Associations of the Single Bovine Embryo Growth Media Metabolome with Successful Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Media
2.2. Experimental Design
2.3. In Vitro Fertilization
2.4. In Vitro Cultivation and Embryo Transfer
2.5. Collection of Media for LC-MS/MS and Categorization of Samples
2.6. Preparation of Culture Media Samples for LC-MS/MS and Spectrometry
2.7. Statistical Analysis
3. Results
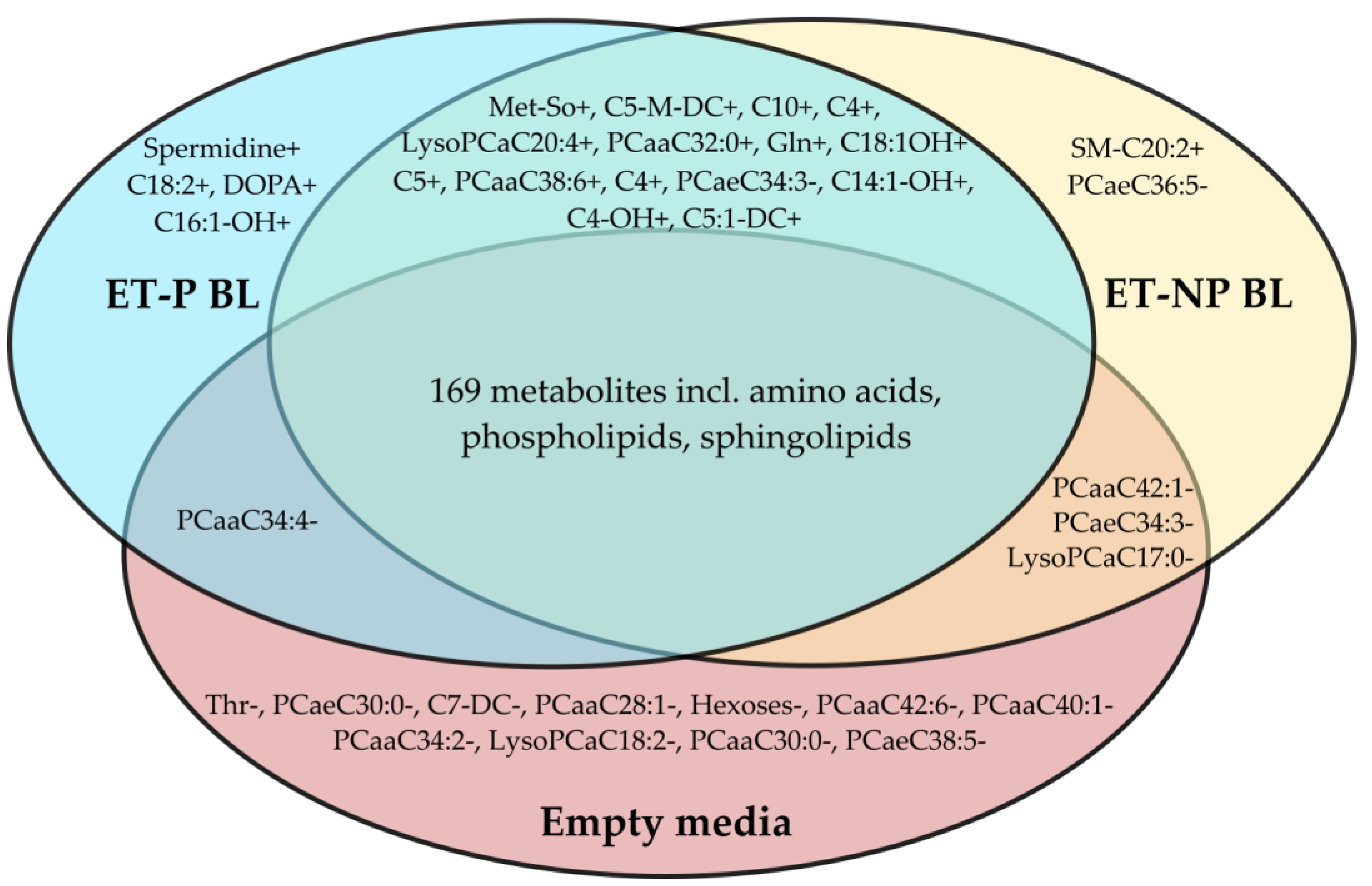
3.1. Metabolites Differing in Culture Media of Blastocysts and Controls
3.2. Derived Indicators Differing between Culture Media of Blastocysts and Controls
3.3. Metabolites Differing between Media of Pregnancy-Yielding and Unyielding Blastocysts
4. Discussion
4.1. Glucose Metabolism
4.2. Amino Acid Metabolism
4.3. Polyamine Metabolism
4.4. Lipid Metabolism
4.5. Phosphatidylcholine Metabolism
4.6. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pohler, K.G.; Reese, S.T.; Franco, G.A.; Oliveira Filho, R.V.; Paiva, R.; Fernandez, L.; de Melo, G.; Moraes Vasconcelos, J.L.; Cooke, R.; Poole, R.K. New Approaches to Diagnose and Target Reproductive Failure in Cattle. Anim. Reprod. 2020, 17, e20200057. [Google Scholar] [CrossRef]
- Mebratu, B. Embryo Transfer in Cattle Production and Its Principle and Applications. Int. J. Pharm. Biomed. Res. 2020, 7, 40–54. [Google Scholar] [CrossRef]
- Nowicki, A. Embryo Transfer as an Option to Improve Fertility in Repeat Breeder Dairy Cows. J. Vet. Res. 2021, 65, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.H.M. 2021 Statistics of Embryo Production and Transfer in Domestic Farm Animals. Embryo Technol. Newsl. 2022, 40, 22–40. [Google Scholar]
- Hansen, P.J. The Incompletely Fulfilled Promise of Embryo Transfer in Cattle-Why Aren’t Pregnancy Rates Greater and What Can We Do about It? J. Anim. Sci. 2020, 98, skaa288. [Google Scholar] [CrossRef] [PubMed]
- Magata, F. Time-Lapse Monitoring Technologies for the Selection of Bovine in Vitro Fertilized Embryos with High Implantation Potential. J. Reprod. Dev. 2023, 69, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Matsuno, Y.; Fujiwara, H. New Roles for EVs, MiRNA and LncRNA in Bovine Embryo Implantation. Front. Vet. Sci. 2022, 9, 944370. [Google Scholar] [CrossRef] [PubMed]
- Cheredath, A.; Uppangala, S.; Asha, C.S.; Jijo, A.; Vani Lakshmi, R.; Kumar, P.; Joseph, D.; Nagana, N.G.; Kalthur, G.; Adiga, S.K. Combining Machine Learning with Metabolomic and Embryologic Data Improves Embryo Implantation Prediction. Reprod. Sci. 2023, 30, 984–994. [Google Scholar] [CrossRef]
- Gomez, E.; Canela, N.; Herrero, P.; Cereto, A.; Gimeno, I.; Carrocera, S.; Martin-gonzalez, D.; Murillo, A.; Muñoz, M. Metabolites Secreted by Bovine Embryos in Vitro Predict Pregnancies That the Recipient Plasma Metabolome Cannot, and Vice Versa. Metabolites 2021, 11, 162. [Google Scholar] [CrossRef]
- Lechniak, D.; Sell-Kubiak, E.; Warzych, E. The Metabolic Profile of Bovine Blastocysts Is Affected by in Vitro Culture System and the Pattern of First Zygotic Cleavage. Theriogenology 2022, 188, 43–51. [Google Scholar] [CrossRef]
- Pallisco, R.; Lazzarino, G.; Bilotta, G.; Marroni, F.; Mangione, R.; Saab, M.W.; Brundo, M.V.; Pittalà, A.; Caruso, G.; Capoccia, E.; et al. Metabolic Signature of Energy Metabolism Alterations and Excess Nitric Oxide Production in Culture Media Correlate with Low Human Embryo Quality and Unsuccessful Pregnancy. Int. J. Mol. Sci. 2023, 24, 890. [Google Scholar] [CrossRef]
- Rubessa, M.; Wheeler, M.B. Label-Free Microscopy: A Non-Invasive New Tool to Assess Gametes and Embryo Quality. Theriogenology 2020, 150, 241–246. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent Advances in Bovine in Vitro Embryo Production: Reproductive Biotechnology History and Methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- McLennan, H.J.; Saini, A.; Dunning, K.R.; Thompson, J.G. Oocyte and Embryo Evaluation by AI and Multi-Spectral Auto-Fluorescence Imaging: Livestock Embryology Needs to Catch-up to Clinical Practice. Theriogenology 2020, 150, 255–262. [Google Scholar] [CrossRef]
- Lipinska, P.; Pawlak, P.; Warzych, E. Species and Embryo Genome Origin Affect Lipid Droplets in Preimplantation Embryos. Front. Cell Dev. Biol. 2023, 11, 1187832. [Google Scholar] [CrossRef] [PubMed]
- Zmuidinaite, R.; Sharara, F.I.; Iles, R.K. Current Advancements in Noninvasive Profiling of the Embryo Culture Media Secretome. Int. J. Mol. Sci. 2021, 22, 2513. [Google Scholar] [CrossRef]
- Merton, J.S.; Vermeulen, Z.L.; Otter, T.; Mullaart, E.; de Ruigh, L.; Hasler, J.F. Carbon-Activated Gas Filtration during in Vitro Culture Increased Pregnancy Rate Following Transfer of in Vitro-Produced Bovine Embryos. Theriogenology 2007, 67, 1233–1238. [Google Scholar] [CrossRef]
- Farin, P.W.; Farin, C.E. Transfer of Bovine Embryos Produced In Vivo or In Vitro: Survival and Fetal Development. Biol. Reprod. 1995, 52, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Miyanaga, S.; Shrestha, R.; Takeda, S.; Kobayashi, S.; Chiba, H.; Kamiya, H.; Hui, S.P. A Fatty Acid Profiling Method Using Liquid Chromatography-High Resolution Mass Spectrometry for Improvement of Assisted Reproductive Technology. Clin. Chim. Acta 2016, 456, 100–106. [Google Scholar] [CrossRef] [PubMed]
- de Melo-Sterza, F.A.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under in Vivo, in Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef] [PubMed]
- Banliat, C.; Mahé, C.; Lavigne, R.; Com, E.; Pineau, C.; Labas, V.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. The Proteomic Analysis of Bovine Embryos Developed in Vivo or in Vitro Reveals the Contribution of the Maternal Environment to Early Embryo. BMC Genom. 2022, 23, 839. [Google Scholar] [CrossRef] [PubMed]
- Ferrick, L.; Lee, Y.S.L.; Gardner, D.K. Metabolic Activity of Human Blastocysts Correlates with Their Morphokinetics, Morphological Grade, KIDScore and Artificial Intelligence Ranking. Hum. Reprod. 2020, 35, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Brison, D.R.; Houghton, F.D.; Falconer, D.; Roberts, S.A.; Hawkhead, J.; Humpherson, P.G.; Lieberman, B.A.; Leese, H.J. Identification of Viable Embryos in IVF by Non-Invasive Measurement of Amino Acid Turnover. Hum. Reprod. 2004, 19, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D.; Hawkhead, J.A.; Humpherson, P.G.; Hogg, J.E.; Balen, A.H.; Rutherford, A.J.; Leese, H.J. Non-Invasive Amino Acid Turnover Predicts Human Embryo Developmental Capacity. Hum. Reprod. 2002, 17, 999–1005. [Google Scholar] [CrossRef]
- de Lima, C.B.; dos Santos, É.C.; Ispada, J.; Fontes, P.K.; Nogueira, M.F.G.; dos Santos, C.M.D.; Milazzotto, M.P. The Dynamics between in Vitro Culture and Metabolism: Embryonic Adaptation to Environmental Changes. Sci. Rep. 2020, 10, 15672. [Google Scholar] [CrossRef]
- Gimeno, I.; García-Manrique, P.; Carrocera, S.; López-Hidalgo, C.; Valledor, L.; Martín-González, D.; Gómez, E. The Metabolic Signature of in Vitro Produced Bovine Embryos Helps Predict Pregnancy and Birth after Embryo Transfer. Metabolites 2021, 11, 484. [Google Scholar] [CrossRef]
- Motiei, M.; Vaculikova, K.; Cela, A.; Tvrdonova, K.; Khalili, R.; Rumpik, D.; Rumpikova, T.; Glatz, Z.; Saha, T. Non-Invasive Human Embryo Metabolic Assessment as a Developmental Criterion. J. Clin. Med. 2020, 9, 4094. [Google Scholar] [CrossRef]
- Leese, H.J.; Brison, D.R.; Sturmey, R.G. The Quiet Embryo Hypothesis: 20 Years On. Front. Physiol. 2022, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J. Amino Acid Transport and Metabolism Regulate Early Embryo Development: Species Differences, Clinical Significance, and Evolutionary Implications. Cells 2021, 10, 3154. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Van Winkle, L.J.; Díaz de la Garza, R.I.; Dubrovsky, J.G. Interkingdom Comparison of Threonine Metabolism for Stem Cell Maintenance in Plants and Animals. Front. Cell Dev. Biol. 2021, 9, 672545. [Google Scholar] [CrossRef]
- Cai, S.; Ye, Q.; Zeng, X.; Yang, G.; Ye, C.; Chen, M.; Yu, H.; Wang, Y.; Wang, G.; Huang, S.; et al. CBS and MAT2A Improve Methionine-Mediated DNA Synthesis through SAMTOR/MTORC1/S6K1/CAD Pathway during Embryo Implantation. Cell Prolif. 2021, 54, e12950. [Google Scholar] [CrossRef]
- Sun, H.; Kang, J.; Su, J.; Zhang, J.; Zhang, L.; Liu, X.; Zhang, J.; Wang, F.; Lu, Z.; Xing, X.; et al. Methionine Adenosyltransferase 2A Regulates Mouse Zygotic Genome Activation and Morula to Blastocyst Transition. Biol. Reprod. 2019, 100, 601–617. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Baez, G.M.; Lobos, N.E. Potential Benefits of Feeding Methionine on Reproduction Efficiency of Lactating Dairy Cows. Four. State Dairy Nutr. Manag. 2014, 4, 19–26. [Google Scholar]
- Hugentobler, S.A.; Diskin, M.G.; Leese, H.J.; Humpherson, P.G.; Watson, T.; Sreenan, J.M.; Morris, D.G. Amino Acids in Oviduct and Uterine Fluid and Blood Plasma during the Estrous Cycle in the Bovine. Mol. Reprod. Dev. 2007, 74, 445–454. [Google Scholar] [CrossRef]
- Drazic, A.; Winter, J. The Physiological Role of Reversible Methionine Oxidation. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 1367–1382. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhou, M.; Zhang, L.; Pang, Y.; Wang, C.; Xiao, Q.; Liu, L. Metabolism and Secretion Mechanism of Catecholamine Syndrome and Related Treatment Strategies. J. Xiangya Med. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Zhuan, Q.; Ma, H.; Chen, J.; Luo, Y.; Luo, Y.; Gao, L.; Hou, Y.; Zhu, S.; Fu, X. Cytoplasm Lipids Can Be Modulated through Hormone-Sensitive Lipase and Are Related to Mitochondrial Function in Porcine IVM Oocytes. Reprod. Fertil. Dev. 2020, 32, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Elmetwally, M.A.; Lenis, Y.; Tang, W.; Wu, G.; Bazer, F.W. Effects of Catecholamines on Secretion of Interferon Tau and Expression of Genes for Synthesis of Polyamines and Apoptosis by Ovine Trophectoderm. Biol. Reprod. 2018, 99, 611–628. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Ren, W.; Rahu, N.; Kalhoro, D.H.; Yin, Y. Exploring Polyamines: Functions in Embryo/Fetal Development. Anim. Nutr. 2017, 3, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Minois, N. Molecular Basis of the “anti-Aging” Effect of Spermidine and Other Natural Polyamines—A Mini-Review. Gerontology 2014, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Muzikova, E.; Clark, D.A. Polyamines May Increase the Percentage of In-Vitro Fertilized Murine Oocytes That Develop into Blastocysts. Hum. Reprod. 1995, 10, 1172–1777. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Cashman, K.; Russell, D.L.; Thompson, J.G.; Norman, R.J.; Robker, R.L. Beta-Oxidation Is Essential for Mouse Oocyte Developmental Competence and Early Embryo Development. Biol. Reprod. 2010, 83, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-González, D.F.; Rodríguez-Osorio, N.; Long, C.R.; Vásquez-Araque, N.A.; Maldonado-Estrada, J.G. L-Carnitine Supplementation during in Vitro Maturation and in Vitro Culture Does Not Affect the Survival Rates after Vitrification and Warming but Alters Inf-T and Ptgs2 Gene Expression. Int. J. Mol. Sci. 2020, 21, 5601. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Inaba, Y.; Somfai, T.; Kaneda, M.; Geshi, M.; Nagai, T.; Manabe, N. Supplementation of Culture Medium with Class L-Carnitine Improves Development and Cryotolerance of Bovine Embryos Produced in Vitro. Reprod. Fertil. Dev. 2013, 25, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, J.; Yu, Y.; Wei, Q.; Deng, W.; Yu, L. L-Carnitine Attenuates Oxidant Injury in HK-2 Cells via ROS-Mitochondria Pathway. Regul. Pept. 2010, 161, 58–66. [Google Scholar] [CrossRef]
- Sudano, M.J.; Santos, V.G.; Tata, A.; Ferreira, C.R.; Paschoal, D.M.; Machado, R.; Buratini, J.; Eberlin, M.N.; Landim-Alvarenga, F.D.C. Phosphatidylcholine and Sphingomyelin Profiles Vary in Bos Taurus Indicus and Bos Taurus Taurus in Vitro- and in Vivo-Produced Blastocysts. Biol. Reprod. 2012, 87, 130. [Google Scholar] [CrossRef]
- Wu, G.; Aoyama, C.; Young, S.G.; Vance, D.E. Early Embryonic Lethality Caused by Disruption of the Gene for Choline Kinase α, the First Enzyme in Phosphatidylcholine Biosynthesis. J. Biol. Chem. 2008, 283, 1456–1462. [Google Scholar] [CrossRef]
- Kühn, T.; Floegel, A.; Sookthai, D.; Johnson, T.; Rolle-Kampczyk, U.; Otto, W.; von Bergen, M.; Boeing, H.; Kaaks, R. Higher Plasma Levels of Lysophosphatidylcholine 18:0 Are Related to a Lower Risk of Common Cancers in a Prospective Metabolomics Study. BMC Med. 2016, 14, 13. [Google Scholar] [CrossRef]
- Boni, R. Ovum Pick-up in Cattle: A 25 Yr Retrospective Analysis. Anim. Reprod. 2012, 9, 362–369. [Google Scholar]
- Smith, A.K.; Grimmer, S.P. Pregnancy Rates for Grade 2 Embryos Following Administration of Synthetic GnRH at the Time of Transfer in Embryo-Recipient Cattle. Theriogenology 2002, 57, 2083–2091. [Google Scholar] [CrossRef]
- Erdem, H.; Karasahin, T.; Alkan, H.; Dursun, S.; Satilmis, F.; Guler, M. Effect of Embryo Quality and Developmental Stages on Pregnancy Rate during Fresh Embryo Transfer in Beef Heifers. Trop. Anim. Health Prod. 2020, 52, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Demetrio, D.G.B.; Benedetti, E.; Demetrio, C.G.B.; Fonseca, J.; Oliveira, M.; Magalhaes, A.; dos Santos, R.M. How Can We Improve Embryo Production and Pregnancy Outcomes of Holstein Embryos Produced in Vitro? (12 Years of Practical Results at a California Dairy Farm). Anim. Reprod. 2020, 17, e20200053. [Google Scholar] [CrossRef] [PubMed]
| Metabolites | Concentrations in Culture Media, μM | Significance | |||
|---|---|---|---|---|---|
| Metabolite | Pregnancy | No Pregnancy | Control Media | p-Value | Test |
| Thr ↑ | 113.5 (±12.7) b | 121.7 (±12.9) b | 205.7 (±43.5) a | 1.20 × 10−7 | ANOVA |
| PC ae C30:0 ↓ | 0.029 (±0.003) b | 0.033 (±0.003) c | 0.041 (±0.005) a | 6.70 × 10−6 | ANOVA |
| C7-DC ↓ | 0.013 (±0.002) b | 0.015 (±0.002) b | 0.02 (±0.003) a | 3.20 × 10−5 | ANOVA |
| Met-SO ↑ | 1.1 (±0.26) b | 0.98 (±0.28) b | 0.33 (±0.28) a | 7.10 × 10−5 | ANOVA |
| PC aa C28:1 ↓ | 0.008 (±0.003) b | 0.009 (±0.003) b | 0.015 (±0.001) a | 0.00019 | ANOVA |
| Spermidine ↑ | 10 (90.91%) | 2 (20%) | 0 (0%) | 0.00035 | Chi-square |
| C5M-DC ↑ | 0.042 (±0.004) b | 0.04 (±0.003) b | 0.032 (±0.005) a | 0.00038 | ANOVA |
| C10 ↑ | 0.114 (±0.013) b | 0.106 (±0.006) b | 0.091 (±0.008) a | 0.00095 | ANOVA |
| DOPA ↑ | 7 (64%) | 2 (20%) | 0 (0%) | 0.021 | Chi-square |
| Metabolites | Concentrations in Culture Media | Significance | |||
|---|---|---|---|---|---|
| Metabolite | Pregnancy | No Pregnancy | Control Media | p-Value | Test |
| Met-SO/Met ↑ | 0.022 (±0.005) b | 0.02 (±0.007) b | 0.006 (±0.005) a | 1.00 × 10−4 | ANOVA |
| SFA/PC ↓ | 0.76 (±0.017) b | 0.77 (±0.024) b | 0.80 (±0.015) a | 0.0011 | ANOVA |
| Total PC/SM ↓ | 1.77 (±0.057) b | 1.77 (±0.043) b | 1.853 (±0.035) a | 0.0083 | ANOVA |
| Total PC aa ↓ | 0.88 (0.86–0.9 a) b | 0.9 (0.89–0.92) b | 0.97 (0.94–0.98) a | 0.00904 | Kruskal |
| Total PC ↓ | 1.69 (±0.063) b | 1.70 (±0.043) b | 1.78 (±0.03) a | 0.013 | ANOVA |
| C2/C0 ↑↓ | 0.17 (0.17–0.19) a | 0.16 (0.15–0.17) b | 0.17 (0.16–0.17) b | 0.0186 | Kruskal |
| (C2 + C3)/C0 ↑↓ | 0.21 (0.20–0.22) b | 0.2 (0.19–0.21) a | 0.2 (0.199–0.211) a | 0.0285 | Kruskal |
| Total AC-DC/Total AC ↓ | 0.19 (±0.008) b | 0.19 (±0.005) b | 0.198 (±0.006) a | 0.043 | ANOVA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsopp, E.; Kilk, K.; Taalberg, E.; Pärn, P.; Viljaste-Seera, A.; Kavak, A.; Jaakma, Ü. Associations of the Single Bovine Embryo Growth Media Metabolome with Successful Pregnancy. Metabolites 2024, 14, 89. https://doi.org/10.3390/metabo14020089
Tsopp E, Kilk K, Taalberg E, Pärn P, Viljaste-Seera A, Kavak A, Jaakma Ü. Associations of the Single Bovine Embryo Growth Media Metabolome with Successful Pregnancy. Metabolites. 2024; 14(2):89. https://doi.org/10.3390/metabo14020089
Chicago/Turabian StyleTsopp, Elina, Kalle Kilk, Egon Taalberg, Pille Pärn, Anni Viljaste-Seera, Ants Kavak, and Ülle Jaakma. 2024. "Associations of the Single Bovine Embryo Growth Media Metabolome with Successful Pregnancy" Metabolites 14, no. 2: 89. https://doi.org/10.3390/metabo14020089
APA StyleTsopp, E., Kilk, K., Taalberg, E., Pärn, P., Viljaste-Seera, A., Kavak, A., & Jaakma, Ü. (2024). Associations of the Single Bovine Embryo Growth Media Metabolome with Successful Pregnancy. Metabolites, 14(2), 89. https://doi.org/10.3390/metabo14020089






