Evaluation of the Metabolite Profile of Fish Oil Omega-3 Fatty Acids (n-3 FAs) in Micellar and Enteric-Coated Forms—A Randomized, Cross-Over Human Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Omega-3 (n-3) Formulations
- Standard (STD) softgel capsules are composed of 1000 mg fish oil (molecularly distilled; anchovy, tuna), containing 600 mg Omega-3 fatty acids which provide: 400 mg of EPA and 200 mg of DHA; other ingredients are gelatin (bovine) and glycerin.
- Enteric-coated (ENT) softgel capsules are composed of 1170 mg fish oil (molecularly distilled, ultra-purified; anchovy, sardine, and/or mackerel), containing 600 mg Omega-3 fatty acids which provide: 400 mg EPA and 200 mg DHA; other ingredients are gelatin, glycerin, purified water, pectin, and natural vitamin E (patented Enteripure® softgels).
- Micellar (LMF) softgel capsules are composed of 585 mg fish oil (molecularly distilled), containing 374 mg of Omega-3 fatty acids which provide: 200 mg EPA, 133 mg DHA and 41 mg DPA3; other ingredients are glycerin, water, gelatin bovine bone, cocoa powder, xylitol, medium chain triglycerides, and methylsulfonylmethane (patent pending LipoMicel®).
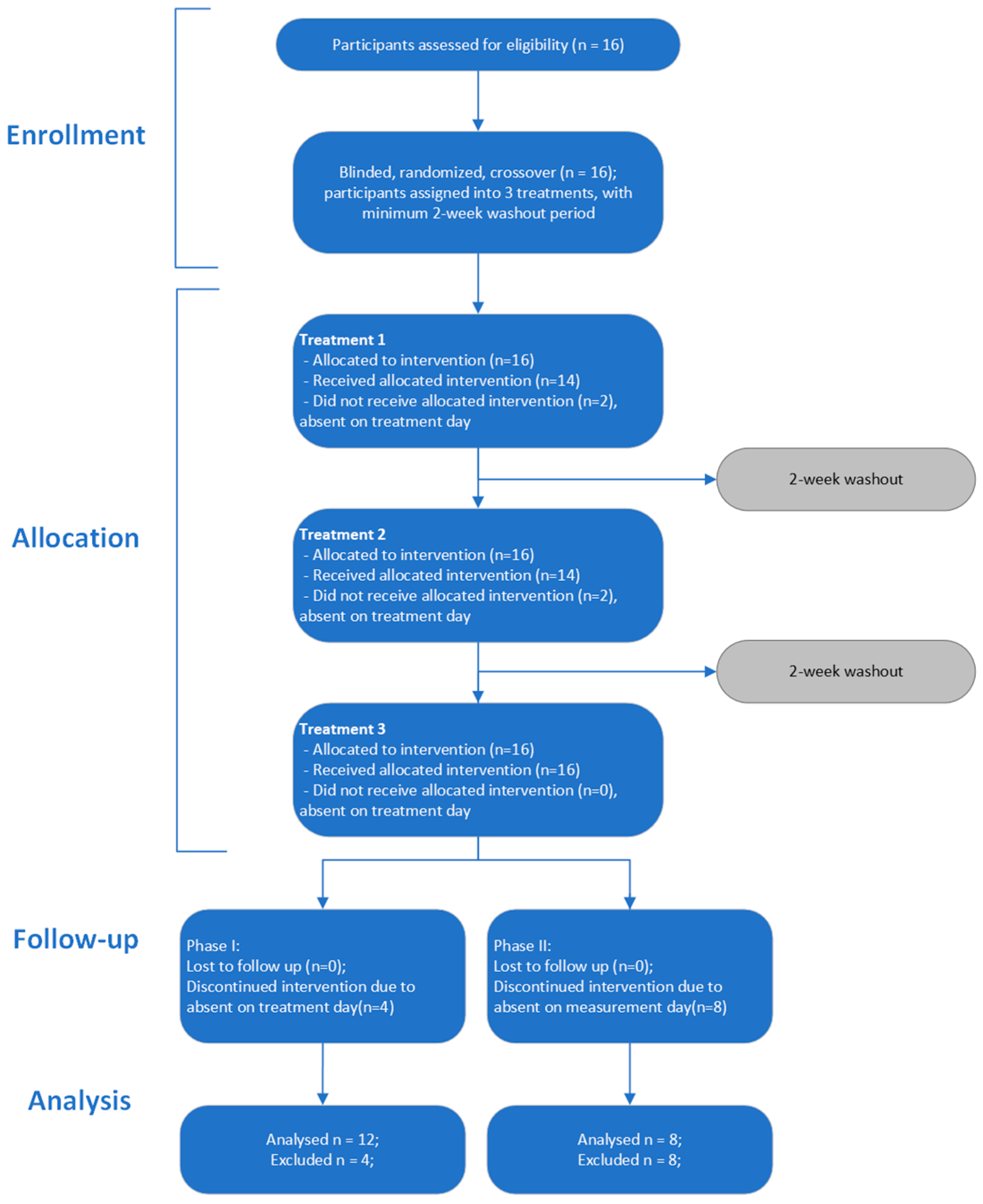
2.2. Study Design
2.3. Determination of Omega-3 Fatty Acids and Metabolites in Blood
2.4. Determination of Blood Lipids
2.5. Data Analysis
2.6. Randomization and Blinding
3. Results
3.1. Baseline Characteristics
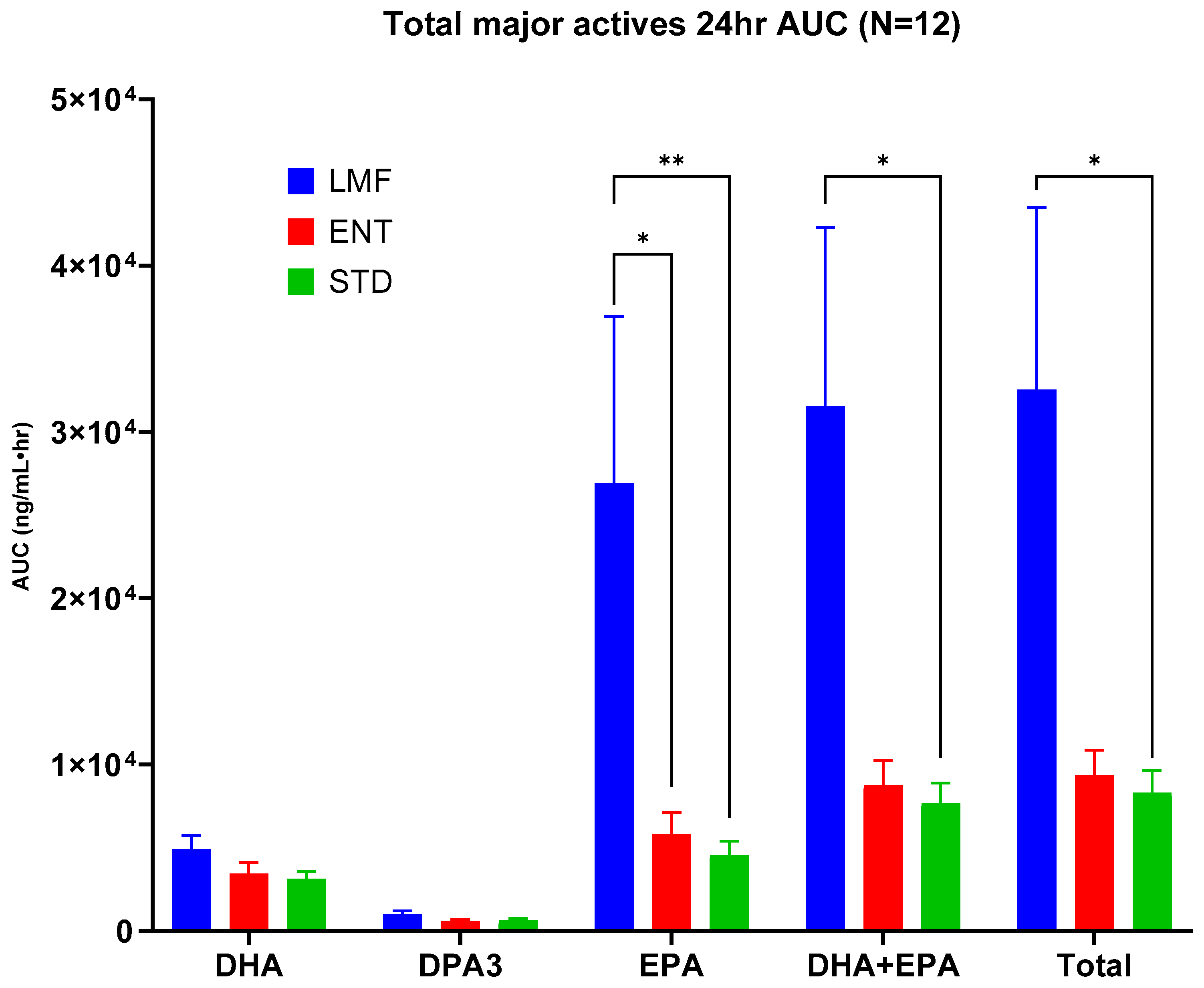
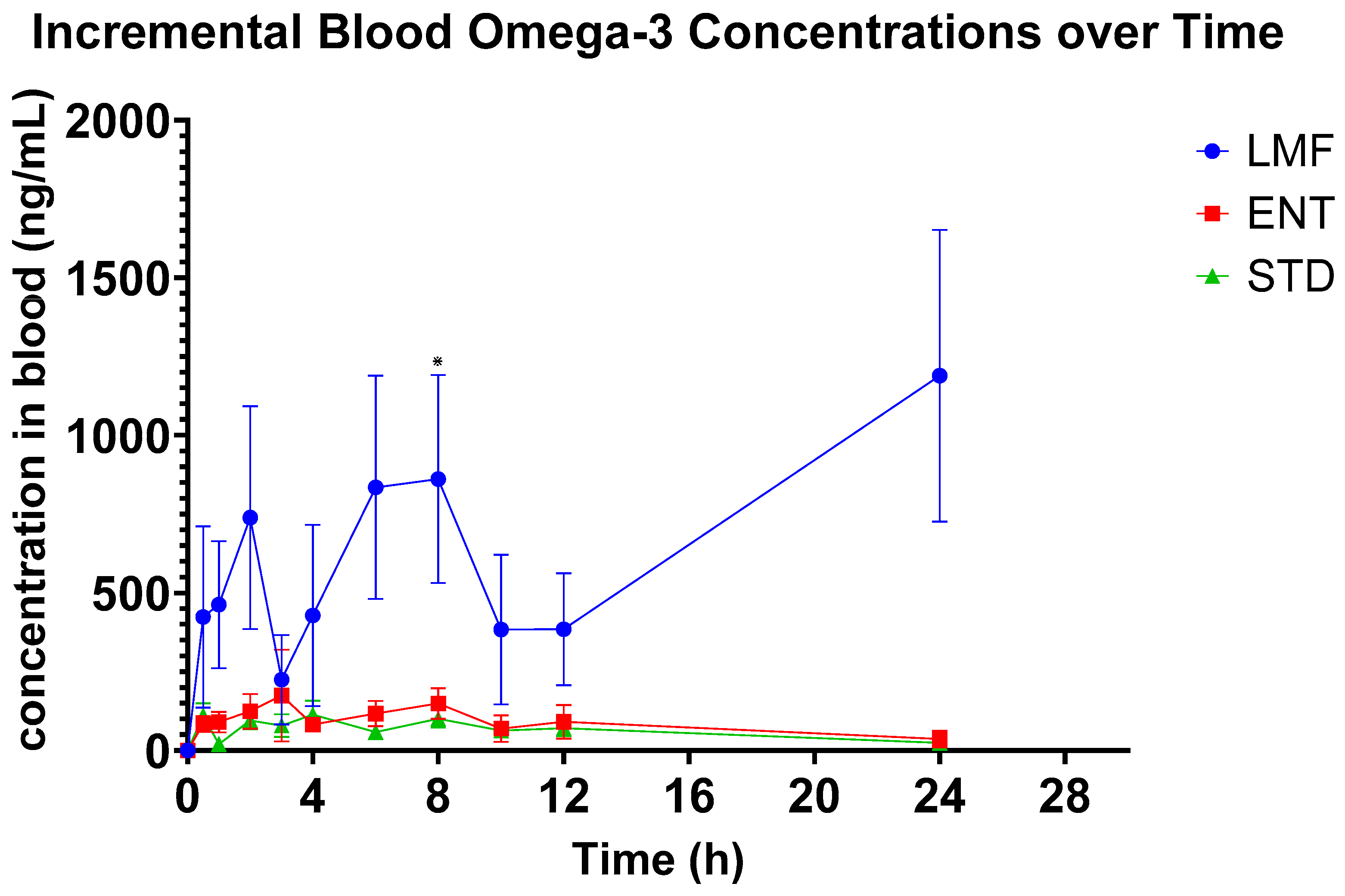
3.2. Pharmacokinetics of n-3 FAs
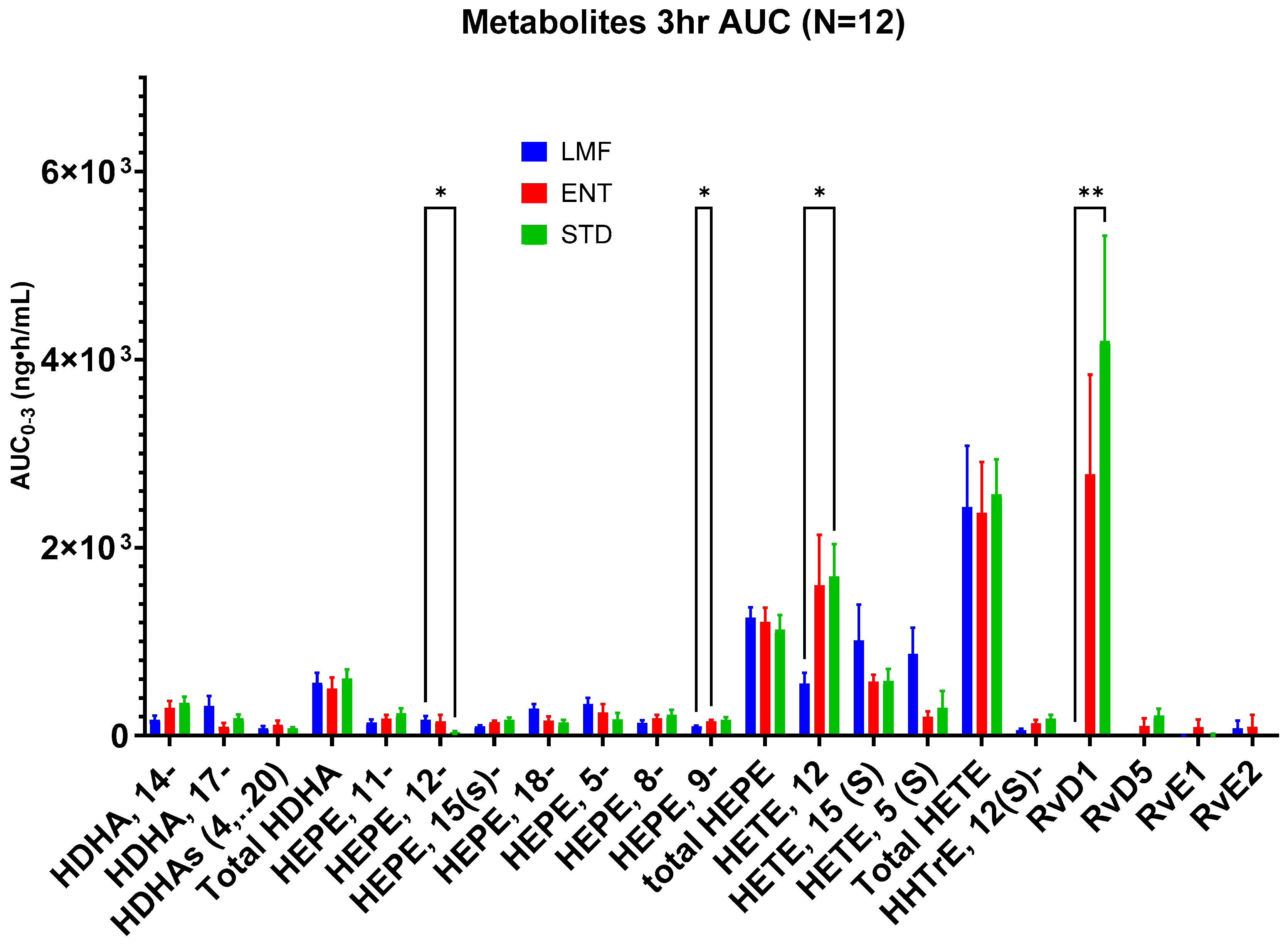
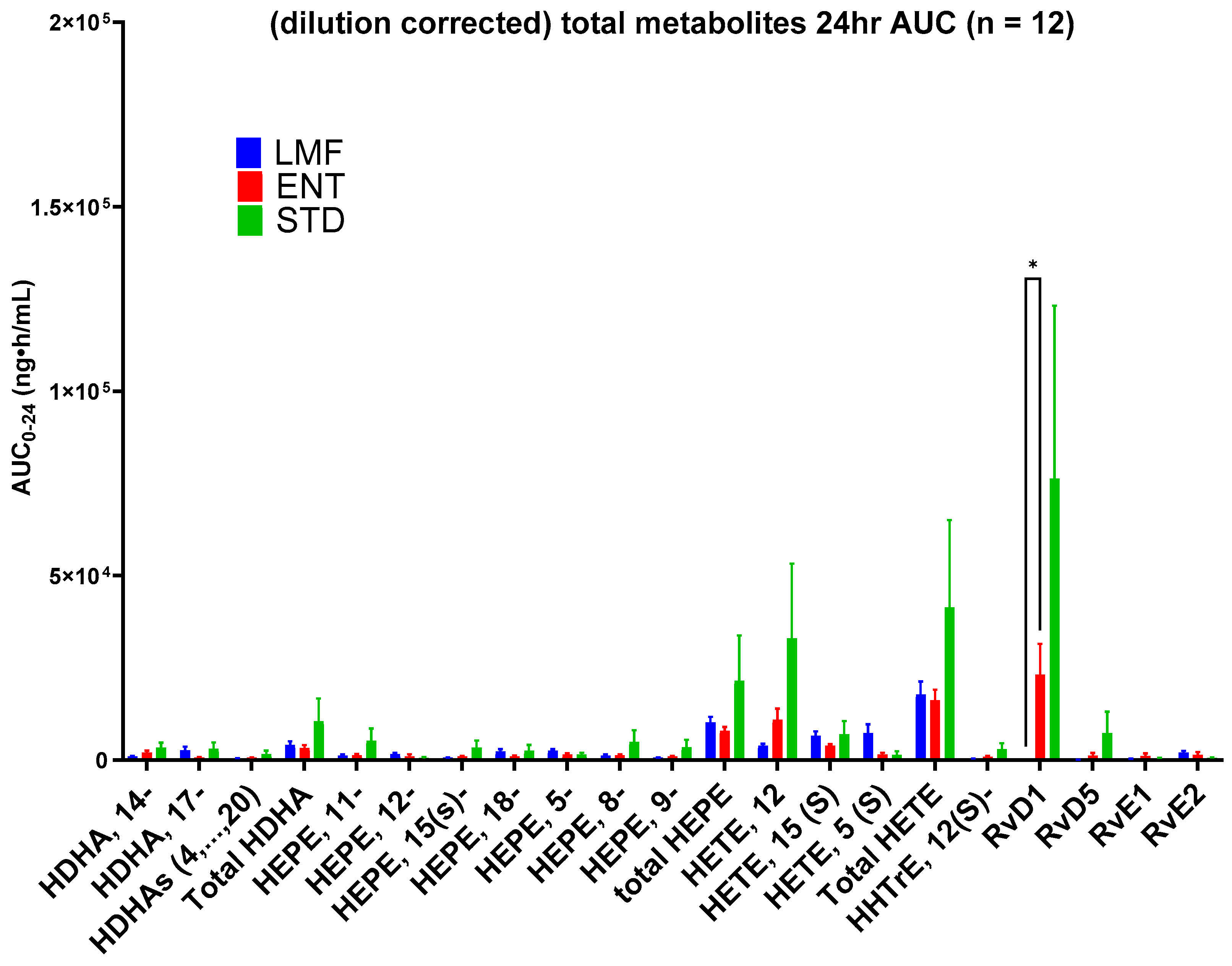
3.3. Blood Concentrations of Metabolites
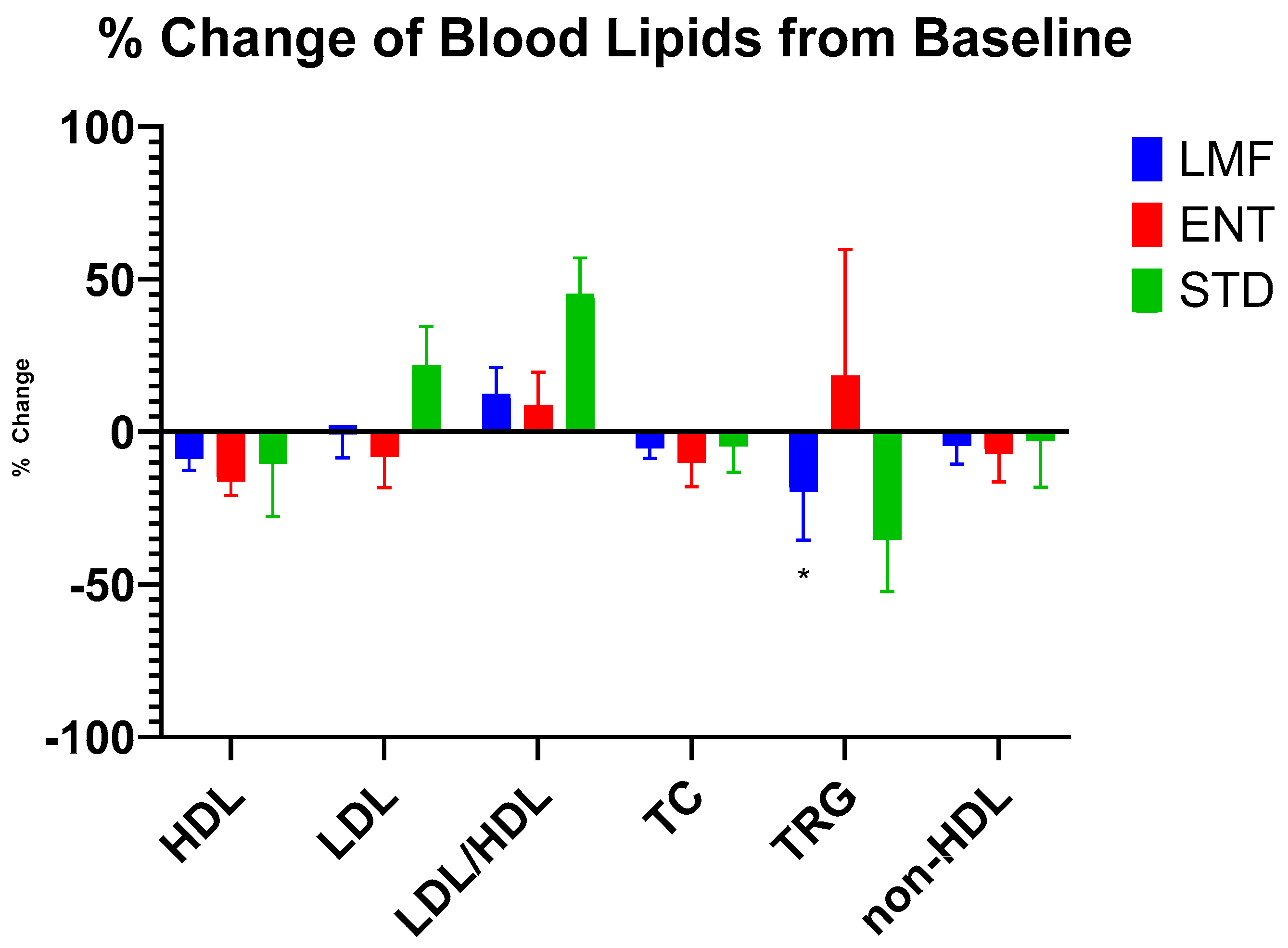
3.4. Blood Lipids
3.5. Side Effects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Dietary Supplement Use among Adults: United States, 2017–2018. Available online: https://www.cdc.gov/nchs/products/databriefs/db399.htm (accessed on 29 November 2023).
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of Omega-3 Fatty Acids on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. eClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.K. Omega-3 Fatty Acids and Cognitive Function. Curr. Opin. Lipidol. 2023, 34, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Kralovec, J.; Bailey-Hall, E.; Smeberg, V.; Stark, J.G.; Salem, N. Similar Eicosapentaenoic Acid and Docosahexaenoic Acid Plasma Levels Achieved with Fish Oil or Krill Oil in a Randomized Double-Blind Four-Week Bioavailability Study. Lipids Health Dis. 2015, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Harris, W.S.; Chung, M.; Lichtenstein, A.H.; Balk, E.M.; Kupelnick, B.; Jordan, H.S.; Lau, J. N−3 Fatty Acids from Fish or Fish-Oil Supplements, but Not α-Linolenic Acid, Benefit Cardiovascular Disease Outcomes in Primary- and Secondary-Prevention Studies: A Systematic Review2. Am. J. Clin. Nutr. 2006, 84, 5–17. [Google Scholar] [CrossRef]
- Gerster, H. Can Adults Adequately Convert Alpha-Linolenic Acid (18:3n-3) to Eicosapentaenoic Acid (20:5n-3) and Docosahexaenoic Acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 1998, 68, 159–173. [Google Scholar] [PubMed]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary Sources, Current Intakes, and Nutritional Role of Omega-3 Docosapentaenoic Acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef]
- Roh, J.; Go, E.J.; Park, J.-W.; Kim, Y.H.; Park, C.-K. Resolvins: Potent Pain Inhibiting Lipid Mediators via Transient Receptor Potential Regulation. Front. Cell Dev. Biol. 2020, 8, 584206. [Google Scholar] [CrossRef]
- Park, J.; Roh, J.; Pan, J.; Kim, Y.H.; Park, C.-K.; Jo, Y.Y. Role of Resolvins in Inflammatory and Neuropathic Pain. Pharmaceuticals 2023, 16, 1366. [Google Scholar] [CrossRef] [PubMed]
- Elajami, T.K.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N.; Welty, F.K. Specialized Proresolving Lipid Mediators in Patients with Coronary Artery Disease and Their Potential for Clot Remodeling. FASEB J. 2016, 30, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Bouhadoun, A.; Manikpurage, H.D.; Deschildre, C.; Zalghout, S.; Dubourdeau, M.; Urbach, V.; Ho-Tin-Noe, B.; Deschamps, L.; Michel, J.-B.; Longrois, D.; et al. DHA, RvD1, RvD5, and MaR1 Reduce Human Coronary Arteries Contractions Induced by PGE2. Prostaglandins Other Lipid Mediat. 2023, 165, 106700. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Immunonutrition Suppresses Acute Inflammatory Responses through Modulation of Resolvin E1 in Patients Undergoing Major Hepatobiliary Resection. Surgery 2016, 160, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Botta, E.; Holinstat, M. Eicosanoids in Inflammation in the Blood and the Vessel. Front. Pharmacol. 2022, 13, 997403. [Google Scholar] [CrossRef]
- Lee, C.-Y.J.; Seet, R.C.S.; Huang, S.H.; Long, L.H.; Halliwell, B. Different Patterns of Oxidized Lipid Products in Plasma and Urine of Dengue Fever, Stroke, and Parkinson’s Disease Patients: Cautions in the Use of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2009, 11, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Zhukareva, V.; Yao, Y.; Uryu, K.; Funk, C.D.; Lawson, J.A.; Trojanowski, J.Q.; Lee, V.M.-Y. 12/15-Lipoxygenase Is Increased in Alzheimer’s Disease. Am. J. Pathol. 2004, 164, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Deol, P.; Fahrmann, J.; Yang, J.; Evans, J.R.; Rizo, A.; Grapov, D.; Salemi, M.; Wanichthanarak, K.; Fiehn, O.; Phinney, B.; et al. Omega-6 and Omega-3 Oxylipins Are Implicated in Soybean Oil-Induced Obesity in Mice. Sci. Rep. 2017, 7, 12488. [Google Scholar] [CrossRef]
- Chiang, K.-M.; Chen, J.-F.; Yang, C.-A.; Xiu, L.; Yang, H.-C.; Shyur, L.-F.; Pan, W.-H. Identification of Serum Oxylipins Associated with the Development of Coronary Artery Disease: A Nested Case-Control Study. Metabolites 2022, 12, 495. [Google Scholar] [CrossRef]
- Chocholoušková, M.; Jirásko, R.; Vrána, D.; Gatěk, J.; Melichar, B.; Holčapek, M. Reversed Phase UHPLC/ESI-MS Determination of Oxylipins in Human Plasma: A Case Study of Female Breast Cancer. Anal. Bioanal. Chem. 2019, 411, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.V.; Davis, B.C.; Garg, M.L. Omega-3 Polyunsaturated Fatty Acids and Vegetarian Diets. Med. J. Aust. 2013, 199, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- Ghasemifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 Long Chain Fatty Acid “Bioavailability”: A Review of Evidence and Methodological Considerations. Prog. Lipid Res. 2014, 56, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Beckermann, B.; Beneke, M.; Seitz, I. Comparative bioavailability of eicosapentaenoic acid and docasahexaenoic acid from triglycerides, free fatty acids and ethyl esters in volunteers. Arzneimittelforschung 1990, 40, 700–704. [Google Scholar]
- Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Enhanced Increase of Omega-3 Index in Response to Long-Term n-3 Fatty Acid Supplementation from Triacylglycerides versus Ethyl Esters. Eur. J. Clin. Nutr. 2011, 65, 247–254. [Google Scholar] [CrossRef]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. New Insights into the Molecular Mechanism of Intestinal Fatty Acid Absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef]
- Goodman, B.E. Insights into Digestion and Absorption of Major Nutrients in Humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of Long-Chain Omega-3 Fatty Acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of Marine Omega-3 Supplements and Human Health. Biomed. Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. Chapter 12: Miccroencapsulation and Delivery of Omega-3 Fatty Acids. In Functional Food Ingredients and Nutraceuticals: Processing Technologies; Functional Foods and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2007; pp. 301–302. ISBN 978-0-8493-2441-3. [Google Scholar]
- Park, E.D.; Park, Y.; Park, S.-S.; Suh, H.-J. Absorption Evaluation of Enteric Coated Capsules Containing Omega 3 Fatty Acids. Korean J. Food Nutr. 2012, 25, 1027–1032. [Google Scholar] [CrossRef]
- Belluzzi, A. N-3 Fatty Acids for the Treatment of Inflammatory Bowel Diseases. Proc. Nutr. Soc. 2002, 61, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Schuchardt, J.P.; Meyer, H.; Hahn, A. Effect of Gastric Acid Resistant Coating of Fish Oil Capsules on Intestinal Uptake of Eicosapentaenoic Acid and Docosahexaenoic Acid. J. Funct. Foods 2011, 3, 129–133. [Google Scholar] [CrossRef]
- Garaiova, I.; Guschina, I.A.; Plummer, S.F.; Tang, J.; Wang, D.; Plummer, N.T. A Randomised Cross-over Trial in Healthy Adults Indicating Improved Absorption of Omega-3 Fatty Acids by Pre-Emulsification. Nutr. J. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Nyheim, H.; Haram, E.M.; Moritz, J.M.; Hustvedt, S.O. A Novel Self-Micro-Emulsifying Delivery System (SMEDS) Formulation Significantly Improves the Fasting Absorption of EPA and DHA from a Single Dose of an Omega-3 Ethyl Ester Concentrate. Lipids Health Dis. 2017, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Serafim, V.; Tiugan, D.-A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and Validation of a LC–MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef]
- Kutzner, L.; Rund, K.M.; Ostermann, A.I.; Hartung, N.M.; Galano, J.-M.; Balas, L.; Durand, T.; Balzer, M.S.; David, S.; Schebb, N.H. Development of an Optimized LC-MS Method for the Detection of Specialized Pro-Resolving Mediators in Biological Samples. Front. Pharmacol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Chuang, J.; Briskey, D.; Dang, J.; Rajgopal, A.; Rao, A. A Randomized Double-Blind Trial to Measure the Absorption Characteristics of Eicosapentaenoic Acid and Docosahexaenoic Acid Rich Oil Blend with Natural Lipid-Based Delivery System. Food Sci. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Wakil, A.; Mir, M.; Mellor, D.D.; Mellor, S.F.; Atkin, S.L. The Bioavailability of Eicosapentaenoic Acid from Reconstituted Triglyceride Fish Oil Is Higher than That Obtained from the Triglyceride and Monoglyceride Forms. Asia Pac. J. Clin. Nutr. 2010, 19, 499–505. [Google Scholar] [PubMed]
- Patan, M.J.; Kennedy, D.O.; Husberg, C.; Hustvedt, S.O.; Calder, P.C.; Khan, J.; Forster, J.; Jackson, P.A. Supplementation with Oil Rich in Eicosapentaenoic Acid, but Not in Docosahexaenoic Acid, Improves Global Cognitive Function in Healthy, Young Adults: Results from Randomized Controlled Trials. Am. J. Clin. Nutr. 2021, 114, 914–924. [Google Scholar] [CrossRef]
- Dasilva, G.; Boller, M.; Medina, I.; Storch, J. Relative Levels of Dietary EPA and DHA Impact Gastric Oxidation and Essential Fatty Acid Uptake. J. Nutr. Biochem. 2018, 55, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.B.; Long, M.A. Emulsions, Microemulsions, and Lipid-Based Drug Delivery Systems for Drug Solubilization and Delivery—Part II: Oral Applications. In Water-Insoluble Drug Formulation; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-12049-2. [Google Scholar]
- Talegaonkar, S.; Azeem, A.; Ahmad, F.J.; Khar, R.K.; Pathan, S.A.; Khan, Z.I. Microemulsions: A Novel Approach to Enhanced Drug Delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 238–257. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the Tolerable Upper Intake Level of Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA) and Docosapentaenoic Acid (DPA). EFSA J. 2012, 10, 2815. [CrossRef]
- Office of Dietary Supplements—Omega-3 Fatty Acids. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-Consumer/ (accessed on 30 April 2024).
- Aarak, K.E.; Kirkhus, B.; Holm, H.; Vogt, G.; Jacobsen, M.; Vegarud, G.E. Release of EPA and DHA from Salmon Oil—A Comparison of in Vitro Digestion with Human and Porcine Gastrointestinal Enzymes. Br. J. Nutr. 2013, 110, 1402–1410. [Google Scholar] [CrossRef]
- Vonach, C.; Viola, K.; Giessrigl, B.; Huttary, N.; Raab, I.; Kalt, R.; Krieger, S.; Vo, T.P.N.; Madlener, S.; Bauer, S.; et al. NF-κB Mediates the 12(S)-HETE-Induced Endothelial to Mesenchymal Transition of Lymphendothelial Cells during the Intravasation of Breast Carcinoma Cells. Br. J. Cancer 2011, 105, 263–271. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase Mediates Invasion of Intrametastatic Lymphatic Vessels and Propagates Lymph Node Metastasis of Human Mammary Carcinoma Xenografts in Mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Duniec, Z.M.; Liu, B.; Hagmann, W.; Gao, X.; Shimoji, K.; Marnett, L.J.; Johnson, C.R.; Honn, K.V. Endogenous 12(S)-HETE Production by Tumor Cells and Its Role in Metastasis. Cancer Res. 1994, 54, 1574–1579. [Google Scholar]
- Leiria, L.O.; Wang, C.-H.; Lynes, M.D.; Yang, K.; Shamsi, F.; Sato, M.; Sugimoto, S.; Chen, E.Y.; Bussberg, V.; Narain, N.R.; et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab. 2019, 30, 768–783.e7. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Virk, R.; Van Dyke, T.E. Potential Mechanisms by Which Hydroxyeicosapentaenoic Acids Regulate Glucose Homeostasis in Obesity. Adv. Nutr. 2022, 13, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of Omega-3 Fatty Acids on Serum Markers of Cardiovascular Disease Risk: A Systematic Review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Briskey, D.; Meola, T.R.; Mallard, A.; Prestidge, C.A.; Rao, A. A Self-Emulsifying Omega-3 Ethyl Ester Formulation (AquaCelle) Significantly Improves Eicosapentaenoic and Docosahexaenoic Acid Bioavailability in Healthy Adults. Eur. J. Nutr. 2020, 59, 2729–2737. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| ≥18 years old | Less than 18 years old |
| Good physical condition | Smokers |
| Provide signed informed consent | Taking prescribed medication |
| Liver disease | |
| Kidney disease | |
| Gastrointestinal disease | |
| Allergy to berberine | |
| Intend to become pregnant, pregnant, or breast-feeding |
| Day | Breakfast | Dinner |
|---|---|---|
| Monday | Bagel with cream cheese and jam | Lasagna and salad |
| Tuesday | Bagel with cream cheese and jam | Pork chops, sweet potatoes, beans, salad |
| Wednesday | Bagel with cream cheese and jam | Chicken skewers, pita bread, tzatziki, cucumbers, tomatoes |
| Thursday | Bagel with cream cheese and jam | Lamb chops, cauliflower, green beans |
| Friday | Bagel with cream cheese and jam | Beef fajita, onions, peppers, wheat tortilla wrap |
| Compounds | Chemical Formula [M] | Scanned Mass [m/z] | Start [min] | End [min] |
|---|---|---|---|---|
| 18-HEPE | C20H30O3 | 317.21222 | 4.00 | 4.60 |
| 15-HETE-d8 | C20H24D8O3 | 327.27808 | 4.50 | 5.10 |
| 17-HDHA/14-HDHA | C22H32O3 | 343.22787 | 7.00 | 8.30 |
| DPA3 | C22H34O2 | 329.2486 | 9.10 | 9.90 |
| EPA | C20H30O2 | 301.2173 | 8.60 | 9.20 |
| DHA | C22H32O2 | 327.23295 | 9.00 | 9.60 |
| (18S-) Resolvin E1 | C20H30O5 | 349.20205 | 3.00 | 4.70 |
| (18S-) Resolvin E2 | C20H30O4 | 333.20713 | 4.30 | 6.50 |
| (AT-)Resolvin D2,3,4 | C22H32O5 | 375.2177 | 4.70 | 7.70 |
| Resolvin D5, 6, Protectin D1, Maresin | C22H32O4 | 359.22278 | 6.00 | 8.20 |
| EPA-d5 | C20H25D5O2 | 306.24869 | 6.80 | 7.25 |
| 15(S), 12(S), 5(S)-HETE | C20H32O3 | 319.22787 | 7.10 | 8.10 |
| 12(S)-HHTrE | C17H28O3 | 279.19657 | 6.30 | 6.90 |
| Prostaglandin D2, E2 | C20H32O5 | 351.2177 | 3.80 | 5.20 |
| Protaglandin F2a | C20H34O5 | 353.23335 | 4.70 | 5.30 |
| Thromboxane B2, 6-keto protaglandin F1a | C20H34O6 | 369.22826 | 3.50 | 5.50 |
| DHA-d5 | C22H27D5O2 | 332.26434 | 9.00 | 9.60 |
| EPA Oxylipins | C20H30O3 | 317.21222 | 6.70 | 7.70 |
| Parameter | |
|---|---|
| N | 16 |
| Males | Females | 9 | 7 |
| Age (years) | 36.1 ± 2.4 |
| Weight (kg) | 61.8 ± 2.4 |
| BMI (kg/m2) | 22.1 ± 0.5 |
| DHA (ng/mL) | 1007.1 ± 131.1 |
| EPA (ng/mL) | 3085.2 ± 833.4 |
| DPA (ng/mL) | 215.0 ± 30.5 |
| AUC0-24 ng/mL·h | STD | ENT | LMF |
|---|---|---|---|
| DHA | 3136.6 ± 433.3 | 3436.8 ± 688.0 | 4910 ± 827.5 |
| DPA3 | 626.4 ± 120.3 | 590.6 ± 93.4 | 1016.8 ± 181.8 |
| EPA | 4545.4 ** ± 839 | 5798 * ± 1317 | 26,920 ** ± 10,021 |
| DHA + EPA | 7682 * ± 1225 | 8734 ± 1503 | 31,529 * ± 10,783 |
| Total | 8309 * ± 1330 | 9325 ± 1555 | 32,546 * ± 10,950 |
| STD | ENT | LMF | |
|---|---|---|---|
| iAUC (ng/mL·h) | 1498.9 **** ± 443.0 | 2057.2 **** ± 813.7 | 16,150 **** ± 5454 |
| Cmax (ng/mL) | 186.0 ± 44.8 | 389 ± 141 | 1732.8 ± 478.3 |
| Tmax (hr) | 3.9 ± 1.1 | 4.7 ± 1.2 | 10.0 ± 2.1 |
| AUC0-3 h ng·h/mL | STD | ENT | LMF |
|---|---|---|---|
| HDHA, 14- | 347.2 ± 68.5 | 292.0 ± 75.6 | 167.1 ± 47.7 |
| HDHA, 17- | 182.9 ± 43.6 | 93.3 ± 41.0 | 316.0 ± 106.2 |
| HDHAs (4…20) | 73.3 ± 15.9 | 113.7 ± 43.8 | 76.2 ± 26.2 |
| Total HDHA | 603.5 ± 101.5 | 498.9 ± 120.0 | 559.3 ± 109.4 |
| HEPE, 11- | 234.9 ± 54.7 | 180.2 ± 39.3 | 140.3 ± 30.0 |
| HEPE, 12- | 33.6 * ± 14.1 | 150.6 ± 73.0 | 166.3 * ± 40.7 |
| HEPE, 15- | 165.3 ± 27.4 | 141.2 ± 16.2 | 95.3 ± 14.3 |
| HEPE, 18- | 135.4 ± 31.3 | 157.6 ± 49.0 | 285.6 ± 51.2 |
| HEPE, 5- | 169.3 ± 70.8 | 246.5 ± 86.9 | 335.9 ± 66.1 |
| HEPE, 8- | 220.7 ± 53.2 | 182.8 ± 37.0 | 134.6 ± 27.9 |
| HEPE, 9- | 165.7 * ± 30.0 | 149.8 * ± 17.3 | 95.8 ± 11.2 |
| total HEPE | 1124.9 ± 158.2 | 1208.8 ± 152.8 | 1253.9 ± 109.2 |
| HETE, 12- | 1692.9 * ± 342.5 | 1597.2 ± 539.4 | 551.1 * ± 118.5 |
| HETE, 15- | 579.2 ± 129.3 | 572.7 ± 75.1 | 1011.7 ± 381.0 |
| HETE, 5- | 293.0 ± 181.5 | 201.5 ± 55.6 | 871.6 ± 274.5 |
| Total HETE | 2565.1 ± 373.7 | 2371.4 ± 538.3 | 2434.3 ± 650.3 |
| HHTrE, 12- | 179.4 ± 43.3 | 131.2 ± 35.4 | 53.9 ± 19.2 |
| RvD1 | 4197 ** ± 112 | 2782 ± 1058 | 0.2 ** ± 0.2 |
| RvD5 | 212.3 ± 74.9 | 100.6 ± 83.7 | −23.1 ± 23.1 |
| RvE1 | −2.7 ± 22.2 | 89.3 ± 80.5 | −16.5 ± 19.9 |
| RvE2 | −91.8 ± 65.7 | 94.8 ± 125.8 | 77.8 ± 80.5 |
| AUC0-24 h ng·h/mL | STD | ENT | LMF |
|---|---|---|---|
| HDHA, 14- | 3410 ± 1296 | 2041.0 ± 493.8 | 998.7 ± 172.2 |
| HDHA, 17- | 3033 ± 1759 | 621.7 ± 228.1 | 2637.3 ± 1031.2 |
| HDHAs (4…20) | 1618.5 ± 951.7 | 630.8 ± 122.9 | 439.6 ± 112.4 |
| Total HDHA | 10,450.5 ± 6273.9 | 3293.4 ± 752.5 | 4075.5 ± 1026.2 |
| HEPE, 11- | 5224.1 ± 3330.8 | 1292.5 ± 329.0 | 1192.1 ± 318.6 |
| HEPE, 12- | 562.9 ± 293.0 | 1016.5 ± 552.0 | 1641.6 ± 371.8 |
| HEPE, 15- | 3345.0 ± 1960.2 | 970.1 ± 144.1 | 636.9 ± 77.6 |
| HEPE, 18- | 2527.3 ± 1561.8 | 947.8 ± 283.6 | 2317.7 ± 631.3 |
| HEPE, 5- | 1465.6 ± 533.4 | 1447.9 ± 448.4 | 2533.6 ± 436.7 |
| HEPE, 8- | 4931.9 ± 3164.2 | 1288.3 ± 310.1 | 1226.0 ± 298.5 |
| HEPE, 9- | 3438.7 ± 2086.5 | 960.1 ± 161.0 | 629.9 ± 64.2 |
| total HEPE | 21,495.5 ± 12,252.5 | 7923.2 ± 1028.3 | 10,177.8 ± 1522.0 |
| HETE, 12- | 32,927.3 ± 20,323.3 | 10,891.8 ± 3080.1 | 3905.1 ± 534.2 |
| HETE, 15- | 6983.2 ± 3602.3 | 3846.1 ± 453.5 | 6596.8 ± 1193.3 |
| HETE, 5- | 1446.9 ± 966.5 | 1510.8 ± 488.4 | 7313.2 ± 2351.5 |
| Total HETE | 41,357.5 ± 23,732.8 | 16,248.7 ± 2824.7 | 17,815.2 ± 3486.3 |
| HHTrE, 12- | 2899.3 ± 1669.9 | 867.6 ± 244.8 | 361.7 ± 58.3 |
| RvD1 | 91,591 * ± 55,336 | 30,786 ± 9988 | 0.19 * ± 0.17 |
| RvD5 | 7336 ± 5788 | 2050 ± 1245 | 39.9 ± 107.7 |
| RvE1 | 662 ± 313 | 1628 ± 1130 | 211.6 ± 201.2 |
| RvE2 | 785.8 ± 341.5 | 2097 ± 1135 | 1854 ± 827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibi, A.; Chang, C.; Kuo, Y.C.; Zhang, Y.; Du, M.; Roh, Y.S.; Gahler, R.; Hardy, M.; Solnier, J. Evaluation of the Metabolite Profile of Fish Oil Omega-3 Fatty Acids (n-3 FAs) in Micellar and Enteric-Coated Forms—A Randomized, Cross-Over Human Study. Metabolites 2024, 14, 265. https://doi.org/10.3390/metabo14050265
Ibi A, Chang C, Kuo YC, Zhang Y, Du M, Roh YS, Gahler R, Hardy M, Solnier J. Evaluation of the Metabolite Profile of Fish Oil Omega-3 Fatty Acids (n-3 FAs) in Micellar and Enteric-Coated Forms—A Randomized, Cross-Over Human Study. Metabolites. 2024; 14(5):265. https://doi.org/10.3390/metabo14050265
Chicago/Turabian StyleIbi, Afoke, Chuck Chang, Yun Chai Kuo, Yiming Zhang, Min Du, Yoon Seok Roh, Roland Gahler, Mary Hardy, and Julia Solnier. 2024. "Evaluation of the Metabolite Profile of Fish Oil Omega-3 Fatty Acids (n-3 FAs) in Micellar and Enteric-Coated Forms—A Randomized, Cross-Over Human Study" Metabolites 14, no. 5: 265. https://doi.org/10.3390/metabo14050265
APA StyleIbi, A., Chang, C., Kuo, Y. C., Zhang, Y., Du, M., Roh, Y. S., Gahler, R., Hardy, M., & Solnier, J. (2024). Evaluation of the Metabolite Profile of Fish Oil Omega-3 Fatty Acids (n-3 FAs) in Micellar and Enteric-Coated Forms—A Randomized, Cross-Over Human Study. Metabolites, 14(5), 265. https://doi.org/10.3390/metabo14050265






