Clinical Efficacy and Metabolomics Modifications Induced by Polyphenol Compound Supplementation in the Treatment of Residual Dizziness following Semont Maneuver in Benign Paroxysmal Positional Vertigo (BPPV) of the Posterior Semicircular Canal (PSC): Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Subjective Symptom Assessement
2.3. Urine Sample Preparation and 1H-NMR Analysis
H-NMR Data Preprocessing
2.4. Statistical Analysis
3. Results
3.1. Visuo-Analog Scale (VAS) of Dizziness
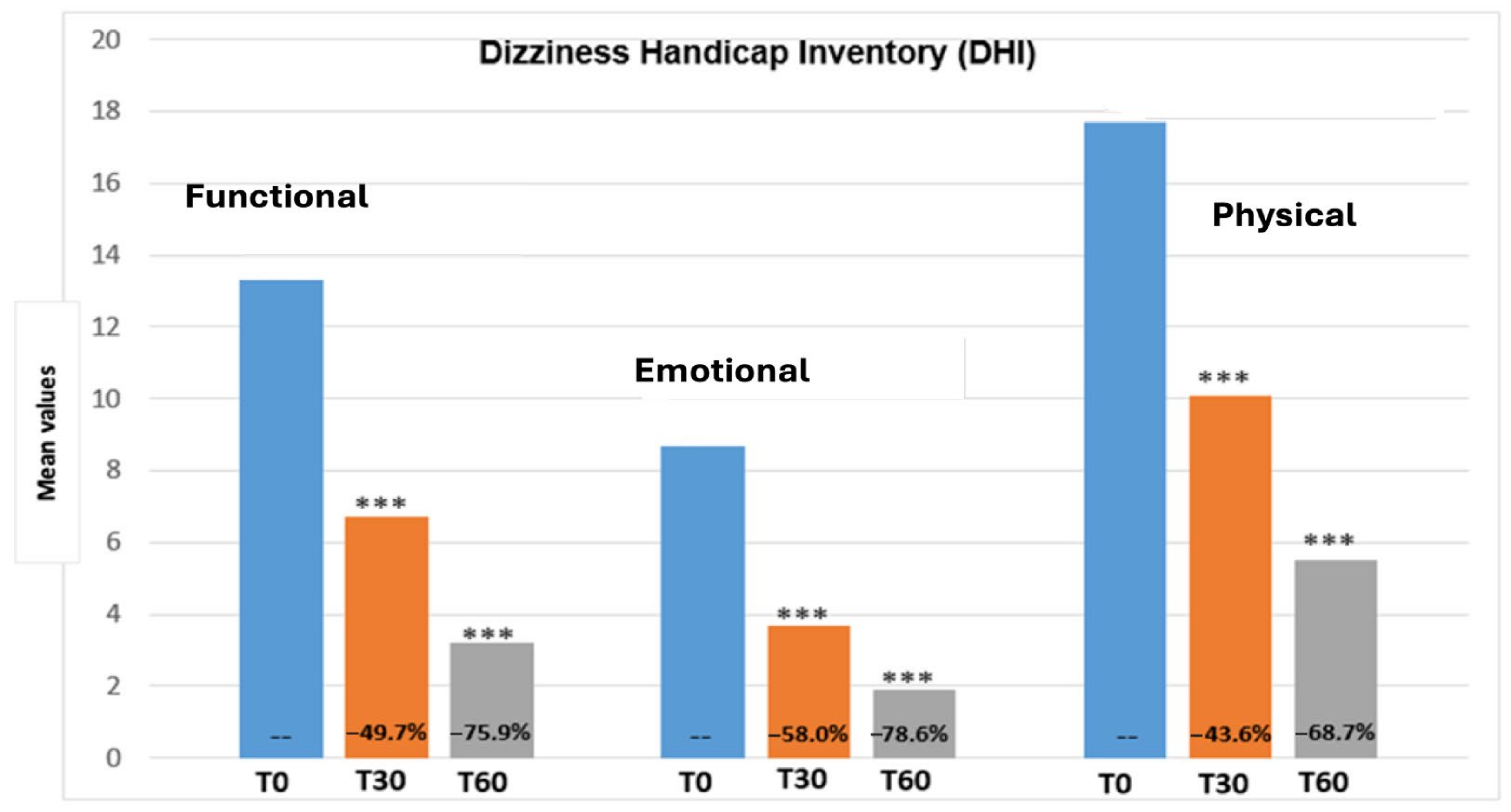
3.2. Dizziness Handicap Inventory (DHI)
3.3. Efficacy and Tolerability Evaluation
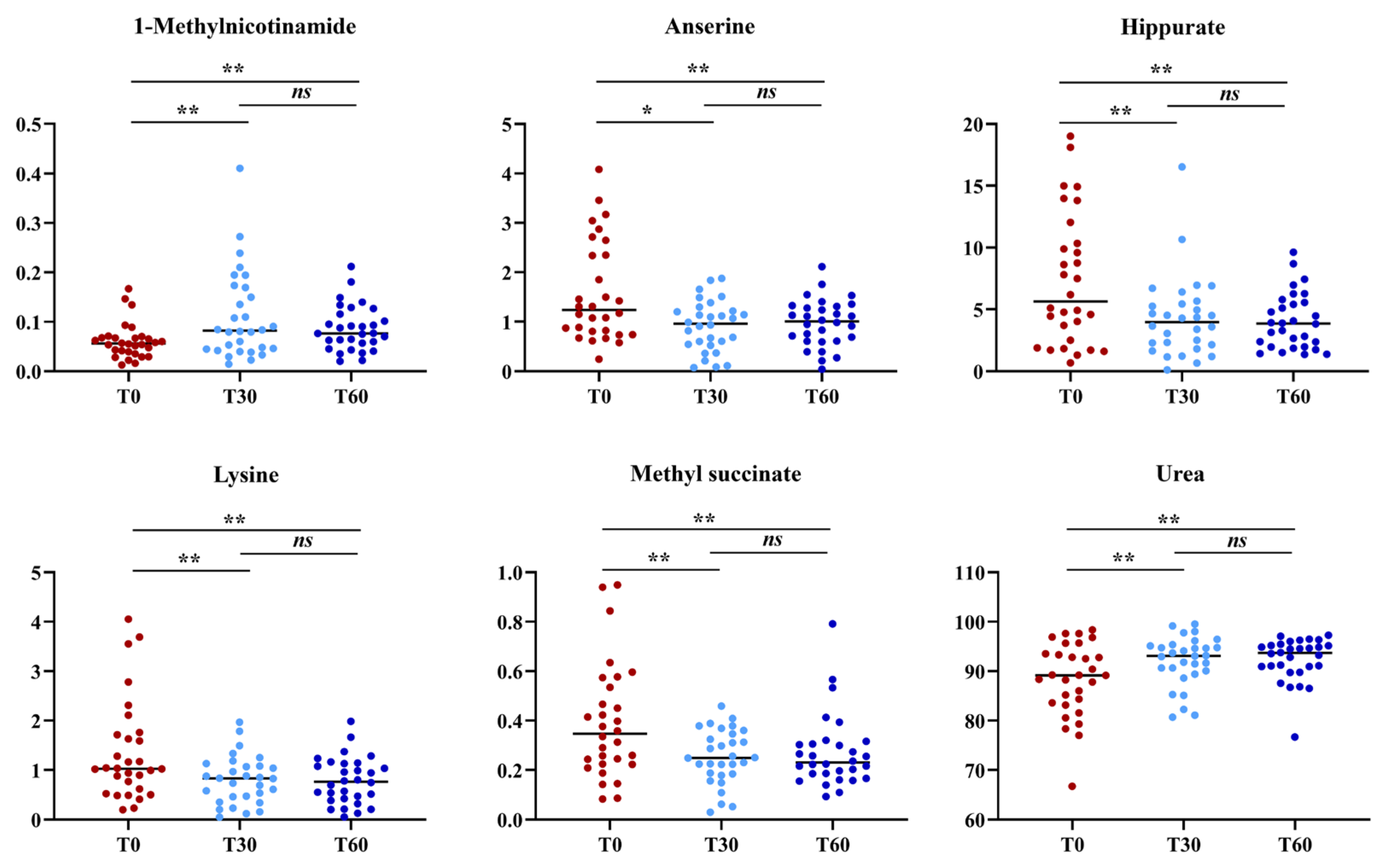
3.4. Urine Metabolomics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol. Head Neck Surg. 2017, 536, S1–S47. [Google Scholar] [CrossRef] [PubMed]
- Von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population based study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Maihoub, S.; Tamás, L.; Szirmai, Á. A possible objective test to detect benign paroxysmal positional vertigo. The role of the caloric and video-head impulse tests in the diagnosis. J. Otol. 2022, 17, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Nuti, D.; Zee, D.S.; Mandalà, M. Benign paroxysmal positional vertigo: What we do and do not know. Semin. Neurol. 2020, 40, 49–58. [Google Scholar] [CrossRef]
- You, P.; Instrum, R.; Parnes, L. Benign paroxysmal positional vertigo Laryngoscope. Investig. Otolaryngol. 2019, 4, 116–123. [Google Scholar]
- Casani, A.P.; Gufoni, M. Recurring benign paroxysmal positional vertigo after successful canalith repositioning manoeuvers. Acta Otorhinolaryngol. Ital. 2023, 43, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M.; Santoro, G.P.; Asprella Libonati, G.; Casani, A.P.; Faralli, M.; Giannoni, B.; Gufoni, M.; Marcelli, V.; Marchetti, P.; Pepponi, E.; et al. Double-blind randomized trial on short-term efficacy of the Semont maneuver for the treatment of posterior canal benign paroxysmal positional vertigo. J. Neurol. 2012, 259, 882–885. [Google Scholar] [CrossRef]
- Casani, A.P.; Nacci, A.; Dallan, I.; Panicucci, E.; Gufoni, M.; Sellari-Franceschini, S. Horizontal semicircular canal benign paroxysmal positional vertigo: Effectiveness of two different methods of treatment. Audiol. Neurootol. 2011, 16, 175–184. [Google Scholar] [CrossRef]
- Albera, A.; Boldreghini, M.; Canale, A.; Albera, R.; Gervasio, C.F. Vertigo returning to the sitting position after the Semont manoeuvre. Is it a prognostic symptom? Acta Otorhinol. Ital. 2018, 38, 145–150. [Google Scholar] [CrossRef]
- Teggi, R.; Giordano, L.; Bondi, S.; Fabiano, B.; Bussi, M. Residual dizziness after successful repositioning maneuvers for idiopathic benign paroxysmal positional vertigo in the elderly. Eur. Arch. Otorhinolaryngol. 2011, 68, 507–511. [Google Scholar] [CrossRef]
- Ke, Y.; Ma, X.; Jing, Y.; Diao, T.; Yu, L. Risk factors for residual dizziness in patients with benign paroxysmal positional vertigo after successful repositioning: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2022, 279, 3237–3256. [Google Scholar] [CrossRef] [PubMed]
- Giommetti, G.; Lapenna, R.; Panichi, R.; Mobaraki, P.D.; Longari, F.; Ricci, G.; Faralli, M. Residual Dizziness after Successful Repositioning Maneuver for Idiopathic Benign Paroxysmal Positional Vertigo: A Review. Audiol. Res. 2017, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, C.; Li, Y.; Zhou, X.; Shui, L.; Han, C. Risk factors and a nomogram model for residual symptoms of cured benign paroxysmal positional vertigo. J. Int. Adv. Otol. 2023, 19, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Noto, A.; Piras, C.; Atzori, L.; Fanos, V. Slotting metabolomics into routine precision medicine. J. Expert Rev. Precis. Med. Drug Dev. 2021, 6, 173–187. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. Metabolomics: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Fanos, V. Metabolomics of autism spectrum disorders: Early insights regarding mammalian-microbial cometabolites. Expert Rev. Mol. Diagn. 2016, 16, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef]
- Noto, A.; Piras, C.; Atzori, L.; Mussap, M.; Albera, A.; Albera, R.; Casani, A.P.; Capobianco, S.; Fanos, V. Metabolomics in Otorhinolaryngology. Front. Mol. Biosci. 2022, 9, 934311. [Google Scholar] [CrossRef]
- Carta, F.; Lussu, M.; Bandin, F.; Noto, A.; Peppi, M.; Chuchueva, N.; Atzori, L.; Fanos, V.; Puxeddu, R. Metabolomic analysis of urine with Nuclear Magnetic Resonance spectroscopy in patients with idiopathic sudden sensorineural hearing loss: A preliminary study. Auris Nasus Larynx 2017, 44, 381–389. [Google Scholar] [CrossRef]
- Di Berardino, F.; Zanetti, D.; Ciusani, E.; Caccia, C.; Leoni, V.; De Grazia, U.; Filipponi, E.; Elli, L. Intestinal permeability and Ménière’s disease. Am. J. Otolaryngol. 2018, 39, 153–156. [Google Scholar] [CrossRef]
- Casani, A.P.; Navari, E.; Albera, R.; Agus, G.; Asprella Libonati, G.; Chiarella, G.; Lombardo, N.; Marcelli, V.; Ralli, G.; Scotto di Santillo, L.; et al. Approach to residual dizziness after successfully treated benign paroxysmal positional vertigo: Effect of a polyphenol compound supplementation. Clin. Pharmacol. 2019, 11, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Maihoub, S.; Mavrogeni, P.; Tamás, L.; Szirmai, Á. Depression scores and quality of life of vertiginous patients, suffering from different vestibular disorders. Eur. Arch. Otorhinolaryngol. 2022, 279, 5173–5179. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ma, X.; Yang, Y.; He, K.; Wang, W.; Shen, J.; Wang, L.; Chen, X.; Jin, Y.; Yang, J.; et al. Associations between cognition, anxiety, depression, and residual dizziness in elderly people with BPPV. Front. Aging Neurosci. 2023, 15, 1208661. [Google Scholar] [CrossRef] [PubMed]
- Secades, J.J.; Gareri, P. Citicoline: Pharmacological and clinical review, update. Rev. Neurol. 2022, 75, S1–S73. [Google Scholar] [PubMed]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Souto, E.B.; Durazzo, A.; Sharifi-Rad, J.; Lucarini, M.; Souto, S.B.; Salehi, B.; Zam, W.; Montanaro, V.; Lucariello, G.; et al. Ginger (Zingiber Officinale Roscoe) as a Nutraceutical: Focus on the Metabolic, Analgesic, and Antiinflammatory Effects. Phytother. Res. 2020, 35, 2403–2417. [Google Scholar] [CrossRef]
- Prakas, D.; Sharma, G. Phytochemicals of Nutraceutical Importance. CABI 2014, 1, 1–19. [Google Scholar]
- Deley, G.; Guillemet, D.; Allaert, F.A.; Babault, N. An Acute Dose of Specific Grape and Apple Polyphenols Improves Endurance Performance: A Randomized, Crossover, Double-Blind versus Placebo Controlled Study. Nutrients 2017, 9, 917. [Google Scholar] [CrossRef]
- Von Brevern, M.; Bertholon, P.; Brandt, T.; Fife, T.; Imai, T.; Nuti, D.; Newman-Toker, D. Benign paroxysmal positional vertigo: Diagnostic criteria. J. Vestib. Res. 2015, 25, 105–117. [Google Scholar] [CrossRef]
- Toupet, M.; Ferrary, E.; Grayeli, A.B. Visual analog scale to assess vertigo and dizziness after repositioning maneuvers for benign paroxysmal positional vertigo. J. Vest. Res. 2011, 2, 235–241. [Google Scholar] [CrossRef]
- Colnaghi, S.; Rezzani, C.; Gnesi, M.; Manfrin, M.; Quaglieri, S.; Nuti, D.; Mandalà, M.; Monti, M.C.; Versino, M. Validation of the Italian Version of the Dizziness Handicap Inventory, the Situational Vertigo Questionnaire, and the Activity-Specific Balance Confidence Scale for Peripheral and Central Vestibular Symptoms. Front. Neurol. 2017, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.P.; Newman, C.W. The development of the Dizziness Handicap Inventory. Arch. Otolaryngol. 1990, 116, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.L.; Marchetti, G.F.; Morris, O. Usefulness of the dizziness handicap inventory in the screening for benign paroxysmal positional vertigo. Otol. Nerotol. 2005, 26, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, B.; Serbetcioglu, B. Discussion of the dizziness handicap inventory. J. Vestib. Res. 2013, 23, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Mercieca-Bebber, R.; King, M.T.; Calvert, M.J.; Stockler, M.R.; Friedlander, M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 2018, 9, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Stanulović, V.; Hodolic, M.; Mitsikostas, D.D.; Papadopoulos, D. Drug tolerability: How much ambiguity can be tolerated? A systematic review of the assessment of tolerability in clinical studies. Br. J. Clin. Pharmacol. 2021, 88, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L. Sample normalization methods in quantitative metabolomics. J. Chromatogr. A 2016, 1430, 80–95. [Google Scholar] [CrossRef]
- Van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; Van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC. Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Piras, C.; Pintus, R.; Pruna, D.; Dessì, A.; Atzori, L.; Fanos, V. Pediatric acute-onset neuropsychiatric syndrome and mycoplasma pneumoniae infection: A case report analysis with a metabolomics approach. Curr. Pediatr. Rev. 2020, 16, 183–193. [Google Scholar]
- Vaduva, C.; Estéban-Sánchez, J.; Sanz-Fernández, R.; Martín-Sanz, E. Prevalence and management of post-BPPV residual symptoms. Eur. Arch. Otorhinolaryngol. 2018, 275, 1429–1437. [Google Scholar] [CrossRef]
- Seok, J.I.; Lee, H.M.; Yoo, J.H.; Lee, D.K. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J. Clin. Neurol. 2008, 4, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Lapenna, R.; Giommetti, G.; Pellegrino, C.; Ricci, G. Residual dizziness after the first BPPV episode: Role of otolithic function and of a delayed diagnosis. Eur. Arch. Otorhinolaryngol. 2016, 273, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Pagliuca, G.; De Vincentiis, M.; Greco, A.; De Virgilio, A.; Nobili Benedetti, F.M.; Gallipoli, C.; Rosato, C.; Clemenzi, V.; Gallo, A. Features of Residual Dizziness after Canalith Repositioning Procedures for Benign Paroxysmal Positional Vertigo. Otolaryngol. Head Neck Surg. 2016, 154, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Casani, A.P.; Gufoni, M.; Capobianco, S. Current insights into treating vertigo in older adults. Drugs Aging 2021, 38, 655–670. [Google Scholar] [CrossRef]
- Wu, P.; Yang, J.; Huang, X.; Ma, Z.; Zhang, T.; Li, H. Predictors of residual dizziness in patients with benign paroxysmal positional vertigo after successful repositioning: A multi-center prospective cohort study. J. Vestib. Res. 2021, 31, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Acar, B.; Karasen, R.M.; Buran, Y. Efficacy of medical therapy in the prevention of residual dizziness after successful repositioning maneuvers for benign paroxysmal positional vertigo (BPPV). B-ENT 2015, 11, 117–121. [Google Scholar] [PubMed]
- Kim, M.B.; Lee, H.S.; Ban, J.H. Vestibular suppressants after canalith repositioning in benign paroxysmal positional vertigo. Laryngos 2014, 124, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Koo, J.W.; Kim, C.S.; Kim, J.S.; Song, J.J. Anxiolytics reduce residual dizziness after successful canalith repositioning maneuvers in benign paroxysmal positional vertigo. Acta Otolaryngol. 2012, 132, 277–284. [Google Scholar] [CrossRef]
- Guneri, E.A.; Kustutan, O. The effects of betahistine in addition to Epley maneuver in posterior canal benign paroxysmal positional vertigo. Otolaryngol. Head Neck Surg. 2012, 146, 104–108. [Google Scholar] [CrossRef]
- Jalali, M.M.; Gerami, H.; Saberi, A.; Razaghi, S. The impact of Betahistine versus dimenhydrinate in the resolution of residual dizziness in patients with benign paroxysmal positional vertigo: A randomized clinical trial. Ann. Otol. Rhinol. Laryngol. 2020, 129, 434–440. [Google Scholar] [CrossRef]
- Piras, C.; Mussap, M.; Noto, A.; De Giacomo, A.; Cristofori, F.; Spada, M.; Fanos, V.; Atzori, L.; Francavilla, R. Alterations of the Intestinal Permeability are Reflected by Changes in the Urine Metabolome of Young Autistic Children: Preliminary Results. Metabolites. 2022, 12, 104. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, A.V.; Bamji, M.S. Nutritional Assessment: Biochemical Tests for Vitamins and Minerals. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press-Elsevier: Amsterdam, The Netherlands, 2003; pp. 4184–4190. [Google Scholar]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Ann. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef]
- Szabadfi, K.; Gabriel, R. Neuropeptides, trophic factors, and other substances providing morphofunctional and metabolic protection in experimental models of diabetic retinopathy. Int. Rev. Cell. Mol. Biol. 2014, 311, 1–121. [Google Scholar]
- Lingchao, J.; Lee, H.J.; Wan, G.; Zhang, L.; Sajjakulnukit, P.; Schacht, J.; Lyssiotis, C.A.; Corfas, G. Auditory metabolomics, an approach to identify acute molecular effects of noise trauma. Sci. Rep. 2019, 9, 9273. [Google Scholar]
- Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; Menni, C. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 20, 13670. [Google Scholar]
- Adibhatla, R.M.; Dempsey, R.J.; Hatcher, J.F. Citicoline: Neuroprotective mechanisms in cerebral ischemia. J. Neurochem. 2002, 80, 12–23. [Google Scholar] [CrossRef]
- Krupinski, J.; Abudawood, M.; Matou-Nasri, S.; Al-Baradie, R.; Petcu, E.; Justicia, C.; Planas, A.; Liu, D.; Rovira, N.; Grau-Slevin, M.; et al. Citicoline induces angiogenesis improving survival of vascular/human brain microvessel endothelial cells through pathways involving ERK1/2 and insulin receptor substrate-1. Vasc. Cell. 2012, 4, 20. [Google Scholar] [CrossRef]
- Petrova, D.; Maslarov, D.; Angelov, I.; Zekin, D. Analysis of Therapeutic Efficacy of Citicoline in Patients with Vertigo of Central Origin and Vascular Aetiology. Am. J. Neuroprot. Neuroregen. 2012, 4, 78–85. [Google Scholar] [CrossRef]
- Chiarella, G.; Marcianò, G.; Viola, P.; Palleria, C.; Pisani, D.; Rania, V.; Casarella, A.; Astorina, A.; Scarpa, A.; Esposito, M.; et al. Nutraceuticals for Peripheral Vestibular Pathology: Properties, Usefulness, Future Perspectives and Medico-Legal Aspects. Nutrients 2021, 13, 3646. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, L.; Maccarrone, M.; Giannini, N.; Ferrari, E.; Caselli, M.C.; Montano, V.; Chico, L.; Casani, A.P.; Navari, E.; Cerchiai, N.; et al. Oxidative Stress in Cerebral Small Vessel Disease Dizziness Patients, Basally and After Polyphenol Compound Supplementation. Curr. Mol. Med. 2018, 18, 160–165. [Google Scholar] [CrossRef] [PubMed]


| VAS Dizziness | |||
|---|---|---|---|
| T0 Mean ± SD | T30 Mean ± SD (Δ%) | T60 Mean ± SD (Δ%) | |
| Mean ± SD (30 subjects) | 45.3 ± 4.2 | 23.2 ± 2.9 (−48.8%) | 8.0 ± 1.8 (−82.5%) |
| Student’s t-test | - | p < 0.0001 *** | p < 0.0001 *** |
| T0 | T30 | T60 | T0 vs. T30 | T0 vs. T60 | T30 vs. T60 | |
|---|---|---|---|---|---|---|
| Metabolites (mM) a | Median (Interquartile Range) | p-Values * | ||||
| 1-Methylnicotinamide | 0.05 (0.03–0.06) | 0.08 (0.04–0.17) | 0.07 (0.05–0.11) | 0.01 | 0.02 | ns |
| Anserine | 1.24 (0.78–2.42) | 0.96 (0.53–1.24) | 1.00 (0.60–1.31) | 0.01 | 0.03 | ns |
| Hippurate | 5.65 (2.35–10.7) | 3.97 (2.05–5.50) | 3.85 (1.96–5.61) | 0.02 | 0.03 | ns |
| Lysine | 1.02 (0.59–1.72) | 0.83 (0.43–1.09) | 0.76 (0.41–1.13) | 0.02 | 0.02 | ns |
| Methyl succinate | 0.34 (0.22–0.549 | 0.24 (0.18–0.35) | 0.23 (0.18–0.30) | 0.02 | 0.01 | ns |
| Urea | 89.1 (83.5–94.0) | 93.1 (89.8–95.1) | 93.6 (90.6–95.5) | 0.04 | 0.02 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casani, A.P.; Albera, R.; Piras, C.; Albera, A.; Noto, A.; Ducci, N.; Atzori, L.; Lucisano, S.; Mussap, M.; Fanos, V. Clinical Efficacy and Metabolomics Modifications Induced by Polyphenol Compound Supplementation in the Treatment of Residual Dizziness following Semont Maneuver in Benign Paroxysmal Positional Vertigo (BPPV) of the Posterior Semicircular Canal (PSC): Preliminary Results. Metabolites 2024, 14, 86. https://doi.org/10.3390/metabo14020086
Casani AP, Albera R, Piras C, Albera A, Noto A, Ducci N, Atzori L, Lucisano S, Mussap M, Fanos V. Clinical Efficacy and Metabolomics Modifications Induced by Polyphenol Compound Supplementation in the Treatment of Residual Dizziness following Semont Maneuver in Benign Paroxysmal Positional Vertigo (BPPV) of the Posterior Semicircular Canal (PSC): Preliminary Results. Metabolites. 2024; 14(2):86. https://doi.org/10.3390/metabo14020086
Chicago/Turabian StyleCasani, Augusto Pietro, Roberto Albera, Cristina Piras, Andrea Albera, Antonio Noto, Nicola Ducci, Luigi Atzori, Sergio Lucisano, Michele Mussap, and Vassilios Fanos. 2024. "Clinical Efficacy and Metabolomics Modifications Induced by Polyphenol Compound Supplementation in the Treatment of Residual Dizziness following Semont Maneuver in Benign Paroxysmal Positional Vertigo (BPPV) of the Posterior Semicircular Canal (PSC): Preliminary Results" Metabolites 14, no. 2: 86. https://doi.org/10.3390/metabo14020086
APA StyleCasani, A. P., Albera, R., Piras, C., Albera, A., Noto, A., Ducci, N., Atzori, L., Lucisano, S., Mussap, M., & Fanos, V. (2024). Clinical Efficacy and Metabolomics Modifications Induced by Polyphenol Compound Supplementation in the Treatment of Residual Dizziness following Semont Maneuver in Benign Paroxysmal Positional Vertigo (BPPV) of the Posterior Semicircular Canal (PSC): Preliminary Results. Metabolites, 14(2), 86. https://doi.org/10.3390/metabo14020086











