Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Ambient Temperature–Humidity Index and Periods of the Experiment
2.3. Body Surface and Rectal Temperatures
2.4. Blood Sampling and Metabolic Parameters Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cincović, M.R.; Majkić, M.; Belić, B.; Plavša, N.; Lakić, I.; Radinović, M. Thermal comfort of cows and temperature humidity index in period of 2005–2016 in Vojvodina region (Serbia). Acta Agric. Serbica 2017, 22, 133–145. [Google Scholar] [CrossRef]
- Pecelj, M.M.; Lukić, M.Z.; Filipović, D.J.; Protić, B.M.; Bogdanović, U.M. Analysis of the Universal Thermal Climate Index during heat waves in Serbia. Nat. Hazards Earth Syst. Sci. 2020, 20, 2021–2036. [Google Scholar] [CrossRef]
- Vranić, P.; Milutinović, S. From local sustainable development towards climate change adaptation: A case study of Serbia. Int. J. Sustain. Dev. World Ecol. 2016, 23, 71–82. [Google Scholar] [CrossRef]
- Collier, R.J.; Gebremedhin, K.G. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Chen, X.; Lu, Y.; Wang, D. Effects of heat stress on body temperature, milk production, and reproduction in dairy cows: A novel idea for monitoring and evaluation of heat stress—A review. Asian-Australas. J. Anim. Sci. 2019, 32, 1332. [Google Scholar] [CrossRef]
- Cincovic, M.R.; Belic, B.; Toholj, B.; Potkonjak, A.; Stevancevic, M.; Lako, B.; Radovic, I. Metabolic acclimation to heat stress in farm housed Holstein cows with different body condition scores. Afr. J. Biotechnol. 2011, 10, 10293–10303. [Google Scholar]
- Yan, G.; Liu, K.; Hao, Z.; Shi, Z.; Li, H. The effects of cow-related factors on rectal temperature, respiration rate, and temperature-humidity index thresholds for lactating cows exposed to heat stress. J. Therm. Biol. 2021, 100, 103041. [Google Scholar] [CrossRef] [PubMed]
- Toledo, I.M.; Fabris, T.F.; Tao, S.; Dahl, G.E. When do dry cows get heat stressed? Correlations of rectal temperature, respiration rate, and performance. JDS Commun. 2020, 1, 21–24. [Google Scholar] [CrossRef]
- Wijffels, G.; Sullivan, M.; Gaughan, J. Methods to quantify heat stress in ruminants: Current status and future prospects. Methods 2021, 186, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Pereira, A.M.F.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Avalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Zhou, M.; Koerkamp, P.G.; Huynh, T.T.T.; Aarnink, A.J.A. Evaporative water loss from dairy cows in climate-controlled respiration chambers. J. Dairy Sci. 2023, 106, 2035–2043. [Google Scholar] [CrossRef]
- Hoffmann, G.; Herbut, P.; Pinto, S.; Heinicke, J.; Kuhla, B.; Amon, T. Animal-related, non-invasive indicators for determining heat stress in dairy cows. Biosyst. Eng. 2020, 199, 83–96. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Samad, H.A.; Shehzad, F.; Qayyum, A. Physiological responses of cattle to heat stress. World Appl. Sci. J. 2010, 8, 38–43. [Google Scholar]
- Majkić, M.; Cincović, M.R.; Belić, B.; Plavša, N.; Lakić, I.; Radinović, M. Relationship between milk production and metabolic adaptation in dairy cows during heat stress. Acta Agric. Serbica 2017, 22, 123–131. [Google Scholar] [CrossRef]
- Tejaswi, V.; Balamurugan, B.; Samad, H.A.; Sarkar, M.; Maurya, V.P.; Singh, G. Differential endocrine and antioxidant responses to heat stress among native and crossbred cattle. J. Vet. Behav. 2020, 39, 1–5. [Google Scholar] [CrossRef]
- Kim, W.S.; Ghassemi Nejad, J.; Roh, S.G.; Lee, H.G. Heat-shock proteins gene expression in peripheral blood mononuclear cells as an indicator of heat stress in beef calves. Animals 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Heck, T.G.; Bittencourt, A.; Scomazzon, S.P.; Newsholme, P.; Curi, R.; Homem de Bittencourt, P.I. The chaperone balance hypothesis: The importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat. Inflamm. 2015, 2015, 249205. [Google Scholar] [CrossRef]
- Ruiz-González, A.; Rico, D.E.; Rico, J.E. Modulation of fecal metabolites by heat stress and diet, and their association with inflammation and leaky gut markers in dairy cows. Metabolites 2022, 12, 142. [Google Scholar] [CrossRef]
- Mezzetti, M.; Cattaneo, L.; Passamonti, M.M.; Lopreiato, V.; Minuti, A.; Trevisi, E. The transition period updated: A review of the new insights into the adaptation of dairy cows to the new lactation. Dairy 2021, 2, 617–636. [Google Scholar] [CrossRef]
- Kendall, P.E.; Webster, J.R. Season and physiological status affects the circadian body temperature rhythm of dairy cows. Livest. Sci. 2009, 125, 155–160. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Godyń, D.; Herbut, P.; Angrecka, S. Measurements of peripheral and deep body temperature in cattle—A review. J. Therm. Biol. 2019, 79, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, K.; Colazo, M.G.; Cook, N.J. Evaluation of infrared thermography compared to rectal temperature to identify illness in early postpartum dairy cows. Res. Vet. Sci. 2019, 125, 315–322. [Google Scholar] [CrossRef]
- Čukić, A.; Rakonjac, S.; Djoković, R.; Cincović, M.; Bogosavljević-Bošković, S.; Petrović, M.; Savić, Ž.; Andjušić, L.; Andjelić, B. Influence of Heat Stress on Body Temperatures Measured by Infrared Thermography, Blood Metabolic Parameters and Its Correlation in Sheep. Metabolites 2023, 13, 957. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle: 2001; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- National Research Council. A Guide to Environmental Research on Animals; National Research Council, National Academic Science: Washington, DC, USA, 1971. [Google Scholar]
- Cincović, M.R.; Belić, B.; Djoković, R.; Ježek, J.; Petrović, M.D.; Božić, A.; Anderson, R.C.; Starič, J. Revised quantitative insulin sensitivity check index: Associations with the metabolic status of cows during early lactation. Vet. Arh. 2019, 89, 613–625. [Google Scholar] [CrossRef]
- Armstrong, D.V. Heat stress interaction with shade and cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I.; Hoogenboom, G. Genetic component of heat stress in dairy cattle, development of heat index function. J. Dairy Sci. 2000, 83, 2120–2125. [Google Scholar] [CrossRef]
- Aguilar, I.; Misztal, I.; Tsuruta, S. Short communication: Genetic trends of milk yield under heat stress for US holsteins. J. Dairy Sci. 2010, 93, 1754–1758. [Google Scholar] [CrossRef]
- Bohmanova, J.; Misztal, I.; Cole, J.B. Temperature-humidity indices as indicators of milk production losses due to heat stress. J. Dairy Sci. 2007, 90, 1947–1956. [Google Scholar] [CrossRef]
- Mbuthia, J.M.; Eggert, A.; Reinsch, N. Comparison of high resolution observational and grid-interpolated weather data and application to thermal stress on herd average milk production traits in a temperate environment. Agric. For. Meteorol. 2022, 322, 108923. [Google Scholar] [CrossRef]
- Mbuthia, J.M.; Mayer, M.; Reinsch, N. Modeling heat stress effects on dairy cattle milk production in a tropical environment using test-day records and random regression models. Animal 2021, 15, 100222. [Google Scholar] [CrossRef]
- Spasojević, J.; Majkić, M.; Cincović, M.; Stanojević, J.; Blond, B.; Radinović, M. Correlation of body surface temperature measured by infrared thermography and air temperature and humidity index (THI) in the assessment of heat stress in cows. Ann. Agron.-Letop. Naučnih Rad. 2023; in press. [Google Scholar]
- Yan, G.; Shi, Z.; Li, H. Critical temperature-humidity index thresholds based on surface temperature for lactating dairy cows in a temperate climate. Agriculture 2021, 11, 970. [Google Scholar] [CrossRef]
- Unruh, E.M.; Theurer, M.E.; White, B.J.; Larson, R.L.; Drouillard, J.S.; Schrag, N. Evaluation of infrared thermography as a diagnostic tool to predict heat stress events in feedlot cattle. Am. J. Vet. Res. 2017, 78, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Shashank, C.G.; Silpa, M.V.; Madhusoodan, A.P.; Devaraj, C.; Koenig, S. Non-Invasive Methods of Quantifying Heat Stress Response in Farm Animals with Special Reference to Dairy Cattle. Atmosphere 2022, 13, 1642. [Google Scholar] [CrossRef]
- Salles, M.S.V.; da Silva, S.C.; Salles, F.A.; Roma, L.C., Jr.; El Faro, L.; Mac Lean, P.A.B.; de Oliveira, C.E.L.; Martello, L.S. Mapping the body surface temperature of cattle by infrared thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef]
- Martello, L.S.; Da Luz, S.S.; Gomes, R.C.; Corte, R.S.R.P.; Leme, P.R. Infrared thermography as a tool to evaluate body surface temperature and its relationship with feed efficiency in Bos indicus cattle in tropical conditions. Int. J. Biometeorol. 2016, 60, 173–181. [Google Scholar] [CrossRef]
- Reece, W.O.; Erickson, H.H.; Goff, J.P.; Uemura, E.E. (Eds.) Dukes’ Physiology of Domestic Animals; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Okada, K.; Takemura, K.; Sato, S. Investigation of various essential factors for optimum infrared thermography. J. Vet. Med. Sci. 2013, 75, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Montanholi, Y.R.; Nicholas, E.O.; Kendall, C.S.; Schenkel, F.S.; Mcbride, B.W.; Miller, S.P. Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- Berry, R.J.; Kennedy, A.D.; Scott, S.L.; Kyle, B.L.; Shaefer, A.L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can. J. Anim. Sci. 2003, 83, 687–693. [Google Scholar] [CrossRef]
- Osei-Amponsah, R.; Dunshea, F.R.; Leury, B.J.; Cheng, L.; Cullen, B.; Joy, A.; Abhijith, A.; Zhang, M.H.; Chauhan, S.S. Heat stress impacts on lactating cows grazing Australian summer pastures on an automatic Robotic Dairy. Animals 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific findings related to changes in vascular microcirculation using infrared thermography in the river buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Peng, D.; Li, G.; Chen, J.; Gu, X. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci. Rep. 2018, 8, 14606. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Johnson, H. Environmental Heat Exposure on Cattle Plasma Catecholamine and Glucocorticoids. J. Dairy Sci. 1973, 56, 189–194. [Google Scholar] [CrossRef]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- de Lima Guimarães Yamada, K.; Dos Santos, G.T.; Damasceno, J.C.; de Almeida, K.V.; Osorio, J.A.; Lourenço, J.C.; Gurgel, A.L.; Dias-Silva, T.P.; de Araújo, M.J.; Ítavo, L.C.; et al. Effects of heat-stress-reducing systems on blood constituents, milk production and milk quality of Holstein and Jersey cows and heifers on pasture. Trop. Anim. Health Prod. 2023, 55, 386. [Google Scholar] [CrossRef]
- Anjali; Gururaj, V.K.; Sarma, L.; Tripathi, M.; Verma, M.R.; Verma, V.; Pathak, M.C.; Samad, H.A.; Maurya, V.P.; Chouhan, V.S.; et al. Thyroid hormone dynamics of Tharparkar and Sahiwal cattle during induced heat stress. Trop. Anim. Health Prod. 2023, 55, 57. [Google Scholar] [CrossRef]
- Hunninck, L.; Jackson, C.R.; May, R.; Røskaft, E.; Palme, R.; Sheriff, M.J. Triiodothyronine (T3) levels fluctuate in response to ambient temperature rather than nutritional status in a wild tropical ungulate. Conserv. Physiol. 2020, 8, coaa105. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Wheelock, J.B.; Sanders, S.R.; Moore, C.E.; Green, H.B.; Waldron, M.R.; Rhoads, R.P. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows. J. Dairy Sci. 2011, 94, 5620–5633. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Majkić, M.; Cincović, M.; Spasojević, J.; Jožef, I.; Blond, B.; Kovačević, D. Resilience curve and cumulative response of cows to heat stress. Ann. Agron. Letop. Naučnih Rad. 2023; in press. [Google Scholar]
- Berian, S.; Gupta, S.K.; Sharma, S.; Ganai, I.; Dua, S.; Sharma, N. Effect of heat stress on physiological and hemato-biochemical profile of cross bred dairy cattle. J. Anim. Res. 2019, 9, 95–101. [Google Scholar] [CrossRef]
- Gao, S.T.; Guo, J.; Quan, S.Y.; Nan, X.M.; Fernandez, M.S.; Baumgard, L.H.; Bu, D.P. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017, 100, 5040–5049. [Google Scholar] [CrossRef]
- Rhoads, R.P.; La Noce, A.J.; Wheelock, J.B.; Baumgard, L.H. Short communication: Alterations in expression of gluconeogenic genes during heat stress and exogenous bovine somatotropin administration. J. Dairy Sci. 2011, 94, 1917–1921. [Google Scholar] [CrossRef]
- Abbas, Z.; Sammad, A.; Hu, L.; Fang, H.; Xu, Q.; Wang, Y. Glucose Metabolism and Dynamics of Facilitative Glucose Transporters (GLUTs) under the Influence of Heat Stress in Dairy Cattle. Metabolites 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, M.; Bionaz, M.; Trevisi, E. Interaction between inflammation and metabolism in periparturient dairy cows. J. Anim. Sci. 2020, 98, S155–S174. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.S.; Lee, S.J.; Park, D.S.; Kim, D.H.; Gu, B.-H.; Park, Y.J.; Rim, C.Y.; Kim, M.; Kim, E.T. Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress. Animals 2021, 11, 974. [Google Scholar] [CrossRef]
- Cincović, M.R.; Djoković, R.; Belić, B.; Potkonjak, A.; Toholj, B.; Stojanac, N.; Stevančević, O.; Starič, J. Inorganic phosphorus decrease after intravenous glucose tolerance test is associated with insulin resistance in dairy cows. Vet. Arh. 2017, 87, 409–418. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Leroy, J.L.M.R.; Opsomer, G. Metabolic Stress in the Transition Period of Dairy Cows: Focusing on the Prepartum Period. Animals 2020, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Krnjaić, S.; Cincović, M.; Djoković, R.; Belić, B.; Ježek, J.; Starič, J. The Influence of Energy Balance, Lipolysis and Ketogenesis on Metabolic Adaptation in Cows Milked Twice and Three Times Daily. Metabolites 2022, 12, 1090. [Google Scholar] [CrossRef]
- Petrović, M.Ž.; Cincović, M.; Starič, J.; Djoković, R.; Belić, B.; Radinović, M.; Majkić, M.; Ilić, Z.Ž. The Correlation between Extracellular Heat Shock Protein 70 and Lipid Metabolism in a Ruminant Model. Metabolites 2022, 12, 19. [Google Scholar] [CrossRef]
- Turk, R.; Rošić, N.; Vince, S.; Perkov, S.; Samardžija, M.; Beer-Ljubić, B.; Belić, M.; Robić, M. The influence of heat stress on energy metabolism in Simmental dairy cows during the periparturient period. Vet. Arh. 2020, 90, 1–10. [Google Scholar] [CrossRef]
- Cincović, M.R.; Belić, B.M.; Toholj, B.D.; Radović, I.V.; Vidović, B.R. The influence of THI values at different periods of lactation on milk quality and characteristics of lactation curve. J. Agric. Sci. 2010, 55, 235–241. [Google Scholar] [CrossRef]
- Sivanandam, S.; Anburajan, M.; Venkatraman, B.; Menaka, M.; Sharath, D. Medical thermography: A diagnostic approach for type 2 diabetes based on non-contact infrared thermal imaging. Endocrine 2012, 42, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, H.; Tarr, G.; Loudon, K.; Lomax, S.; White, P.; McGreevy, P.; Polkinghorne, R.; González, L.A. Using infrared thermography on farm of origin to predict meat quality and physiological response in cattle (Bos taurus) exposed to transport and marketing. Meat Sci. 2020, 169, 108173. [Google Scholar] [CrossRef]
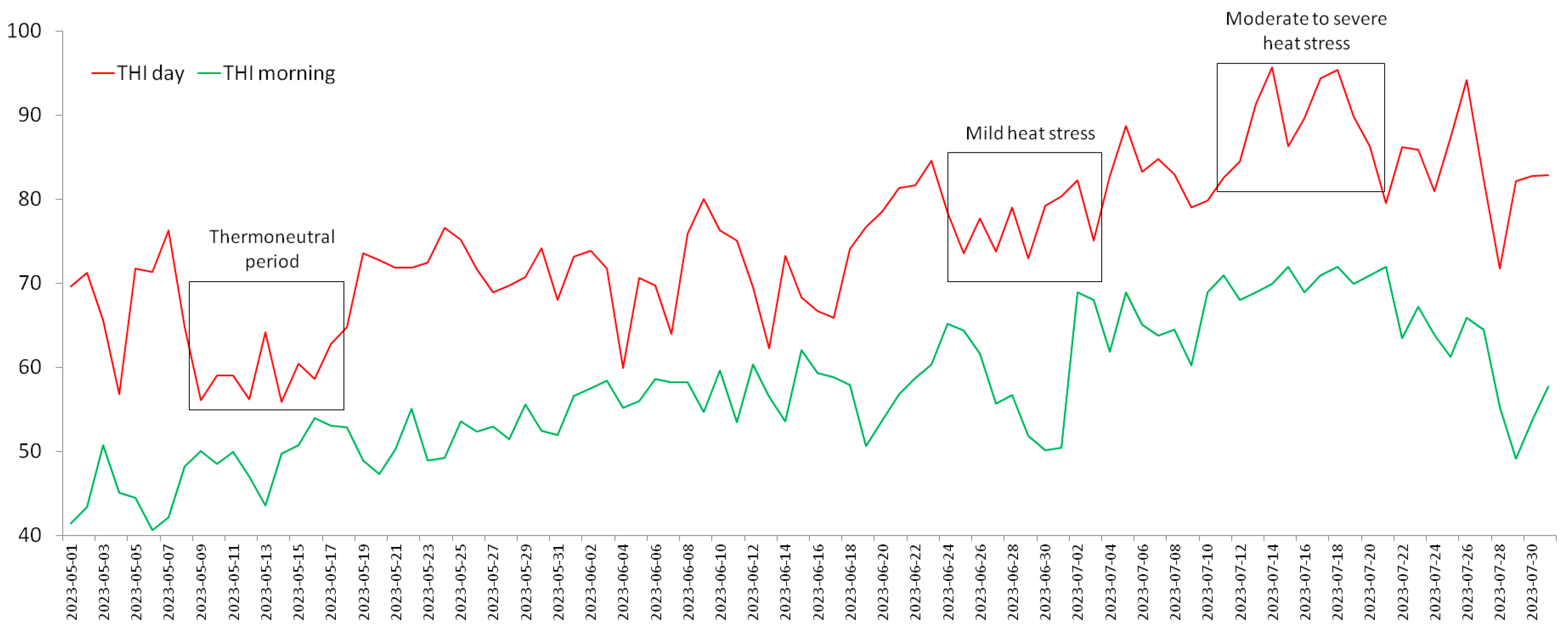
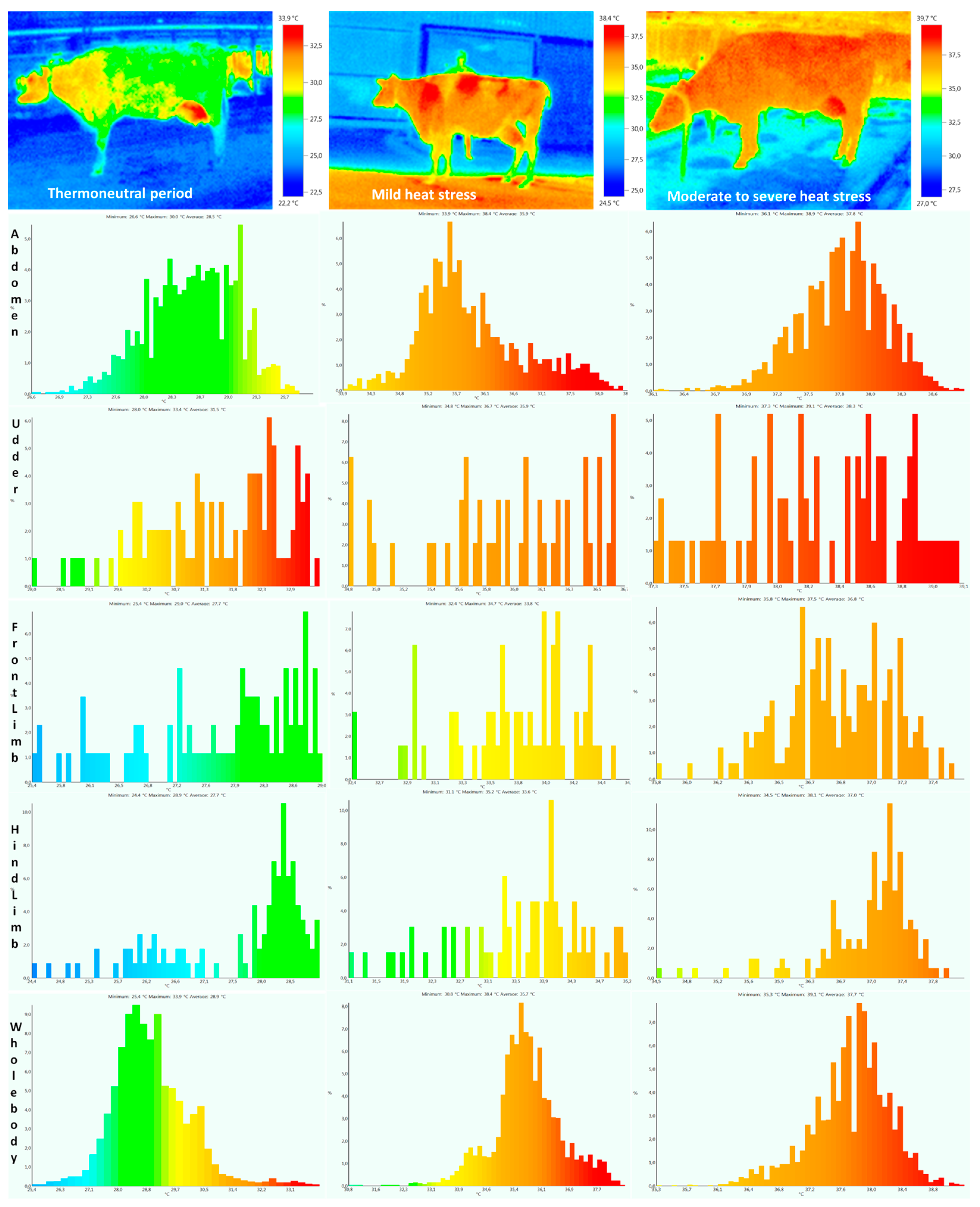
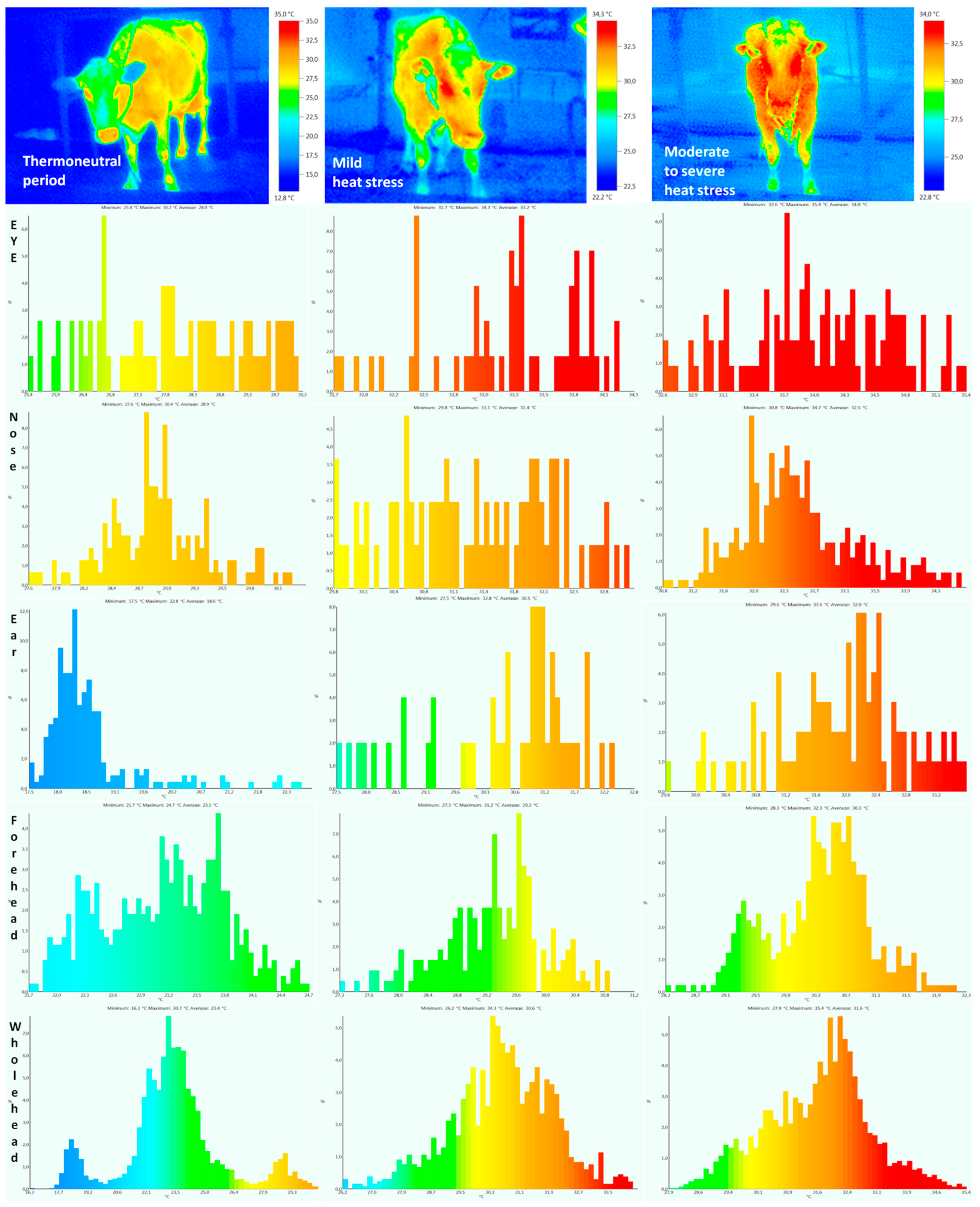
| Body Temperature (°C) | Thermoneutral Period | Mild Heat Stress | Moderate to Severe Heat Stress | Heat Stress Period | Lactation Period | Heat Stress × Lactation Period | |||
|---|---|---|---|---|---|---|---|---|---|
| Early Lactation | Mid-Lactation | Early Lactation | Mid-Lactation | Early Lactation | Mid-Lactation | ||||
| Rectal | 38.5 ± 0.38 a | 38.4 ± 0.42 a | 38.9 ± 0.51 b | 38.9 ± 0.56 b | 39.3 ± 0.48 c | 39.4 ± 0.49 c | 0.018 | 0.59 | 0.48 |
| Eye | 27.8 ± 0.42 a | 28.1 ± 0.41 a | 33.0 ± 0.36 b | 33.2 ± 0.38 b | 33.9 ± 0.39 c | 34.1 ± 0.40 c | 0.0013 | 0.49 | 0.36 |
| Ear | 18.6 ± 0.65 a | 18.9 ± 0.63 a | 30.4 ± 0.69 b | 30.6 ± 0.56 b | 32.0 ± 0.34 c | 32.2 ± 0.35 c | 0.0001 | 0.23 | 0.26 |
| Nose | 28.9 ± 0.32 a | 28.7 ± 0.31 a | 31.2 ± 0.35 b | 31.4 ± 0.37 b | 32.3 ± 0.30 c | 32.4 ± 0.34 c | 0.0015 | 0.19 | 0.58 |
| Forehead | 23.1 ± 0.38 a | 23.4 ± 0.41 a | 29.1 ± 0.59 b | 29.4 ± 0.57 b | 30.2 ± 0.50 c | 30.3 ± 0.57 c | 0.0021 | 0.31 | 0.32 |
| Whole head | 23.2 ± 0.95 a | 23.5 ± 0.99 a | 30.6 ± 0.78 b | 30.9 ± 0.81 b | 31.4 ± 0.86 c | 31.6 ± 0.79 c | 0.0002 | 0.52 | 0.37 |
| Abdomen | 28.5 ± 0.61 a | 28.6 ± 0.60 a | 35.9 ± 0.70 b | 36.1 ± 0.68 b | 37.8 ± 0.32 c | 37.9 ± 0.32 c | 0.0001 | 0.56 | 0.42 |
| Udder | 31.5 ± 0.44 a | 31.3 ± 0.42 a | 36.1 ± 0.51 b | 35.8 ± 0.49 b | 38.5 ± 0.27 c | 38.4 ± 0.25 c | 0.0003 | 0.19 | 0.13 |
| Front limb | 27.9 ± 0.42 a | 27.9 ± 0.31 a | 33.8 ± 0.63 b | 33.9 ± 0.59 b | 36.8 ± 0.42 c | 36.9 ± 0.41 c | 0.0002 | 0.28 | 0.15 |
| Hind limb | 27.7 ± 0.43 a | 27.5 ± 0.50 a | 33.5 ± 0.59 b | 33.7 ± 0.48 b | 37.2 ± 0.60 c | 37.4 ± 0.58 c | 0.0001 | 0.31 | 0.19 |
| Whole body | 28.8 ± 1.21 a | 28.9 ± 1.11 a | 35.7 ± 1.13 b | 35.9 ± 1.17 b | 37.7 ± 0.56 c | 37.6 ± 0.62 c | 0.0001 | 0.62 | 0.28 |
| Blood Parameters | Thermoneutral Period | Mild Heat Stress | Moderate to Severe Heat Stress | Heat Stress Period | Lactation Period | Heat Stress × Lactation Period | |||
|---|---|---|---|---|---|---|---|---|---|
| Early Lactation | Mid-Lactation | Early Lactation | Mid-Lactation | Early Lactation | Mid-Lactation | ||||
| eHsp70(ng/mL) * | 3.99 ± 0.65 a | 3.14 ± 0.78 b | 7.69 ± 1.15 c | 5.11 ± 0.61 d | 10.13 ± 1.03 e | 6.32 ± 0.95 c | 0.001 | 0.005 | 0.005 |
| TNFα(ng/mL) | 7.55 ± 2.1 a | 5.02 ± 1.4 b | 10.14 ± 2.1 c | 4.03 ± 1.62 d | 12.5 ± 1.98 e | 8.07 ± 1.2 f | 0.001 | 0.005 | 0.005 |
| CORT (nmol/L) | 19.9 ± 5.1 a | 18.1 ± 4.2 a | 26.5 ± 3.9 b | 22.3 ± 4.2 b | 25.6 ± 3.5 b | 23.1 ± 3.4 b | 0.001 | 0.751 | 0.624 |
| T3 (nmol/L) | 1.02 ± 0.15 a | 1.13 ± 0.16 a | 0.84 ± 0.11 b | 0.93 ± 0.12 b | 0.67 ± 0.15 c | 0.70 ± 0.14 c | 0.001 | 0.382 | 0.412 |
| T4 (nmol/L) | 36.3 ± 5.2 a | 41.5 ± 6.9 a | 32.2 ± 4.2 b | 36.7 ± 4.8 b | 25.5 ± 3.9 c | 26.9 ± 4.07 c | 0.001 | 0.561 | 0.513 |
| INS (mU/L) | 5.55 ± 1.1 a | 7.03 ± 0.98 b | 7.21 ± 0.98 b | 8.14 ± 1.25 c | 6.11 ± 1.33 b | 9.12 ± 1.41 c | 0.009 | 0.048 | 0.046 |
| NEFA(mmol/L) | 0.75 ± 0.08 a | 0.55 ± 0.7 b | 0.49 ± 0.07 b | 0.46 ± 0.08 b | 0.62 ± 0.05 c | 0.38 ± 0.07 d | 0.001 | 0.005 | 0.005 |
| GLU (mmol/L) | 3.14 ± 0.21 a | 3.51 ± 0.28 b | 2.24 ± 0.21 c | 2.59 ± 0.23 d | 2.01 ± 0.27 e | 2.31 ± 0.20 c | 0.001 | 0.045 | 0.009 |
| RQUICKI | 0.41 ± 0.05 a | 0.42 ± 0.04 b | 0.47 ± 0.04 c | 0.45 ± 0.04 d | 0.47 ± 0.05 c | 0.46 ± 0.05 e | 0.001 | 0.012 | 0.007 |
| BHB (mmol/L) | 0.75 ± 0.10 a | 0.63 ± 0.09 b | 0.54 ± 0.08 c | 0.49 ± 0.07 c | 0.81 ± 0.09 d | 0.70 ± 0.08 d | 0.008 | 0.314 | 0.43 |
| Ca (mmol/L) | 2.47 ± 0.09 a | 2.55 ± 0.11 b | 2.31 ± 0.10 c | 2.42 ± 0.09 d | 2.19 ± 0.08 e | 2.35 ± 0.07 c | 0.001 | 0.005 | 0.005 |
| P (mmol/L) | 2.99 ± 0.25 a | 2.81 ± 0.21 a | 2.45 ± 0.19 b | 2.41 ± 0.21 b | 2.29 ± 0.18 c | 2.30 ± 0.16 c | 0.001 | 0.822 | 0.752 |
| TPROT (g/L) | 63.21 ± 6.5 a | 68.2 ± 5.2 a | 55.4 ± 4.61 b | 53.2 ± 5.02 b | 52.5 ± 4.33 c | 51.8 ± 3.28 c | 0.001 | 0.692 | 0.513 |
| ALB (g/L) | 35.4 ± 2.99 a | 38.6 ± 2.86 a | 30.5 ± 2.41 b | 33.6 ± 2.43 b | 32.5 ± 2.39 c | 36.1 ± 2.41 c | 0.001 | 0.683 | 0.413 |
| Urea (mmol/L) | 4.71 ± 0.78 a | 4.29 ± 0.71 a | 5.92 ± 0.89 b | 4.94 ± 0.91 a | 6.31 ± 0.95 b | 5.11 ± 0.78 a | 0.045 | 0.324 | 0.294 |
| Creat (µmol/L) | 85.5 ± 6.3 a | 91.5 ± 6.5 a | 106.2 ± 7.2 b | 101.4 ± 7.3 b | 118.6 ± 7.5 c | 116.8 ± 6.9 c | 0.045 | 0.214 | 0.352 |
| TGC (mmol/L) | 0.28 ± 0.04 a | 0.35 ± 0.03 b | 0.21 ± 0.03 c | 0.28 ± 0.04 a | 0.15 ± 0.04 d | 0.28 ± 0.04 a | 0.007 | 0.043 | 0.048 |
| CHOL (mmol/L) | 3.73 ± 0.29 a | 4.21 ± 0.30 b | 3.42 ± 0.29 c | 3.96 ± 0.25 d | 3.01 ± 0.21 e | 3.9 ± 0.27 d | 0.008 | 0.046 | 0.045 |
| TBIL (µmol/L) | 5.91 ± 1.13 a | 4.22 ± 1.11 a | 7.12 ± 1.21 b | 6.99 ± 1.14 b | 8.03 ± 1.56 c | 7.86 ± 1.57 b | 0.009 | 0.761 | 0.672 |
| AST (U/L) | 86.5 ± 9.53 a | 75.4 ± 8.99 b | 105.8 ± 10 c | 89.1 ± 9.45 a | 114.3 ± 11.2 d | 99.3 ± 10.6 c | 0.001 | 0.05 | 0.332 |
| GGT (U/L) | 27.4 ± 3.21 a | 23.9 ± 2.99 b | 36.7 ± 3.04 c | 28.3 ± 3.14 a | 40.2 ± 4.56 d | 30.7 ± 4.02 e | 0.001 | 0.005 | 0.005 |
| LDH (U/L) | 789 ± 40 a | 756 ± 65 a | 1235 ± 50 b | 1199 ± 52 b | 1211 ± 53 c | 1204 ± 42 c | 0.046 | 0.622 | 0.421 |
| ALP (U/L) | 84.5 ± 15.2 a | 98.6 ± 13.5 a | 99.2 ± 14.1 a | 104 ± 15.3 a | 99.5 ± 14.5 a | 102 ± 12.8 a | 0.653 | 0.534 | 0.521 |
| CK (U/L) | 146 ± 25.3 a | 151 ± 21.4 a | 172 ± 25 a | 181 ± 30.4 a | 189 ± 30 a | 178 ± 32.2 a | 0.05 | 0.274 | 0.391 |
| Rectal Temp. | Eye Temp. | Ear Temp. | Nose Temp. | Forehead Temp. | Whole Head t. | Abdomen Temp. | Udder Temp. | Front Limb t. | Hind Limb t. | Whole Body t. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| eHsp70 a | 0.36 **F | 0.85 ***S | 0.86 ***S | 0.81 ***S | 0.89 ***S | 0.95 ***S | 0.93 ***S | 0.9 ***S | 0.81 ***S | 0.79 ***M | 0.96 ***S |
| TNFα | 0.38 **F | 0.69 ***M | 0.61 ***M | 0.74 ***M | 0.75 ***M | 0.84 ***S | 0.89 ***S | 0.93 ***S | 0.63 ***M | 0.65 ***M | 0.84 ***S |
| CORT | 0.16 P | 0.56 ***F | 0.58 ***F | 0.59 ***F | 0.63 ***M | 0.7 ***M | 0.65 ***M | 0.49 ***F | 0.52 ***F | 0.32 **F | 0.72 ***M |
| T3 | 0.12 P | −0.51 **F | −0.48 **F | −0.39 **F | −0.51 **F | −0.62 ***M | −0.6 ***M | −0.44 **F | −0.36 **F | −0.32 **F | −0.51 **F |
| T4 | 0.15 P | −0.48 **F | −0.45 **F | −0.43 **F | −0.42 **F | −0.7 ***M | −0.61 ***M | −0.51 **F | −0.38 **F | −0.19 P | −0.45 **F |
| INS | 0.16 P | 0.61 ***M | 0.52 **F | 0.59 **F | 0.63 ***M | 0.78 ***M | 0.8 ***S | 0.49 **F | 0.32 **F | 0.22 *P | 0.82 ***S |
| NEFA | 0.12 P | −0.53 **F | −0.49 **F | −0.5 **F | −0.52 **F | −0.68 ***M | −0.7 ***M | −0.52 **F | −0.41 **F | −0.36 **F | −0.65 ***M |
| GLU | 0.11 P | −0.62 ***M | −0.55 **F | −0.58 **F | −0.61 ***M | −0.8 ***S | −0.74 ***M | −0.61 ***M | −0.38 **F | −0.33 **F | −0.79 ***M |
| RQUICKI | 0.03 P | 0.51 **F | 0.59 **F | 0.39 **F | 0.42 **F | 0.58 **F | 0.49 **F | 0.35 **F | 0.42 **F | 0.29 **P | 0.83 ***S |
| BHB | 0.06 P | −0.26 *P | −0.18 P | −0.23 *P | −0.29 **P | −0.3 **F | −0.39 **F | −0.3 **F | −0.25 *P | −0.19 P | −0.57 **F |
| Ca | 0.02 P | −0.15 P | −0.2 P | −0.19 P | −0.16 P | −0.25 *P | −0.3 **F | −0.15 P | −0.11 P | −0.1 P | −0.51 **F |
| P | 0.03 P | −0.1 P | −0.09 N | −0.08 N | −0.1 P | −0.16 P | −0.2 *P | −0.08 N | −0.02 N | −0.02 N | −0.29 **P |
| TPROT | 0.05 P | −0.34 **F | −0.31 **F | −0.35 **F | −0.41 **F | −0.46 **F | −0.4 **F | −0.33 **F | −0.21 *P | −0.18 P | −0.53 **F |
| ALB | 0.07 P | −0.21 *P | −0.2 P | −0.19 P | −0.25 *P | −0.39 **F | −0.3 **F | −0.25 *P | −0.18 P | −0.15 P | −0.37 **F |
| Urea | 0.14 P | 0.48 **F | 0.45 **F | 0.43 **F | 0.5 **F | 0.57 ***F | 0.58 **F | 0.39 **F | 0.4 **F | 0.42 **F | 0.62 ***M |
| Creat | 0.03 P | 0.59 **F | 0.55 **F | 0.58 **F | 0.61 ***M | 0.7 ***M | 0.78 ***M | 0.68 ***M | 0.59 **F | 0.55 **F | 0.81 ***S |
| TGC | 0.05 P | −0.42 **F | −0.45 **F | −0.41 **F | −0.55 **F | −0.55 **F | −0.57 **F | −0.49 **F | −0.32 **F | −0.28 **F | −0.64 **M |
| CHOL | 0.08 P | −0.35 **F | −0.36 **F | −0.38 **F | −0.39 **F | −0.4 **F | −0.45 **F | −0.5 **F | −0.32 **F | −0.29 **F | −0.55 **F |
| TBIL | 0.06 P | 0.21 *P | 0.21 *P | 0.19 P | 0.15 P | 0.21 *P | 0.36 **F | 0.38 **F | 0.18 P | 0.17 P | 0.36 **F |
| AST | 0.13 P | 0.39 **F | 0.42 **F | 0.45 **F | 0.4 **F | 0.48 **F | 0.52 **F | 0.65 ***M | 0.47 **F | 0.39 **F | 0.72 ***M |
| GGT | 0.09 P | 0.4 **F | 0.38 **F | 0.36 **F | 0.39 **F | 0.45 **F | 0.52 **F | 0.3 **F | 0.25 *P | 0.19 P | 0.69 ***M |
| LDH | 0.11 P | 0.23 *P | 0.25 *P | 0.28 **P | 0.35 **F | 0.29 **P | 0.41 **F | 0.48 **F | 0.32 **F | 0.25 *P | 0.63 ***M |
| ALP | 0.09 P | 0.08 N | 0.06 N | 0.05 N | 0.12 P | 0.15 P | 0.2 P | 0.21 *P | 0.07 N | 0.03 F | 0.15 P |
| CK | 0.04 P | 0.11 P | 0.15 P | 0.21 *P | 0.15 P | 0.23 *P | 0.21 *P | 0.18 P | 0.25 *P | 0.26 *P | 0.31 **F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blond, B.; Majkić, M.; Spasojević, J.; Hristov, S.; Radinović, M.; Nikolić, S.; Anđušić, L.; Čukić, A.; Došenović Marinković, M.; Vujanović, B.D.; et al. Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows. Metabolites 2024, 14, 104. https://doi.org/10.3390/metabo14020104
Blond B, Majkić M, Spasojević J, Hristov S, Radinović M, Nikolić S, Anđušić L, Čukić A, Došenović Marinković M, Vujanović BD, et al. Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows. Metabolites. 2024; 14(2):104. https://doi.org/10.3390/metabo14020104
Chicago/Turabian StyleBlond, Bojan, Mira Majkić, Jovan Spasojević, Slavča Hristov, Miodrag Radinović, Sandra Nikolić, Ljiljana Anđušić, Aleksandar Čukić, Maja Došenović Marinković, Biljana Delić Vujanović, and et al. 2024. "Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows" Metabolites 14, no. 2: 104. https://doi.org/10.3390/metabo14020104
APA StyleBlond, B., Majkić, M., Spasojević, J., Hristov, S., Radinović, M., Nikolić, S., Anđušić, L., Čukić, A., Došenović Marinković, M., Vujanović, B. D., Obradović, N., & Cincović, M. (2024). Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows. Metabolites, 14(2), 104. https://doi.org/10.3390/metabo14020104








