Roles of Myokines and Muscle-Derived Extracellular Vesicles in Musculoskeletal Deterioration under Disuse Conditions
Abstract
1. Introduction
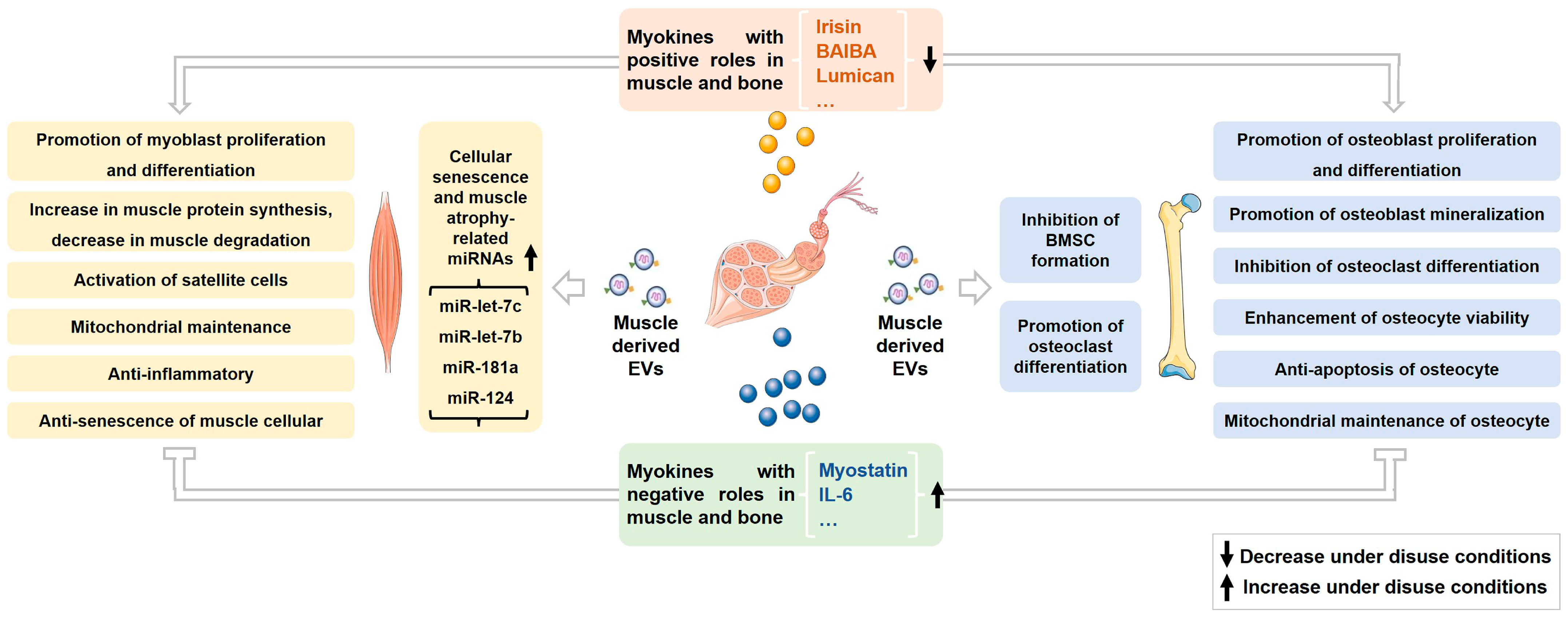
2. Roles of Myokines on Musculoskeletal Metabolism and Homeostasis under Normal and Disuse Conditions
2.1. Irisin
2.2. Myostatin
2.3. Other Myokines
3. Roles of Muscle-Derived EVs on Musculoskeletal Metabolism and Homeostasis under Normal and Disuse Conditions
3.1. Effects of Muscle-Derived EVs on Muscle
3.2. Effects of Muscle-Derived EVs on Bone
3.3. Changes and Effects of Muscle-Derived EVs under Disuse Conditions
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase protein |
| AMPK | AMP-activated protein kinase |
| BAIBA | β-aminoisobutyric acid |
| BMP | bone morphogenetic protein |
| BMSCs | bone marrow stromal cells |
| Bsp | bone sialoprotein |
| CCL-7 | chemokine (C-C motif) ligand 7 |
| COL-1 | collagen I |
| DKK-1 | dickkopf-related protein 1 |
| eEF-2 | eukaryotic elongation factor 2 |
| Erα | estrogen receptor alpha |
| ERK | extracellular signal-regulated kinase |
| EVs | extracellular vesicles |
| FNDC-5 | fibronectin type III domain-containing 5 |
| G6PD | glucose-6-phosphate dehydrogenase |
| IGF-1 | insulin-like growth factor 1 |
| IL-6 | interleukin 6 |
| ILVs | intraluminal vesicles |
| JNK | c-Jun N-terminal kinase |
| Lrp-5 | low density lipoprotein receptor-related protein 5 |
| MAPK | mitogen-activated protein kinase |
| MEK-2 | mitogen-activated protein kinase-extracellular signal-regulated kinase |
| MHC | myosin heavy chain |
| miRNAs | microRNAs |
| Mmp-9 | matrix metalloproteinase 9 |
| MRGPRD | mas-related G protein-coupled receptor type D |
| mTOR | mammalian target of rapamycin |
| MuRF-1 | muscle RING finger 1 |
| MVEs | multivesicular endosomes |
| Myf-5 | myogenic factor-5 |
| MyoD | myogenic differentiation antigen |
| MyoG | myogenin |
| NFATc1 | nuclear factor of activated T-Cells, cytoplasmic 1 |
| NF-κB | nuclear factor kappa-B |
| OCN | osteocalcin |
| OPG | osteoprotegerin |
| OPN | osteopontin |
| Pax-7 | paired box 7 |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator-1alpha |
| PI3K | phosphatidylinositol 3-kinase |
| PKB, Akt | protein kinase B |
| PPARδ | peroxisome proliferator-activated receptor δ |
| RANK | receptor activator of nuclear factor-κ-gene binding |
| Rrbp-1 | Ribosomal binding protein 1 |
| RUNX-2 | runt-related transcription factor 2 |
| SHP-2 | Src-homology domain 2 containing protein-tyrosine phosphatase |
| SOST | sclerostin |
| STAT-5 | signal transducers and activators of transduction 5 |
| Trap | tartrate-resistant acid phosphatase |
| YAP-1 | yes-associated protein 1 |
References
- Lloyd, S.A.; Lang, C.H.; Zhang, Y.; Paul, E.M.; Laufenberg, L.J.; Lewis, G.S.; Donahue, H.J. Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J. Bone Miner. Res. 2014, 29, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.A.; Allen, M.R.; Hogan, H.A.; Delp, M.D. Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone 2002, 31, 149–157. [Google Scholar] [CrossRef]
- Kim, B.J. Effects of muscles on bone metabolism-with a focus on myokines. Ann. Geriatr. Med. Res. 2022, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W. A role for myokines in muscle-bone interactions. Exerc. Sport Sci. Rev. 2011, 39, 43–47. [Google Scholar] [CrossRef]
- Lau, P.; Vico, L.; Rittweger, J. Dissociation of bone resorption and formation in spaceflight and simulated microgravity: Potential role of myokines and osteokines? Biomedicines 2022, 10, 342. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Hershfield, M.R.; Linsenbardt, H.R.; Smith, J.; Mack, J.; Natesan, S.; Averitt, D.L.; Stark, T.R.; Sosanya, N.M. Biological function of extracellular vesicles (EVs): A review of the field. Mol. Biol. Rep. 2023, 10, 8639–8651. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.S.; Chen, C.A.; Zhou, Q.A. Exosomes horizontal line nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS. Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, A.; Van Pelt, D.W.; Hettinger, Z.R.; Fu, X.; Richards, C.I.; Butterfield, T.A.; Petrocelli, J.J.; Vechetti, I.J.; Confides, A.L.; Drummond, M.J.; et al. Extracellular vesicle distribution and localization in skeletal muscle at rest and following disuse atrophy. Skelet. Muscle 2023, 13, 6. [Google Scholar] [CrossRef]
- Huang, H.; Ma, S.; Xing, X.; Su, X.; Xu, X.; Tang, Q.; Gao, X.; Yang, J.; Li, M.; Liang, C.; et al. Muscle-derived extracellular vesicles improve disuse-induced osteoporosis by rebalancing bone formation and bone resorption. Acta Biomater. 2023, 157, 609–624. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Grube, L.; Dellen, R.; Kruse, F.; Schwender, H.; Stuhler, K.; Poschmann, G. Mining the secretome of C2C12 muscle cells: Data dependent experimental approach to analyze protein secretion using label-free quantification and peptide based analysis. J. Proteome Res. 2018, 17, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Song, K.M. Irisin promotes C2C12 myoblast proliferation via ERK-dependent CCL7 upregulation. PLoS ONE 2019, 14, e0222559. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Sanesi, L.; Storlino, G.; Dicarlo, M.; Oranger, A.; Zerlotin, R.; Pignataro, P.; Suriano, C.; Guida, G.; Grano, M.; Colaianni, G.; et al. Time-dependent unloading effects on muscle and bone and involvement of FNDC5/irisin axis. NPJ. Microgravity 2023, 9, 4. [Google Scholar] [CrossRef]
- Alzoughool, F.; Al-Zghoul, M.B.; Ghanim, B.Y.; Atoum, M.; Aljawarneh, Y.; Idkaidek, N.; Qinna, N.A. Impact of sustained exogenous irisin myokine administration on muscle and myocyte integrity in Sprague Dawley rats. Metabolites 2022, 12, 939. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Mermier, C.M.; Conn, C.A. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J. Physiol. Biochem. 2015, 71, 679–689. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin enhances osteoblast differentiation in vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Wang, X.; Chen, T.; Tao, F.; Liu, C.; Tu, Q.; Shen, G.; Chen, J.J. Irisin deficiency disturbs bone metabolism. J. Cell Physiol. 2021, 236, 664–676. [Google Scholar] [CrossRef]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, D.M.; Yang, J.; Tan, L.; Zhang, D.Y. Irisin enhances osteogenic differentiation of mouse MC3T3-E1 cells via upregulating osteogenic genes. Exp. Ther. Med. 2021, 21, 580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.F.; Murray, D.; Wu, Y.W.; Yu, L.M.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.W.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Lin, D.; Ding, Z. The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int. J. Biol. Macromol. 2020, 146, 25–35. [Google Scholar] [CrossRef]
- Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Zhang, J.; Luo, Y.; Nie, Y.; Hu, Y.; Yang, Z.; Zhang, J.; et al. Irisin promotes proliferation but inhibits differentiation in osteoclast precursor cells. FASEB J. 2018, 32, 5813–5823. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xia, X.; Wang, Q.; Hu, D.; Zhang, L.; Li, X.; Ding, X.; Guo, H.; Guo, Y. Myostatin mutation enhances bovine myogenic differentiation through PI3K/AKT/mTOR signalling via removing DNA methylation of RACK1. Cells 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Q.; Zeng, W.; Ma, M.; Wei, Z.X.; Liu, H.B.; Liu, X.F.; Wang, M.; Shi, X.; Zeng, J.H.; Yang, L.F.; et al. Precise editing of myostatin signal peptide by CRISPR/Cas9 increases the muscle mass of Liang Guang Small Spotted pigs. Transgenic Res. 2020, 29, 149–163. [Google Scholar] [CrossRef]
- Sheng, H.; Guo, Y.; Zhang, L.; Zhang, J.; Miao, M.; Tan, H.; Hu, D.; Li, X.; Ding, X.; Li, G.; et al. Proteomic studies on the mechanism of myostatin regulating cattle skeletal muscle development. Front. Genet. 2021, 12, 752129. [Google Scholar] [CrossRef]
- Perie, L.; Parente, A.; Brun, C.; Magnol, L.; Pelissier, P.; Blanquet, V. Enhancement of C2C12 myoblast proliferation and differentiation by GASP-2, a myostatin inhibitor. Biochem. Biophys. Rep. 2016, 6, 39–46. [Google Scholar]
- Zhu, L.; Wang, X.; Wei, Z.; Yang, M.; Zhou, X.; Lei, J.; Bai, C.; Su, G.; Liu, X.; Yang, L.; et al. Myostatin deficiency enhances antioxidant capacity of bovine muscle via the SMAD-AMPK-G6PD pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3497644. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, P.; Lai, W.; Song, T.; Peng, J.; Wei, H.K. Myostatin inhibits eEF2K-eEF2 by regulating AMPK to suppress protein synthesis. Biochem. Biophys. Res. Commun. 2017, 494, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, M.; Wu, P.; Zhang, X.; Zhou, K.; Li, T.; Zhang, T.; Xie, K.; Dai, G.; Wang, J. MicroRNA-27b-3p targets the myostatin gene to regulate myoblast proliferation and is involved in myoblast differentiation. Cells 2021, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Guo, Q.; Guo, L.J.; Liu, T.; Wu, X.P.; Lin, Z.Y.; He, H.B.; Jiang, T.J. GDF8 inhibits bone formation and promotes bone resorption in mice. Clin. Exp. Pharmacol. Physiol. 2017, 44, 500–508. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Divieti Pajevic, P.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef]
- Dankbar, B.; Fennen, M.; Brunert, D.; Hayer, S.; Frank, S.; Wehmeyer, C.; Beckmann, D.; Paruzel, P.; Bertrand, J.; Redlich, K.; et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat. Med. 2015, 21, 1085–1090. [Google Scholar] [CrossRef]
- Jung, T.W.; Hwang, H.J.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Choi, K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia 2015, 58, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.W.; Ding, K.; Dai, X.Y.; Lin, W.Q. beta-aminoisobutyric acid accelerates the proliferation and differentiation of MC3T3-E1 cells via moderate activation of ROS signaling. J. Chin. Med. Assoc. 2018, 81, 611–618. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E. Blocking bone loss with l-BAIBA. Trends Endocrinol. Metab. 2018, 29, 284–286. [Google Scholar] [CrossRef]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jahn, K.; Yi, J.X.; Zhou, J.S.; Brotto, M.; Bonewald, L.F. beta-aminoisobutyric Acid, L-BAIBA, is a muscle-derived osteocyte survival factor. Cell. Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, Y.S.; Kim, D.A.; Moon, S.A.; Lee, S.E.; Lee, S.H.; Koh, J.M. Lumican, an exerkine, protects against skeletal muscle loss. Int. J. Mol. Sci. 2022, 23, 10031. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, S.J.; Kim, D.A.; Lee, S.H.; Koh, J.M.; Kim, B.J. Muscle-derived lumican stimulates bone formation via integrin alpha2beta1 and the downstream ERK signal. Front. Cell. Dev. Biol. 2020, 8, 565826. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, D.A.; Kim, E.Y.; Chang, E.J.; Park, S.J.; Kim, B.J. Lumican inhibits osteoclastogenesis and bone resorption by suppressing Akt activity. Int. J. Mol. Sci. 2021, 22, 4717. [Google Scholar] [CrossRef]
- Haddad, F.; Zaldivar, F.; Cooper, D.M.; Adams, G.R. IL-6-induced skeletal muscle atrophy. J. Appl. Physiol. 2005, 98, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Fix, D.K.; Wang, X.W.; Goldsmith, F.C.; Koh, H.J.; Carson, J.A. Systemic IL-6 regulation of eccentric contraction-induced muscle protein synthesis. Am. J. Physiol.-Cell Physiol. 2018, 315, C91–C103. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, F.; Rucci, N.; Del Fattore, A.; Peruzzi, B.; Paro, R.; Longo, M.; Vivarelli, M.; Muratori, F.; Berni, S.; Ballanti, P.; et al. Impaired skeletal development in interleukin-6-transgenic mice: A model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 2006, 54, 3551–3563. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C.; Hirao, M.; Okamoto, M.; Koizumi, K.; Morimoto, T.; Yoshikawa, H.; Hashimoto, J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Cui, F.Q.; Ning, K.T.; Wang, Z.; Fu, P.Y.; Wang, D.E.; Xu, H.Y. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef]
- Wu, L.F.; Zhu, D.C.; Tang, C.H.; Ge, B.; Shi, J.; Wang, B.H.; Lu, Y.H.; He, P.; Wang, W.Y.; Lu, S.Q.; et al. Association of plasma Irisin with bone mineral density in a large Chinese population using an extreme sampling design. Calcif. Tissue Int. 2018, 103, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, H.J.; Guo, W.C.; Yang, J. Low serum concentrations of Irisin are associated with increased risk of hip fracture in Chinese older women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kawao, N.; Moritake, A.; Tatsumi, K.; Kaji, H. Roles of Irisin in the linkage from muscle to bone during mechanical unloading in mice. Calcif. Tissue Int. 2018, 103, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Oranger, A.; Storlino, G.; Dicarlo, M.; Zerlotin, R.; Pignataro, P.; Sanesi, L.; Narici, M.; Pisot, R.; Simunic, B.; Colaianni, G.; et al. Impact of 10-day bed rest on serum levels of irisin and markers of musculoskeletal metabolism. FASEB J. 2023, 37, e22668. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Zhao, F.; Yin, C.; Yang, C.; Wang, X.; Wu, Z.; Liang, S.; Li, D.; Lin, X.; et al. Recombinant Irisin prevents the reduction of osteoblast differentiation induced by stimulated microgravity through increasing beta-catenin expression. Int. J. Mol. Sci. 2020, 21, 1259. [Google Scholar] [CrossRef]
- Colucci, S.; Colaianni, G.; Brunetti, G.; Ferranti, F.; Mascetti, G.; Mori, G.; Grano, M. Irisin prevents microgravity-induced impairment of osteoblast differentiation in vitro during the space flight CRS-14 mission. FASEB J. 2020, 34, 10096–10106. [Google Scholar] [CrossRef] [PubMed]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin prevents disuse-induced osteocyte apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McPherron, A.C.; Lovejoy, C.O. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif. Tissue Int. 2002, 71, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McPherron, A.C.; Lovejoy, C.O.; Hudson, J. Femoral morphology and cross-sectional geometry of adult myostatin-deficient mice. Bone 2000, 27, 343–349. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Gu, M.; Zhu, L.; Hai, C.; Di, A.; Wu, D.; Bai, C.; Su, G.; Liu, X.; et al. Loss of myostatin alters mitochondrial oxidative phosphorylation, TCA cycle activity, and ATP production in skeletal muscle. Int. J. Mol. Sci. 2022, 23, 15707. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W. Increased bone mineral density in the femora of GDF8 knockout mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 272, 388–391. [Google Scholar] [CrossRef]
- Takayama, K.; Hitachi, K.; Okamoto, H.; Saitoh, M.; Odagiri, M.; Ohfusa, R.; Shimada, T.; Taguchi, A.; Taniguchi, A.; Tsuchida, K.; et al. Development of myostatin inhibitory D-Peptides to enhance the potency, increasing skeletal muscle mass in mice. ACS Med. Chem. Lett. 2022, 13, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Pirruccello-Straub, M.; Jackson, J.; Wawersik, S.; Webster, M.T.; Salta, L.; Long, K.; McConaughy, W.; Capili, A.; Boston, C.; Carven, G.J.; et al. Blocking extracellular activation of myostatin as a strategy for treating muscle wasting. Sci. Rep. 2018, 8, 2292. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kuramochi, T.; Katada, H.; Ueyama, A.; Ruike, Y.; Ohmine, K.; Shida-Kawazoe, M.; Miyano-Nishizawa, R.; Shimizu, Y.; Okuda, M.; et al. Novel myostatin-specific antibody enhances muscle strength in muscle disease models. Sci. Rep. 2021, 11, 2160. [Google Scholar] [CrossRef]
- Grobet, L.; Martin, L.R.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J. A deletion in the bovine myostatin gene causes the double|[ndash]|muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef]
- Kambadur, R.; Sharma, M.; Smith, T.P.L.; Bass, J.J. Mutations in myostatin (GDF8) in double-muscled belgian blue and piedmontese cattle. Genome Res. 1997, 7, 910–915. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S.; Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E.A. A mutation in the myostatin gene increases muscle mass and enhances racing performance in Heterozygote dogs. PLoS Genet. 2007, 3, e79. [Google Scholar]
- Qian, L.L.; Xie, J.Y.; Gao, T.; Cai, C.B.; Jiang, S.W.; Bi, H.F.; Xie, S.S.; Cui, W.T. Targeted myostatin loss-of-function mutation increases type II muscle fibers in Meishan pigs. J. Integr. Agric. 2022, 21, 188–198. [Google Scholar] [CrossRef]
- Qian, L.L.; Tang, M.X.; Yang, J.Z.; Wang, Q.Q.; Cai, C.B.; Jiang, S.W.; Li, H.G.; Jiang, K.; Gao, P.F.; Ma, D.Z.; et al. Targeted mutations in myostatin by zinc-finger nucleases result in double-muscled phenotype in Meishan pigs. Sci. Rep. 2015, 5, srep14435. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, N.; Ozaki, E.; Koyama, T.; Matsui, D.; Watanabe, I.; Tomida, S.; Nagamitsu, R.; Hashiguchi, K.; Inaba, M.; Yamada, S.; et al. Evaluation of myostatin as a possible regulator and marker of skeletal muscle-cortical bone interaction in adults. J. Bone Miner. Metab. 2021, 39, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Bialek, P.; Parkington, J.; Li, X.; Gavin, D.; Wallace, C.; Zhang, J.; Root, A.; Yan, G.; Warner, L.; Seeherman, H.J.; et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone 2014, 60, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Wehling, M.; Cai, B.; Tidball, J.G. Modulation of myostatin expression during modified muscle use. FASEB J. 2000, 14, 103–110. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.; Dolmans, J.; van Loon, L.J. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611. [Google Scholar] [CrossRef]
- Lalani, R.; Bhasin, S.; Byhower, F.; Tarnuzzer, R.; Grant, M.; Shen, R.; Asa, S.; Ezzat, S.; Gonzalez-Cadavid, N.F. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J. Endocrinol. 2000, 167, 417–428. [Google Scholar] [CrossRef]
- Hanson, A.M.; Young, M.H.; Harrison, B.C.; Zhou, X.; Han, H.Q.; Stodieck, L.S.; Ferguson, V.L. Inhibiting myostatin signaling partially mitigates structural and functional adaptations to hindlimb suspension in mice. NPJ Microgravity 2023, 9, 2. [Google Scholar] [CrossRef]
- Lee, S.J.; Lehar, A.; Meir, J.U.; Koch, C.; Morgan, A.; Warren, L.E.; Rydzik, R.; Youngstrom, D.W.; Chandok, H.; George, J.; et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc. Natl. Acad. Sci. USA 2020, 117, 23942–23951. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Shi, X.; Zhang, W.; Pennington, C.; Thakore, H.; Haque, M.; Kang, B.; Isales, C.M.; Fulzele, S.; Wenger, K.H. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone 2007, 40, 1544–1553. [Google Scholar] [CrossRef]
- Roberts, L.D.; Bostrom, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell. Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.J.; Yang, Y.; Li, T.; Li, M.H.; Yao, T.T.; Hu, G.X.; Wan, G.M.; Chang, B. Signaling metabolite beta-aminoisobutyric acid as a metabolic regulator, biomarker, and potential exercise pill. Front. Endocrinol. 2023, 14, 1192458. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Raastad, T.; Thiede, B.; Rustan, A.C.; Drevon, C.A.; Haugen, F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1013–E1021. [Google Scholar] [CrossRef]
- Murgia, M.; Brocca, L.; Monti, E.; Franchi, M.V.; Zwiebel, M.; Steigerwald, S.; Giacomello, E.; Sartori, R.; Zampieri, S.; Capovilla, G.; et al. Plasma proteome profiling of healthy subjects undergoing bed rest reveals unloading-dependent changes linked to muscle atrophy. J. Cachexia Sarcopenia Muscle 2023, 14, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Blottner, D.; Moriggi, M.; Trautmann, G.; Hastermann, M.; Capitanio, D.; Torretta, E.; Block, K.; Rittweger, J.; Limper, U.; Gelfi, C.; et al. Space omics and tissue response in astronaut skeletal muscle after short and long duration missions. Int. J. Mol. Sci. 2023, 24, 4095. [Google Scholar] [CrossRef] [PubMed]
- Belizario, J.E.; Fontes-Oliveira, C.C.; Borges, J.P.; Kashiabara, J.A.; Vannier, E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. Springerplus 2016, 5, 619. [Google Scholar] [CrossRef] [PubMed]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front. Endocrinol. 2019, 9, 788. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.; Mehta, S.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Sams, C. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 2013, 33, 456–465. [Google Scholar] [CrossRef]
- Bosutti, A.; Malaponte, G.; Zanetti, M.; Castellino, P.; Heer, M.; Guarnieri, G.; Biolo, G. Calorie restriction modulates inactivity-induced changes in the inflammatory markers C-reactive protein and pentraxin-3. J. Clin. Endocr. Metab. 2008, 93, 3226–3229. [Google Scholar] [CrossRef]
- Yakabe, M.; Ogawa, S.; Ota, H.; Iijima, K.; Eto, M.; Ouchi, Y.; Akishita, M. Inhibition of interleukin-6 decreases atrogene expression and ameliorates tail suspension-induced skeletal muscle atrophy. PLoS ONE 2018, 13, e0191318. [Google Scholar] [CrossRef]
- Drummond, M.J.; Timmerman, K.L.; Markofski, M.M.; Walker, D.K.; Dickinson, J.M.; Jamaluddin, M.; Brasier, A.R.; Rasmussen, B.B.; Volpi, E. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R216–R223. [Google Scholar] [CrossRef]
- Cavey, T.; Pierre, N.; Nay, K.; Allain, C.; Ropert, M.; Loreal, O.; Derbre, F. Simulated microgravity decreases circulating iron in rats: Role of inflammation-induced hepcidin upregulation. Exp. Physiol. 2017, 102, 291–298. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Choi, H.; Jun, H.S. Preventive Effects of dulaglutide on disuse muscle atrophy through inhibition of inflammation and apoptosis by induction of Hsp72 expression. Front. Pharmacol. 2020, 11, 90. [Google Scholar] [CrossRef]
- Kim, D.S.; Cha, H.N.; Jo, H.J.; Song, I.H.; Baek, S.H.; Dan, J.M.; Kim, Y.W.; Kim, J.Y.; Lee, I.K.; Seo, J.S.; et al. TLR2 deficiency attenuates skeletal muscle atrophy in mice. Biochem. Biophys. Res. Commun. 2015, 459, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.X.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.K.; Lee, C.H.; Kwon, O.; Kim, M.-S. Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c). Diabetes Metab. J. 2022, 46, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hu, G.; Yang, Y.; Li, J.; Jin, J.; Chang, B. Role of MOTS-c in the regulation of bone metabolism. Front. Physiol. 2023, 14, 711. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 640. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell. Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- van den Boorn, J.G.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug. Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr. Opin. Cell. Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Lam, N.T.; Gartz, M.; Thomas, L.; Haberman, M.; Strande, J.L. Influence of microRNAs and exosomes in muscle health and diseases. J. Muscle Res. Cell Motil. 2020, 41, 269–284. [Google Scholar] [CrossRef]
- Choi, J.S.; Yoon, H.I.; Lee, K.S.; Choi, Y.C.; Yang, S.H.; Kim, I.S.; Cho, Y.W. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control Release 2016, 222, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Sudo, Y.; Makino, T.; Kimura, S.; Tomita, K.; Noguchi, M.; Sakurai, H.; Shimizu, M.; Takahashi, Y.; Sato, R.; et al. Skeletal muscle releases extracellular vesicles with distinct protein and microRNA signatures that function in the muscle microenvironment. PNAS Nexus 2022, 1, pgac173. [Google Scholar] [CrossRef]
- Alfonzo, M.C.; Al Saedi, A.; Fulzele, S.; Hamrick, M.W. Extracellular vesicles as communicators of senescence in musculoskeletal aging. JBMR Plus 2022, 6, e10686. [Google Scholar] [CrossRef] [PubMed]
- Youssef, E.l.; Baradie, K.B.; Hamrick, M.W. Therapeutic application of extracellular vesicles for musculoskeletal repair & regeneration. Connect. Tissue Res. 2021, 62, 99–114. [Google Scholar]
- Vechetti, I.J., Jr.; Valentino, T.; Mobley, C.B.; McCarthy, J.J. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J. Physiol. 2021, 599, 845–861. [Google Scholar] [CrossRef]
- Aoi, W.; Tanimura, Y. Roles of skeletal muscle-derived exosomes in organ metabolic and immunological communication. Front. Endocrinol. 2021, 12, 697204. [Google Scholar] [CrossRef]
- Xu, Q.; Cui, Y.Z.; Luan, J.; Zhou, X.Y.; Li, H.Y.; Han, J.X. Exosomes from C2C12 myoblasts enhance osteogenic differentiation of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem. Biophys. Res. Commun. 2018, 498, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Forterre, A.; Jalabert, A.; Chikh, K.; Pesenti, S.; Euthine, V.; Granjon, A.; Errazuriz, E.; Lefai, E.; Vidal, H.; Rome, S. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle 2014, 13, 78–89. [Google Scholar] [CrossRef]
- Mytidou, C.; Koutsoulidou, A.; Katsioloudi, A.; Prokopi, M.; Kapnisis, K.; Michailidou, K.; Anayiotos, A.; Phylactou, L.A. Muscle-derived exosomes encapsulate myomiRs and are involved in local skeletal muscle tissue communication. FASEB J. 2021, 35, e21279. [Google Scholar] [CrossRef]
- Takafuji, Y.; Tatsumi, K.; Kawao, N.; Okada, K.; Muratani, M.; Kaji, H. MicroRNA-196a-5p in extracellular vesicles secreted from myoblasts suppresses osteoclast-like cell formation in mouse cells. Calcif. Tissue Int. 2021, 108, 364–376. [Google Scholar] [CrossRef]
- Forterre, A.; Jalabert, A.; Berger, E.; Baudet, M.; Chikh, K.; Errazuriz, E.; De Larichaudy, J.; Chanon, S.; Weiss-Gayet, M.; Hesse, A.M.; et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: A new paradigm for myoblast-myotube cross talk? PLoS ONE 2014, 9, e84153. [Google Scholar] [CrossRef]
- Fry, C.S.; Kirby, T.J.; Kosmac, K.; McCarthy, J.J.; Peterson, C.A. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 2017, 20, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Maeshige, N.; Yan, J.W.; Ma, X.Q.; Uemura, M.; Matsuda, M.; Nishimura, Y.; Hasunuma, T.; Kondo, H.; Fujino, H.; et al. Skeletal myotube-derived extracellular vesicles enhance itaconate production and attenuate inflammatory responses of macrophages. Front. Immunol. 2023, 14, 1099799. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Pan, C.; Yuan, H.; Li, X.; Chen, Z.; He, H. Myoblast-derived exosomal Prrx2 attenuates osteoporosis via transcriptional regulation of lncRNA-MIR22HG to activate Hippo pathway. Mol. Med. 2023, 29, 54. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, Y.; Tatsumi, K.; Ishida, M.; Kawao, N.; Okada, K.; Kaji, H. Extracellular vesicles secreted from mouse muscle cells suppress osteoclast formation: Roles of mitochondrial energy metabolism. Bone 2020, 134, 115298. [Google Scholar] [CrossRef]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef]
- Watanabe, S.; Sudo, Y.; Sakurai, H.; Sato, R.; Yamauchi, Y. Identification of protein markers for skeletal muscle-derived extracellular vesicles (SkM-EVs) by quantitative proteomics reveals how SkM-EVs function in vivo. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Van Pelt, D.; Butterfield, T.; Dupont-Versteegden, E. Disuse atrophy elevates skeletal muscle CD63 expression and ex vivo release of extracellular vesicles in a muscle-specific manner. FASEB J. 2021, 35, 3995. [Google Scholar] [CrossRef]
- Van Pelt, D.W.; Butterfield, T.A.; Dupont-Versteegden, E.E. Disuse atrophy elevates the release of skeletal muscle-derived extracellular vesicles but lowers serum EV concentration in rats. FASEB J. 2020, 34, 4121. [Google Scholar] [CrossRef]
- Parker, E.; Mendhe, B.; Ruan, L.; Marshall, B.; Zhi, W.; Liu, Y.; Fulzele, S.; Tang, Y.L.; McGee-Lawrence, M.; Lee, T.J.; et al. MicroRNA cargo of extracellular vesicles released by skeletal muscle fibro-adipogenic progenitor cells is significantly altered with disuse atrophy and IL-1beta deficiency. Physiol. Genom. 2022, 54, 296–304. [Google Scholar] [CrossRef]
| Myokine | Target Cell/Tissue | Effect and Mechanism | References | |
|---|---|---|---|---|
| Irisin | C2C12 myoblasts | Activates satellite cells Enhances protein synthesis through activating Akt/mTOR pathway and down-regulates protein degradation through suppressing protein expression of Atrogin-1 and MuRF-1 | [12] | |
| C2C12 myoblasts | Enhances myoblast proliferation and fusion through up-regulating mRNA expression of ERK-dependent chemokine (C-C motif) ligand 7 (CCL-7) | [13] | ||
| Human skeletal muscle cell | Stimulates muscle growth through up-regulating mRNA expression of IGF-1 and down-regulating mRNA expression of myostatin | [14] | ||
| C2C12 myoblast | Preserves muscle cell from senescence through inhibiting mRNA expression of senescence marker, p53 | [15] | ||
| Hind muscle of female SD rats | Promotes mitochondrial fusion Increases mRNA expression of main regulatory genes for mitochondrial fusion, DPL1, and Mfn | [16] | ||
| C2C12 myotubes | Increases mitochondrial content and oxygen consumption through up-regulating mRNA and protein expression of several genes including peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1α) | [17] | ||
| BMSCs | Enhances osteoblast differentiation via increasing mRNA expression of Alp and Col-1 | [18] | ||
| BMSCs | Promotes osteogenesis through up-regulating mRNA expression of osteogenic markers, including Runx-2, bone sialoprotein (Bsp), Col-1, and Alp Promotes BMSCs mineralization Inhibits osteoclastogenesis through decreasing mRNA expression of osteoclastogenesis markers, including tartrate-resistant acid phosphatase (Trap), matrix metalloproteinase 9 (Mmp-9), and NFATc1 | [19] | ||
| Murine osteoblastic MC3T3-E1 cells | Promotes osteoblast proliferation and differentiation through activating P38/ERK MAPK signaling pathway | [20] | ||
| MC3T3-E1 osteoblasts | Enhances osteogenic differentiation via increasing mRNA expression of osteogenic genes, Alp, Col-1, Runx-2, osterix, Opn, Ocn, Opg, and ERα | [21] | ||
| MC3T3-E1 osteoblast precursor cells RAW264.7 osteoclast precursor cells | Increases osteoblastogenesis and mineralization through activating β-catenin signaling Inhibits RANKL-induced osteoclastogenesis through decreasing mRNA expression of nuclear factor of activated T-Cells, cytoplasmic 1 (NFATc1) | [22] | ||
| Tibia of young male mice | Stimulates bone formation through up-regulating mRNA expression of Atf-4, Runx-2, Osx, low density lipoprotein receptor-related protein 5 (Lrp-5), β-catenin, Alp, and Col-1a1 Inhibits osteoclastogenesis and reduces osteoclast numbers | [23] | ||
| MC3T3-E1 cells | Enhances M2 polarization of osteoblasts through activating AMPK signaling pathway | [24] | ||
| Mouse bone marrow monocytes RAW264.7 cells | Promotes osteoclast precursor cell proliferation through activating p38 and JNK signaling pathway Inhibits differentiation of osteoclast cells through suppressing NF-κB pathway | [25] | ||
| Osteocyte-like cells (MLO-Y4) | Prevents apoptosis of osteocyte-like cells (MLO-Y4) | [26] | ||
| Myostatin | Deletion | Luxi yellow cattle muscle | Promotes myogenic differentiation through activating PI3K/Akt/mTOR signaling pathway | [27] |
| Longissimus dorsi of Liang Guang Small Spotted pigs | Promotes proliferation and myogenic differentiation of skeletal muscle cells through elevating protein expression of myogenic regulatory factors, MyoD, MyoG, and Myf-5 | [28] | ||
| Bovine skeletal muscle satellite cells (BSMSCs) | Promotes proliferation and myogenic differentiation of BSMSCs through increasing mRNA and protein expression of extracellular matrix and ribosome-related proteins, COL-1A1, activating focal adhesion, PI3K-Akt, and ribosomal pathways | [29] | ||
| C2C12 myoblasts | Promotes C2C12 proliferation and differentiation through inhibiting myostatin canonical signaling pathway | [30] | ||
| Bovine muscle | Enhances antioxidant capacity through activating SMAD-AMPK-G6PD signaling pathway | [31] | ||
| Administration | C2C12 myoblasts | Inhibits protein synthesis through suppressing eukaryotic elongation factor 2 (eEF-2) through AMPK signaling pathway | [32] | |
| C2C12 myoblasts | Inhibits myoblast differentiation | [33] | ||
| Primary mouse osteoblasts osteoclasts | Inhibits osteoblastic differentiation and mineralization through decreasing ALP activity, mRNA expression of osteoblast transcription factors osterix and Runx-2, as well as OCN secretion Promotes RANKL-induced osteoclastogenesis through increasing number of TRAP+ multinucleated giant cells, TRAP activity, and mRNA expression of NFATc1 | [34] | ||
| RANKL-induced osteoclasts Cultured osteocytic (Ocy454) cells | Inhibits osteoblastic differentiation through suppressing osteocyte-derived exosomal miR-218 Weakens osteocyte function via promoting mRNA expression of several bone regulators such as sclerostin (SOST), dickkopf-related protein 1 (DKK-1), and RANKL | [35] | ||
| Bone marrow-derived macrophages (BMMs) | Promotes osteoclastogenesis through activating MAPK pathways and SMAD2 signaling | [36] | ||
| BAIBA | C2C12 cells | Attenuates insulin resistance and suppresses inflammation through activating AMPK–PPARδ signaling pathway | [37] | |
| MC3T3-E1 cells | Promotes proliferation and differentiation of osteoprogenitor cells through activating NAD(P)H oxidase/ROS signaling pathway | [38] | ||
| Osteocytes | Increases osteocyte viability through blocking mitochondrial fission and preserving mitochondrial integrity | [39] | ||
| Osteocytes | Prevents ROS induced mitochondria breakdown through activating Mas-related G protein-coupled receptor type D (MRGPRD) | [40] | ||
| Lumican | C2C12 myoblasts | Promotes myogenesis through activating p38 MAPK-mediated myoblast differentiation | [41] | |
| C2C12 myoblasts | Maintains positive protein balance through up-regulating protein synthesis and down-regulating protein degradation | [41] | ||
| Murine preosteoblast MC3T3-E1 cells | Stimulates bone formation via integrin α2β1 and the downstream ERK signal | [42] | ||
| Primary bone marrow cells | Inhibits osteoclastogenesis and bone resorption through suppressing Akt activity | [43] | ||
| IL-6 | TA and EDL muscles of rats | Decreases total protein and myofibrillar protein content through decreasing phosphorylation of ribosomal S6 kinase and signal transducers and activators of transduction 5 (STAT-5) | [44] | |
| Skeletal muscle of mice | Inhibits basal protein synthesis through suppressing mTORC1 signaling | [45] | ||
| Primary osteoblasts and osteoclasts of mice | Decreases osteoblast and increases osteoclast number and activity | [46] | ||
| MC3T3-E1 osteoblastic cells | Negatively regulates osteoblast differentiation through activating Src-homology domain 2 containing protein-tyrosine phosphatase (SHP-2)/mitogen-activated protein kinase-extracellular signal–regulated kinase kinase (MEK-2)/ERK and SHP-2/PI3K/Akt-2 pathways, as well as reducing mRNA expression of osteoblastic differentiation related genes, including Alp, Runx-2, and Ocn | [47] | ||
| Muscle-Derived EVs Containing miRNAs | Target Cell/Tissue | Effect and Mechanism | References |
|---|---|---|---|
| C2C12 myotube-derived exosomal miR-133a | C2C12 myoblasts | Inhibits myoblast proliferation and promotes myoblast differentiation into myotube through silencing Sirt-1 | [118] |
| C2C12 myotube-derived exosomal proteins | C2C12 myoblasts | Inhibits myoblast proliferation through down-regulating mRNA expression of cyclin-D1 Promotes myoblast differentiation into myotubes through up-regulating mRNA expression of MyoG | [121] |
| Exosomes released from differentiating human skeletal myoblasts | Human adipose-derived stem cells Hindlimb muscles of mice | Promotes myogenesis through increasing expression of myogenic proteins (myosin heavy chain and desmin) Alleviates skeletal muscle fibrosis through reducing collagen deposition | [111] |
| Muscle interstitium-derived exosomal miR-1, -206, -431, and -486 | C2C12 myoblasts | Promotes muscle differentiation through inhibiting mRNA expression of Pax-7 and promotes mRNA expression of MHC | [112] |
| Myogenic progenitor cell-derived exosomal miR-206 | Extracellular matrix | Inhibits excessive extracellular matrix generation through suppressing protein expression of Rrbp-1 and down-regulates mRNA expression of collagen proteins involved in biosynthesis | [122] |
| miR-206-3p, miR-378a-3p, miR-30d-5p, and miR-21a-5p in myotube-derived EVs | Mouse bone marrow-derived macrophages | Exhibits anti-inflammatory effects in macrophages through activating PI3K-Akt and JAK-STAT pathways | [123] |
| Myoblast-derived exosomal miR-27a-3p | MC3T3-E1 pre-osteoblasts | Promotes MC3T3-E1 pre-osteoblast differentiation and bone mineralization through activating Wnt/β-catenin signaling pathway | [117] |
| Differentiating C2C12 cell-derived exosomal Prrx-2 | BMSCs | Promotes osteogenesis differentiation through alleviating inhibitory effects of miR-128 on YAP-1 via up-regulating lncRNA MIR22HG | [124] |
| Skeletal muscle-derived EVs | Primary BMSCs and osteoclasts of C57BL/6J mice | Promotes osteogenesis differentiation of BMSCs through inhibiting osteoclast formation | [9] |
| C2C12 myoblast- and myotube-derived EV miR-196a-5p | Raw264.7 cells | Suppresses osteoclast formation through weakening mitochondrial function of osteoclasts | [120,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gao, Y.; Yan, J. Roles of Myokines and Muscle-Derived Extracellular Vesicles in Musculoskeletal Deterioration under Disuse Conditions. Metabolites 2024, 14, 88. https://doi.org/10.3390/metabo14020088
Zhang J, Gao Y, Yan J. Roles of Myokines and Muscle-Derived Extracellular Vesicles in Musculoskeletal Deterioration under Disuse Conditions. Metabolites. 2024; 14(2):88. https://doi.org/10.3390/metabo14020088
Chicago/Turabian StyleZhang, Jie, Yunfang Gao, and Jiangwei Yan. 2024. "Roles of Myokines and Muscle-Derived Extracellular Vesicles in Musculoskeletal Deterioration under Disuse Conditions" Metabolites 14, no. 2: 88. https://doi.org/10.3390/metabo14020088
APA StyleZhang, J., Gao, Y., & Yan, J. (2024). Roles of Myokines and Muscle-Derived Extracellular Vesicles in Musculoskeletal Deterioration under Disuse Conditions. Metabolites, 14(2), 88. https://doi.org/10.3390/metabo14020088






