stability
2.7. Comparison of Serum and VAMS® Samples
2.8. Proof of Concept
3. Results and Discussion
3.1. Method Development and Validation
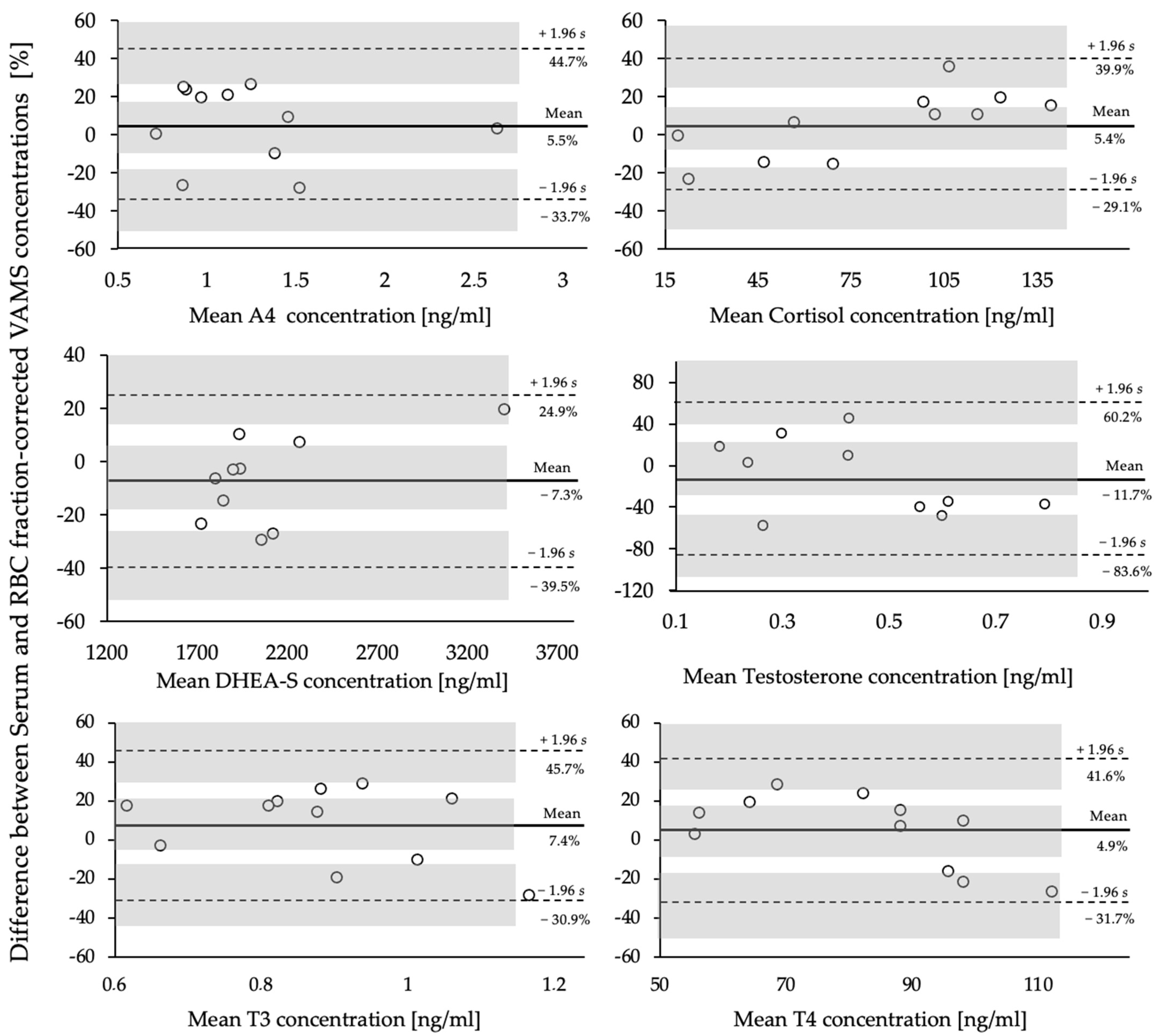
3.2. Comparison of VAMS® and Serum Samples
3.3. Proof of Concept
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Areta, J.L.; Taylor, H.L.; Koehler, K. Low Energy Availability: History, Definition and Evidence of Its Endocrine, Metabolic and Physiological Effects in Prospective Studies in Females and Males. Eur. J. Appl. Physiol. 2021, 121, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Ackerman, K.E.; Bailey, D.M.; Burke, L.M.; Constantini, N.; Hackney, A.C.; Heikura, I.A.; Melin, A.; Pensgaard, A.M.; Stellingwerff, T.; et al. 2023 International Olympic Committee’s (IOC) Consensus Statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sport. Med. 2023, 57, 1073–1097. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; Tenforde, A.S.; Parziale, A.L.; Holtzman, B.; Ackerman, K.E. Endocrine Effects of Relative Energy Deficiency in Sport. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 335–349. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Koltun, K.J.; Williams, N.I. The Role of Energy Availability in Reproductive Function in the Female Athlete Triad and Extension of Its Effects to Men: An Initial Working Model of a Similar Syndrome in Male Athletes. Sports Med. 2019, 49, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Van Uytfanghe, K.; Heughebaert, L.; Stove, C.P. Self-Sampling at Home Using Volumetric Absorptive Microsampling: Coupling Analytical Evaluation to Volunteers’ Perception in the Context of a Large Scale Study. Clin. Chem. Lab. Med. 2021, 59, e185–e187. [Google Scholar] [CrossRef] [PubMed]
- Heikura, I.A.; Uusitalo, A.L.T.; Stellingwerff, T.; Bergland, D.; Mero, A.A.; Burke, L.M. Low Energy Availability Is Difficult to Assess but Outcomes Have Large Impact on Bone Injury Rates in Elite Distance Athletes. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Dipla, K.; Kraemer, R.R.; Constantini, N.W.; Hackney, A.C. Relative Energy Deficiency in Sports (RED-S): Elucidation of Endocrine Changes Affecting the Health of Males and Females. Hormones 2021, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Tuma, C.; Thomas, A.; Braun, H.; Thevis, M. Quantification of 25-Hydroxyvitamin D2 and D3 in Mitra® Devices with Volumetric Absorptive Microsampling Technology (VAMS®) by UHPLC-HRMS for Regular Vitamin D Status Monitoring. J. Pharm. Biomed. Anal. 2023, 228, 115314. [Google Scholar] [CrossRef] [PubMed]
- Taves, M.D.; Schmidt, K.L.; Ruhr, I.M.; Kapusta, K.; Prior, N.H.; Soma, K.K. Steroid Concentrations in Plasma, Whole Blood and Brain: Effects of Saline Perfusion to Remove Blood Contamination from Brain. PLoS ONE 2010, 5, e15727. [Google Scholar] [CrossRef]
- Ochi, Y.; Hachiya, T.; Yoshimura, M.; Miyazaki, T.; Majima, T.; Takahashi, H. Determination of Triiodothyronine in Red Blood Cells by Radioimmunoassay. Endocrinol. Jpn. 1976, 23, 207–213. [Google Scholar] [CrossRef]
- Mendel, C.M.; Cavalieri, R.R. Red Blood Cell Thyroxine in Nonthyroid Illness and in Heparin-Treated Patients. J. Clin. Endocrinol. Metab. 1984, 58, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- FDA; Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine(CVM). Bioanalytical Method Validation Guidance for Industry. Biopharm. Drug Dispos. 2018, 44. [Google Scholar] [CrossRef]
- Fragala, M.S.; Goldman, S.M.; Goldman, M.M.; Bi, C.; Colletti, J.D.; Arent, S.M.; Walker, A.J.; Clarke, N.J. Measurement of Cortisol and Testosterone in Athletes: Accuracy of Liquid Chromatography-Tandem Mass Spectrometry Assays for Cortisol and Testosterone Measurement in Whole-Blood Microspecimens. J. Strength. Cond. Res. 2018, 32, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.J.; Adaway, J.E.; Hawley, J.M.; Keevil, B.G. Quantification of Testosterone, Androstenedione and 17-Hydroxyprogesterone in Whole Blood Collected Using Mitra Microsampling Devices. Ann. Clin. Biochem. 2020, 57, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ponzetto, F.; Parasiliti-Caprino, M.; Leoni, L.; Marinelli, L.; Nonnato, A.; Nicoli, R.; Kuuranne, T.; Ghigo, E.; Mengozzi, G.; Settanni, F. LC-MS/MS Measurement of Endogenous Steroid Hormones and Phase II Metabolites in Blood Volumetric Absorptive Microsampling (VAMS) for Doping Control Purposes. Clin. Chim. Acta 2024, 557, 117890. [Google Scholar] [CrossRef] [PubMed]
- Billett, H.H. Hemoglobin and Hematocrit. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Brun, J.-F. Exercise Hemorheology as a Three Acts Play with Metabolic Actors: Is It of Clinical Relevance? Clin. Hemorheol. Microcirc. 2002, 26, 155–174. [Google Scholar] [PubMed]
- Belviranli, M.; Okudan, N.; Kabak, B. The Effects of Acute High-Intensity Interval Training on Hematological Parameters in Sedentary Subjects. Med. Sci. 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Chace, D.H.; Singleton, S.; DiPerna, J.; Aiello, M.; Foley, T. Rapid Metabolic and Newborn Screening of Thyroxine (T4) from Dried Blood Spots by MS/MS. Clin. Chim. Acta 2009, 403, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Hofman, L.F.; Foley, T.P.; Henry, J.J.; Naylor, E.W. Assays for Thyroid-Stimulating Hormone Using Dried Blood Spotted Filter Paper Specimens to Screen for Hypothyroidism in Older Children and Adults. J. Med. Screen. 2003, 10, 5–10. [Google Scholar] [CrossRef]
- Iwayama, H.; Kakita, H.; Iwasa, M.; Adachi, S.; Takano, K.; Kikuchi, M.; Fujisawa, Y.; Osaka, H.; Yamada, Y.; Okumura, A.; et al. Measurement of Reverse Triiodothyronine Level and the Triiodothyronine to Reverse Triiodothyronine Ratio in Dried Blood Spot Samples at Birth May Facilitate Early Detection of Monocarboxylate Transporter 8 Deficiency. Thyroid 2021, 31, 1316–1321. [Google Scholar] [CrossRef]
- De Souza, M.J.; Lee, D.K.; VanHeest, J.L.; Scheid, J.L.; West, S.L.; Williams, N.I. Severity of Energy-Related Menstrual Disturbances Increases in Proportion to Indices of Energy Conservation in Exercising Women. Fertil. Steril. 2007, 88, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Vanheest, J.L.; Rodgers, C.D.; Mahoney, C.E.; De Souza, M.J. Ovarian Suppression Impairs Sport Performance in Junior Elite Female Swimmers. Med. Sci. Sports Exerc. 2014, 46, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Harber, V.J.; Petersen, S.R.; Chilibeck, P.D. Thyroid Hormone Concentrations and Muscle Metabolism in Amenorrheic and Eumenorrheic Athletes. Can. J. Appl. Physiol. 1998, 23, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Heath, E.M. Induction of Low-T3 Syndrome in Exercising Women Occurs at a Threshold of Energy Availability. Am. J. Physiol. 1994, 266, R817–R823. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.M.; Ackerman, K.E. Endocrine and Metabolic Repercussions of Relative Energy Deficiency in Sport. Curr. Opin. Endocr. Metab. Res. 2019, 9, 56–65. [Google Scholar] [CrossRef]
- Tornberg, Å.B.; Melin, A.; Koivula, F.M.; Johansson, A.; Skouby, S.; Faber, J.; Sjödin, A. Reduced Neuromuscular Performance in Amenorrheic Elite Endurance Athletes. Med. Sci. Sports Exerc. 2017, 49, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.L.; Mortola, J.F.; Girton, L.; Suh, B.; Laughlin, G.; Pham, P.; Yen, S.S. Neuroendocrine Aberrations in Women with Functional Hypothalamic Amenorrhea. J. Clin. Endocrinol. Metab. 1989, 68, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Laughlin, G.A.; Mortola, J.F.; Girton, L.; Nelson, J.C.; Yen, S.S. Hypothalamic-Pituitary-Thyroidal Function in Eumenorrheic and Amenorrheic Athletes. J. Clin. Endocrinol. Metab. 1992, 75, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Nys, G.; Cobraiville, G.; Kok, M.G.M.; Wéra, O.; Servais, A.-C.; Fillet, M. Comparison of Nanofluidic and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry for High Sensitive Pharmacokinetic Studies of Estrogens Starting from Whole Blood Microsampling. J. Chromatogr. A 2017, 1524, 160–168. [Google Scholar] [CrossRef]
- Nys, G.; Gallez, A.; Kok, M.G.M.; Cobraiville, G.; Servais, A.-C.; Piel, G.; Pequeux, C.; Fillet, M. Whole Blood Microsampling for the Quantitation of Estetrol without Derivatization by Liquid Chromatography-Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2017, 140, 258–265. [Google Scholar] [CrossRef]
- Verdonk, S.J.E.; Vesper, H.W.; Martens, F.; Sluss, P.M.; Hillebrand, J.J.; Heijboer, A.C. Estradiol Reference Intervals in Women during the Menstrual Cycle, Postmenopausal Women and Men Using an LC-MS/MS Method. Clin. Chim. Acta 2019, 495, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Keski-Rahkonen, P.; Desai, R.; Jimenez, M.; Harwood, D.T.; Handelsman, D.J. Measurement of Estradiol in Human Serum by LC-MS/MS Using a Novel Estrogen-Specific Derivatization Reagent. Anal. Chem. 2015, 87, 7180–7186. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, M.T.; de Kleijne, V.; Martens, F.; Heijboer, A.C. Hematocrit and Standardization in DBS Analysis: A Practical Approach for Hormones Mainly Present in the Plasma Fraction. Clin. Chim. Acta 2021, 520, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.V.; Wald, J.A.; Swerdloff, R.S.; Wang, C.; Wu, F.C.W.; Bowers, L.D.; Matsumoto, A.M. Large Divergence in Testosterone Concentrations between Men and Women: Frame of Reference for Elite Athletes in Sex-Specific Competition in Sports, a Narrative Review. Clin. Endocrinol. 2019, 90, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Goldman, N.; Glei, D.A. Sex Differences in the Relationship between DHEAS and Health. Exp. Gerontol. 2007, 42, 979–987. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.A.K.J.; Ackerman, K.E.; Sipilä, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological Considerations for Studies in Sport and Exercise Science with Women as Participants: A Working Guide for Standards of Practice for Research on Women. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef]

| Analytes | Precursor Ion [M+H]+ [m/z] | Precursor Ion [M-H]− [m/z] | Quantifier Ion [m/z] | Qualifier Ion [m/z] | Retention Time (RT) [min] | Collision Energy (CE) [eV] |
|---|---|---|---|---|---|---|
| 4-Androstenedione C19H26O2 | 287.2011 | 97.07 | 109.06 | 8.36 | 30 | |
| Cortisol (F) C21H30O5 | 363.2171 | 121.07 | 327.20 | 7.45 | 25 | |
| D4-Cortisol C21H26D4O5 | 366.2417 | 121.07 | 7.40 | 35 | ||
| DHEA-S C19H28O5S | 367.1578 | 96.96 | 8.22 | 35 | ||
| Progesterone (P4) C21H30O2 | 315.2324 | 109.06 | 97.06 | 9.36 | 20 | |
| D9-Progesterone C21H21D9O2 | 324.2887 | 100.08 | 9.25 | 22 | ||
| Testosterone (T) C19H28O2 | 289.2167 | 97.07 | 109.06 | 8.64 | 25 | |
| D3-Testosterone C19D3H25O2 | 292.2355 | 97.06 | 8.68 | 25 | ||
| Triiodothyronine (T3) C15H12I3NO4 | 651.7978 | 605.79 | 507.87 | 7.83 | 20 | |
| C13T3 13C6C9H12I3NO4 | 657.7438 | 611.81 | 8.15 | 25 | ||
| Thyroxine (T4) C15H11I4NO4 | 777.6866 | 731.69 | 633.76 | 8.37 | 25 | |
| C13T4 13C6C9H11I4NO4 | 783.7068 | 737.70 | 8.31 | 25 | ||
| 17β-Estradiol + DMIS C23H30N2O4S | 431.1926 | 367.2 | 9.26 | 50 | ||
| D4-Estradiol + DMIS C23D4H26N2O4S | 435.2177 | 371.2 | 9.30 | 45 |
| Substance | Intraday Precision [%] | Interday Precision [%] | Accuracy [%] | Linearity [R2] | LOQ [ng/mL] | Recovery [%] | Matrix Effects [%] | Stability [Days] | |
|---|---|---|---|---|---|---|---|---|---|
| (20 °C) | (−18 °C) | ||||||||
| 4-Androstenedione (A4) | 5 | 7 | 87 | 0.99 | 0.5 | 81 | 91 | >28 | >28 |
| Cortisol (F) | 4 | 7 | 92 | 0.99 | 2.5 | 66 | 85 | 14 | >28 |
| DHEA-S | 4 | 10 | 102 | 0.99 | 2.5 | 63 | 95 | >28 | >28 |
| Progesterone (P4) | 8 | 12 | 93 | 0.99 | 0.8 | 77 | 88 | >28 | >28 |
| Testosterone (T) | 2 | 3 | 96 | 0.99 | 0.02 | 84 | 92 | >28 | >28 |
| Triiodothyronine (T3) | 3 | 10 | 101 | 0.99 | 0.1 | 31 | 87 | >28 | >28 |
| Thyroxine (T4) | 5 | 9 | 119 | 0.99 | 0.5 | 27 | 89 | >28 | >28 |
| 17β-Estradiol + DMIS (E2) | 3 | 4 | 108 | 0.99 | 0.04 | 87 | 90 | >28 | >28 |
| Analyte | Serum [ng/mL] | VAMS® [ng/mL] | Mean Bias [ng/mL] | 95% Limits of Agreement (LoA) | Significance (p-Value) |
|---|---|---|---|---|---|
| A4 | 1.24 (±0.5) | 1.19 (±0.6) | 0.05 | −0.40–0.50 | 0.457 |
| F | 81.54 (±46.6) | 73.69 (±35.8) | −9.28 | −19.97–38.54 | 0.130 |
| DHEA-S | 1932.07 (±675.4) | 2050.00 (±496.0) | −92.15 | −820.87–636.57 | 0.193 |
| T | 0.40 (±0.15) | 0.48 (±0.27) | −0.10 | −0.44–0.24 | 0.167 |
| T3 | 0.92 (±0.17) | 0.87 (±0.22) | 0.06 | −0.32–0.43 | 0.473 |
| T4 | 84.76 (±15.5) | 83.79 (±24.9) | 2.00 | −31.05–35.04 | 0.865 |
| Male (n = 18) | Female (n = 32) | Significance (p-Value) | |
|---|---|---|---|
| Age [years] | 27.2 (±5.8) | 24.0 (±5.0) | 0.001 |
| Height [cm] | 182.8 (±7.0) | 170.3 (±5.4) | 0.001 |
| Body Weight [kg] | 80.8 (±13.6) | 63.1 (±6.8) | 0.001 |
| BMI | 24.1 (±3.5) | 21.7 (±2.4) | 0.009 |
| A4 [ng/mL] | 1.2 (±0.5) | 1.7 (±0.7) | 0.019 |
| Cortisol (F) [ng/mL] | 136.3 (±23.5) | 136.5 (±39.5) | 0.980 |
| DHEA-S [ng/mL] | 1826.7 (±843.8) | 1535.8 (±728.4) | 0.228 |
| 17β-Estradiol [ng/mL] | N/A | 0.07 (±0.07) | N/A |
| Progesterone [ng/mL] | N/A | 4.0 (±4.7) | N/A |
| Testosterone (T) [ng/mL] | 5.2 (±1.4) | 0.2 (±0.1) | 0.001 |
| T3 [ng/mL] | 0.59 (±0.2) | 0.56 (±0.1) | 0.471 |
| T4 [ng/mL] | 37.3 (±12.6) | 36.3 (±7.6) | 0.728 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuma, C.; Thomas, A.; Braun, H.; Thevis, M. Development of an LC-HRMS/MS Method for Quantifying Steroids and Thyroid Hormones in Capillary Blood: A Potential Tool for Assessing Relative Energy Deficiency in Sport (RED-S). Metabolites 2024, 14, 328. https://doi.org/10.3390/metabo14060328
Tuma C, Thomas A, Braun H, Thevis M. Development of an LC-HRMS/MS Method for Quantifying Steroids and Thyroid Hormones in Capillary Blood: A Potential Tool for Assessing Relative Energy Deficiency in Sport (RED-S). Metabolites. 2024; 14(6):328. https://doi.org/10.3390/metabo14060328
Chicago/Turabian StyleTuma, Chiara, Andreas Thomas, Hans Braun, and Mario Thevis. 2024. "Development of an LC-HRMS/MS Method for Quantifying Steroids and Thyroid Hormones in Capillary Blood: A Potential Tool for Assessing Relative Energy Deficiency in Sport (RED-S)" Metabolites 14, no. 6: 328. https://doi.org/10.3390/metabo14060328
APA StyleTuma, C., Thomas, A., Braun, H., & Thevis, M. (2024). Development of an LC-HRMS/MS Method for Quantifying Steroids and Thyroid Hormones in Capillary Blood: A Potential Tool for Assessing Relative Energy Deficiency in Sport (RED-S). Metabolites, 14(6), 328. https://doi.org/10.3390/metabo14060328






