Analysis of Immunosuppression and Antioxidant Damage in Diploid and Triploid Crucian Carp (Carassius auratus) Induced by Saline-Alkaline Environmental Stress: From Metabolomic Insight
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Subjects
2.3. Experimental Methods and Sample Collection
2.4. Metabolite Extraction
2.5. UPLC-QTOF-MS Analysis
2.6. Data Processing
3. Results
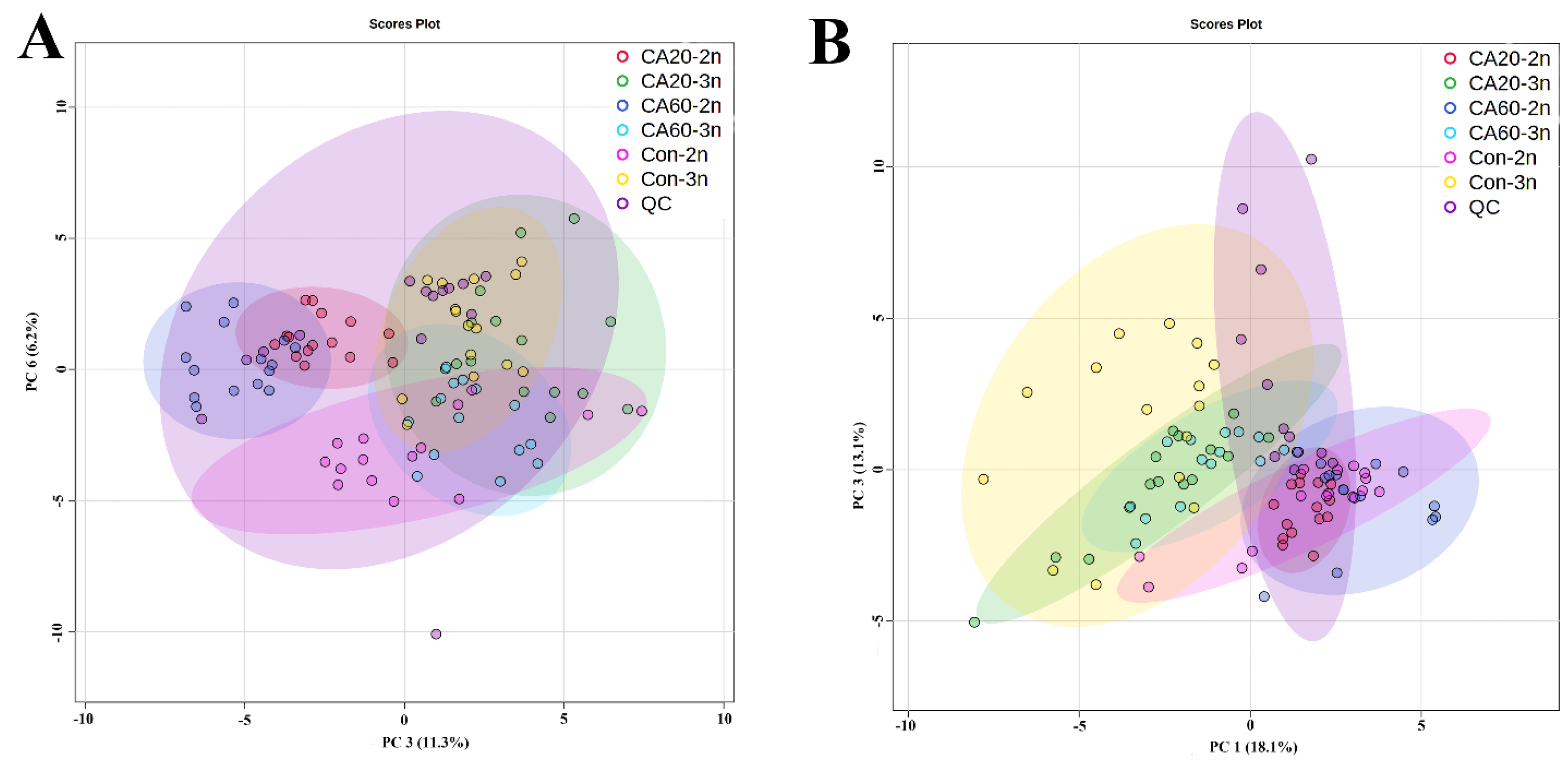
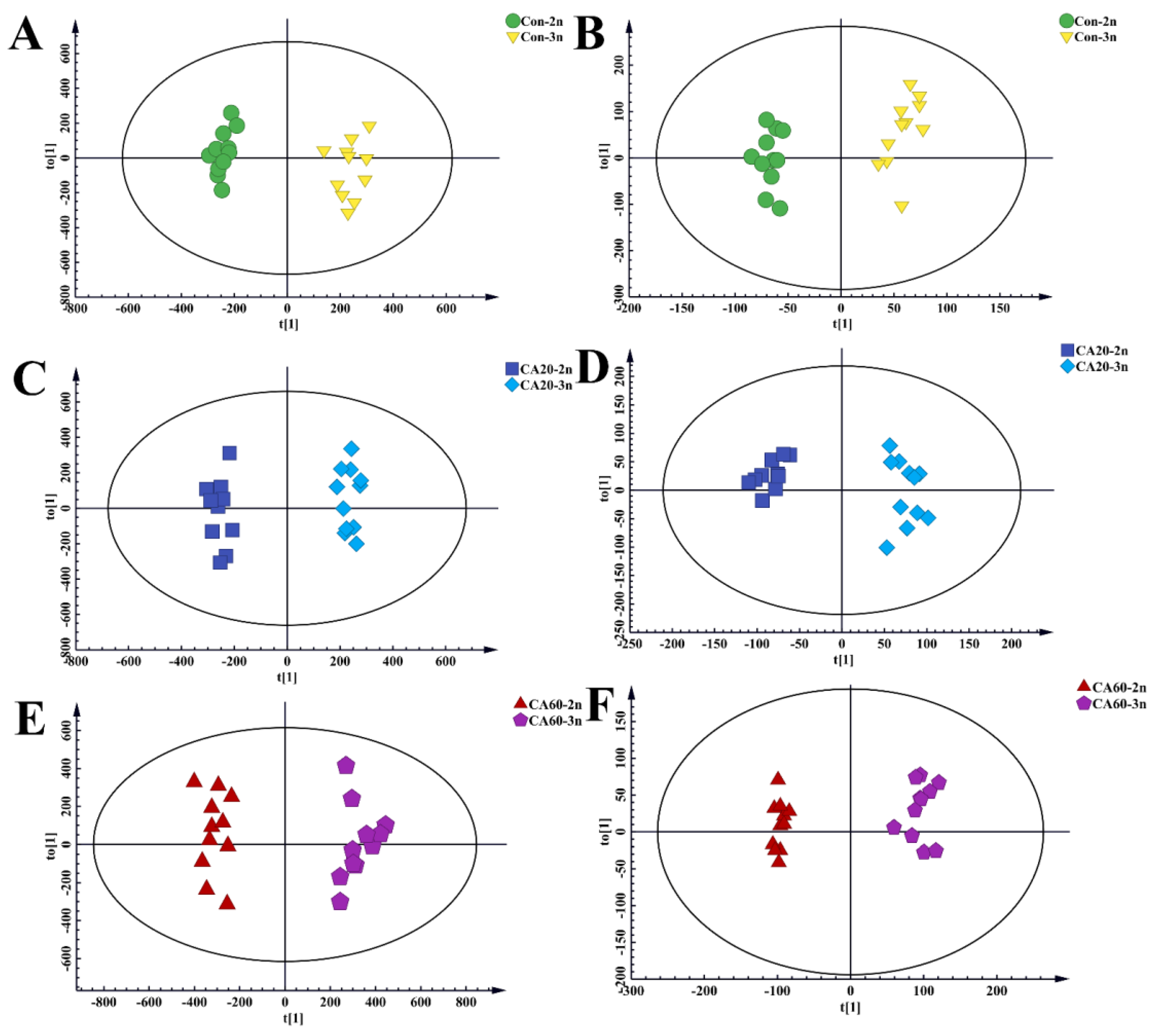
3.1. Metabolic Profiles
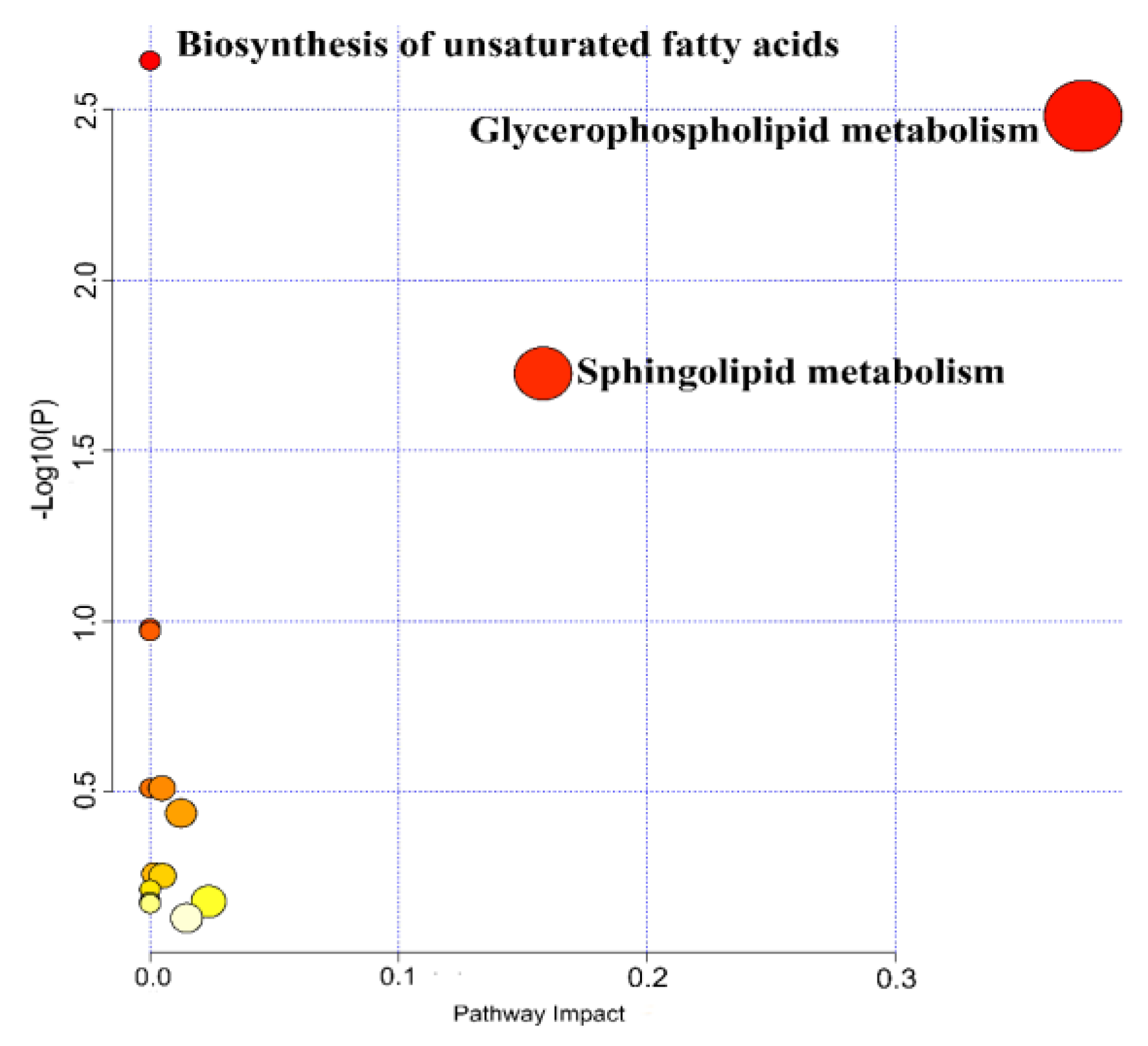
3.2. Selection of Differentially Expressed Metabolites and Metabolic Pathway Analysis
4. Discussion
4.1. Glycerophospholipid Metabolism Pathway
4.2. Sphingolipid Metabolism Pathway
4.3. Biosynthesis Metabolic Pathway of Polyunsaturated Fatty Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.H.; Guo, K.; Luo, L.; Zhang, R.; Xu, W.; Song, Y.Y.; Zhao, Z.G. Fattening in saline and alkaline water improves the color, nutritional and taste quality of adult Chinese mitten crab Eriocheir sinensis. Foods 2022, 11, 2573. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.B.; Zhang, C.F.; Guo, J.T.; Zhang, L. Analysis and Countermeasures of the Development Status and Existing Problems of Freshwater Aquaculture. Henan Fish 2023, 2, 4–6. [Google Scholar]
- Yu, Y.L.; Li, Z.; Zhang, L.; Li, Q.; Sun, Y.H.; Wei, H.J.; Wang, G.Y.; Gan, J.H. Effects of saline-alkali water on the nutritional quality of Bo fang“Xianfeng No.2”. Anim. Breed. Feed. 2024, 10, 37–45. [Google Scholar]
- Liu, Y.X.; Fang, H.; Lai, Q.F.; Liang, L.Q. The Current State and Development Strategy for China’s Saline-Alkaline Fisheries. Strateg. Study CAE 2016, 18, 74–78. [Google Scholar]
- Yi, X.F.; Lai, Q.F.; Shi, J.Q.; Gao, P.C.; Zhou, K.; Qi, H.F.; Wang, H.; Me, Z.L. Nitrogenous waste excretion and gene expression of nitrogen transporter in Gymnocypris przewalskii in high alkaline environment. J. Fish. Sci. China 2017, 24, 681–689. [Google Scholar]
- Jiang, K.M.; Wang, W.Q.; Li, J.L.; Feng, W.R.; Kamunga, E.M.; Zhang, Z.H.; Tang, Y.K. Physiological and molecular responses of juvenile silver crucian carp (Carassius gibelio) to long-term high alkaline stress: Growth performance, histopathology, and transcriptomic analysis. Aquac. Rep. 2024, 39, 102393. [Google Scholar] [CrossRef]
- Zhu, S.Q. Research on Fishery Development Countermeasures in Raohe County. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2022. [Google Scholar]
- Wei, X.F.; Liu, Y.J.; Li, S.W.; Ding, L.; Han, S.C.; Chen, Z.X.; Lu, H.; Wang, P.; Sun, Y.C. Stress response and tolerance mechanisms of NaHCO3 exposure based on biochemical assays and multi-omics approach in the liver of crucian carp (Carassius auratus). Ecotoxicol. Environ. Saf. 2023, 253, 114633. [Google Scholar] [CrossRef]
- Yin, X.L. Production Systems, Efficiency and the Impacts on Environment: Evidence Based on Yancheng Freshwater Fish Production; Nanjing Agricultural University: Nanjing, China, 2017. [Google Scholar]
- Purcell, K.M.; Klerks, P.L.; Leberg, P.L. Adaptation to sea level rise: Does local adaptation influence the demography of coastal fish populations? J. Fish Biol. 2010, 77, 1209–1218. [Google Scholar] [CrossRef]
- Yang, J. Tolerance of 5 Species of Fish and Changes of Immune Related Indicators under Salinity and Immunological Stress Reaction. Master’s thesis, Shanghai Ocean University, Shanghai, China, 2014.
- Lu, M.; Li, X.Y.; Li, Z.; Du, W.X.; Zhou, L.; Wang, Y.; Zhang, X.J.; Wang, Z.W.; Gui, J.F. Regain of sex determination system and sexual reproduction ability in a synthetic octoploid male fish. Sci. China Life Sci. 2020, 64, 77–87. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Feng, C.; Liu, X.F.; Liu, S.J. 3nLcn2, a teleost lipocalin 2 that possesses antimicrobial activity and inhibits bacterial infection in triploid crucian carp. Fish Shellfish Immun. 2020, 102, 47–55. [Google Scholar] [CrossRef]
- Cai, L.; Ao, Z.P.; Tang, T.; Tong, F.L.; Wei, Z.H.; Yang, F.Z.; Shu, Y.Q.; Liu, S.J.; Mai, K.S. Characterization of difference in muscle volatile compounds between triploid and diploid crucian carp. Aquacult. Rep. 2021, 20, 100641. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, K.; Zhang, X.; Liu, F.; Qin, Q.; Tao, M.; Wen, M.; Tang, C.; Liu, S. A new type of triploid fish derived from the diploid hybrid crucian carp (♀) × autotetraploid fish (♂). Reprod. Breed. 2021, 1, 122–127. [Google Scholar] [CrossRef]
- Li, S.N.; Zhou, Y.; Yang, C.H.; Fan, S.Y.; Huang, L.; Zhou, T.; Wang, Q.B.; Zhao, R.R.; Tang, C.C.; Tao, M.; et al. Comparative analyses of hypothalamus transcriptomes reveal fertility-, growth-, and immune-related genes and signal pathways in different ploidy cyprinid fish. Genomics 2021, 113, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Min, E.K.; Lee, H.; Sung, E.J.; Seo, S.W.; Song, M.; Wang, S.; Kim, S.S.; Bae, M.A.; Kim, T.Y.; Lee, S.; et al. Integrative multi-omics reveals analogous developmental neurotoxicity mechanisms between perfluorobutanesulfonic acid and perfluorooctanesulfonic acid in zebrafish. J. Hazard. Mater. 2023, 457, 131714. [Google Scholar] [CrossRef]
- Baranasic, D.; Hörtenhuber, M.; Balwierz, P.J.; Zehnder, T.; Mukarram, A.K.; Nepal, C.; Várnai, C.; Hadzhiev, Y.; Jimenez-Gonzalez, A.; Li, N.; et al. Multiomic atlas with functional stratification and developmental dynamics of zebrafish cis-regulatory elements. Nat. Genet. 2022, 54, 1037–1050. [Google Scholar] [CrossRef]
- Curdy, N.; Lanvin, O.; Cadot, S.; Laurent, C.; Fournié, J.-J.; Franchini, D.-M. Stress Granules in the Post-transcriptional Regulation of Immune Cells. Front. Cell Dev. Biol. 2020, 8, 611186. [Google Scholar] [CrossRef]
- Shahjahan, M.; Islam, M.J.; Hossain, M.T.; Mishu, M.A.; Hasan, J.; Brown, C. Blood biomarkers as diagnostic tools: An overview of climate-driven stress responses in fish. Sci. Total Environ. 2022, 843, 156910. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Grabicov, K.; Brooks, B.W.; Grabic, R. Inffuence of time-dependent sampling on the plasma metabolome and exposome of ffsh collected from an efffuent-dependent pond. Sci. Total Environ. 2023, 906, 167446. [Google Scholar] [CrossRef]
- Sun, Y.C.; Han, S.C.; Yao, M.Z.; Liu, H.B.; Wang, Y.M. Exploring the metabolic biomarkers and pathway changes in crucian under carbonate alkalinity exposure using high-throughput metabolomics analysis based on UPLC-ESI-QTOF-MS. RSC Adv. 2020, 10, 1552–1571. [Google Scholar] [CrossRef]
- Sun, Y.C.; Wu, S.; Du, N.N.; Song, Y.; Xu, W. High-throughput metabolomics enables metabolite biomarkers and metabolic mechanism discovery of fish in response to alkalinity stress. RSC Adv. 2018, 8, 14983–14990. [Google Scholar] [CrossRef]
- Sun, Y.C.; Han, S.C.; Yao, M.Z.; Wang, Y.M.; Geng, L.W.; Wang, P.; Liu, W.H.; Liu, H.B. High-throughput metabolomics method based on liquid chromatography-mass spectrometry: Insights into the underlying mechanisms of salinity–alkalinity exposure-induced metabolites changes in Barbus capito. J. Sep. Sci. 2021, 44, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liu, Y.J.; Wei, X.F.; Geng, C.Y.; Liu, W.Z.; Han, L.; Yuan, F.Y.; Wang, P.; Sun, Y.C. Effects of α-ketoglutarate supplementation on serum metabolism of crucian carp under carbonate alkaline stress based on UPLC-Q-TOF/MS metabolomics. J. Fish. Sci. China 2023, 30, 138–149. [Google Scholar]
- Li, Y.; Gao, P.C.; Zhou, K.; Yao, Z.L.; Sun, Z.; Qin, H.C.; Li, Q.F. Effects of saline and alkaline stresses on the survival, growth, and physiological responses in juvenile mandarin fish (Siniperca chuatsi). Aquaculture 2024, 591, 741143. [Google Scholar] [CrossRef]
- Huang, Z.; Dai, L.Y.; Peng, F.Y.; Tang, L.W.; Wang, X.J.; Chen, J.Y.; Liu, J.H.; Fu, W.; Peng, L.Y.; Liu, W.B.; et al. mTOR signaling pathway regulates embryonic development and rapid growth of triploid crucian carp. Aquacult. Rep. 2023, 33, 101860. [Google Scholar] [CrossRef]
- Ristovski, M.; Farhat, D.; Ellaine, M.; Bancud, S.; Lee, J.-Y. Lipid Transporters Beam Signals from Cell Membranes. Membranes 2021, 11, 2–18. [Google Scholar] [CrossRef]
- Stojanović, O.; Miguel-Aliaga, I.; Trajkovski, M. Intestinal plasticity and metabolism as regulators of organismal energy homeostasis. Nat. Metab. 2022, 4, 1444–1458. [Google Scholar] [CrossRef]
- Liu, X.H.; Li, C.; Hou, C.M.; Jiang, Y.; Chen, F.; Zhu, Y.M.; Zou, L.H. Dissecting the effects of paraquat-induced pulmonary injury in rats using UPLC-Q-TOF-MS/MS-based metabonomics. Toxicol. Res. 2023, 12, 527–538. [Google Scholar] [CrossRef]
- Ma, S.; Yang, K.; Li, Z.; Li, Z.; Feng, Y.; Wang, X.; Wang, J.; Zhu, Z.; Wang, Z.; Wang, J.; et al. A retro-inverso modiffed peptide alleviated ovalbumin-induced asthma model by affecting glycerophospholipid and purine metabolism of immune cells. Pulm. Pharmacol. Ther. 2023, 78, 102185. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, Y.; Yang, C.; Gong, W.; Wang, C.; Wu, L.; Wang, D. Short-Chain Fatty Acids Attenuate 5-Fluorouracil-Induced THP-1 Cell Inflammation through Inhibiting NF-κB/NLRP3 Signaling via Glycerolphospholipid and Sphingolipid Metabolism. Molecules 2023, 28, 2–18. [Google Scholar] [CrossRef]
- Tei, R.; Morstein, J.; Shemet, A.; Trauner, D.; Baskin, J.M. Optical Control of Phosphatidic Acid Signaling. ACS Cent. Sci. 2021, 7, 1205–1215. [Google Scholar] [CrossRef]
- Bellot, P.E.N.R.; Moia, M.N.; Reis, B.Z.; Pedrosa, L.F.C.; Tasic, L.F.B., Jr.; Sena-Evangelista, K.C.M. Are Phosphatidylcholine and Lysophosphatidylcholine Body Levels Potentially Reliable Biomarkers in Obesity? A Review of Human Studies. Mol. Nutr. Food Res. 2023, 67, 2200568. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.S.; Iraji, R.; Bakovic, M. Phosphatidylethanolamine homeostasis under conditions of impaired CDP-ethanolamine pathway or phosphatidylserine decarboxylation. Front. Nutr. 2023, 9, 1094273. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.F.; Wei, F.; Dong, X.Y.; Chen, H.; Huang, F.H. Progress in Analysis Methods of Glycerophospholipid Based on Chemical Derivatization. J. Instrum. Anal. 2018, 37, 1396–1404. [Google Scholar]
- Luo, Z.F.; Wu, C.; Jin, S.Y.; Zhang, Y.; He, S.F. Effects of lysophosphatidic acid on ischemia/reperfusion injury and TRPV1 expression in isolated mouse heart. Chin. Pharmacol. Bull. 2022, 38, 417–421. [Google Scholar]
- Zhang, T.X.; Yan, Z.G.; Zheng, X.; Wang, S.P.; Fan, J.T.; Liu, Z.T. Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea). Fish Shellfish Immunol. 2020, 99, 514–525. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.Y.; Bae, Y.-S. Functional roles of sphingolipids in immunity and their implication in disease. Exp. Mol. Med. 2023, 55, 1110–1130. [Google Scholar] [CrossRef]
- Jamil, M.; Cowart, L.A. Sphingolipids in mitochondria—From function to disease. Front. Cell Dev. Biol. 2023, 11, 1302472. [Google Scholar] [CrossRef]
- Cartier, A.; Leigh, T.; Liu, C.H.; Hla, T. Endothelial sphingosine 1-phosphate receptors promote vascular normalization and antitumor therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3157–3166. [Google Scholar] [CrossRef]
- Li, F.M.; Yu, Z.X. Functions of ceramide and its role in pulmonary arterial hypertension. Basic Clin. Med. 2021, 41, 267–271. [Google Scholar]
- Liu, T.; Li, J.; Bao, C.Y. Role of endoplasmic reticulum stress pathway in palmitic acid -induced apoptosis of human periodontal membrane stem cells. China Med. Her. 2022, 19, 4–8. [Google Scholar]
- Liu, Y.J.; Yao, M.Z.; Li, S.W.; Wei, X.F.; Ding, L.; Han, S.C.; Wang, P.; Lv, B.C.; Chen, Z.X.; Sun, Y.C. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total Environ. 2022, 822, 153622. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.Q.; Liu, Y.Y.; Wu, Y.F.; Chen, Y. Dihydrocurcumin ameliorates the lipid accumulation, oxidative stress and insulin resistance in oleic acid-induced L02 and Hep G2 cells. Biomed. Pharmacother. 2018, 103, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh-Smith, W.A.C.; Abdallah, A.; Cunningham, C.J. The potential effects of polyunsaturated ω-3 fatty acids on spinal cord injury: A systematic review & meta-analysis of preclinical evidence. Prostaglandins Leukot. Essent. Fat. Acids 2023, 191, 102554. [Google Scholar]
- Tojjari, A.; Choucair, K.; Sadeghipour, A.; Saeed, A.; Saeed, A. Anti-Inflammatory and Immune Properties of Polyunsaturated Fatty Acids (PUFAs) and Their Impact on Colorectal Cancer (CRC) Prevention and Treatment. Cancers 2023, 15, 2–17. [Google Scholar] [CrossRef]
- Munson, P.V.; Adamik, J.; Hartmann, F.J.; Favaro PM, B.; Ho, D.; Bendall, C.S.; Combes, A.J.; Krummel, M.F.; Zhang, K.; Kelley, R.K.; et al. Polyunsaturated Fatty Acid–Bound α-Fetoprotein Promotes Immune Suppression by Altering Human Dendritic Cell Metabolism. Cancer Res. 2023, 83, 1543–1557. [Google Scholar] [CrossRef]
- Wang, X.; Yan, S.M. Regulation and Mechanism of Lipid Metabolism by Polyunsaturated Fatty Acids of Animals. Chin. J. Anim. Nutr. 2019, 31, 2471–2478. [Google Scholar]
- Murota, K. Digestion and absorption of dietary glycerophospholipids in the small intestine: Their significance as carrier molecules of choline and n-3 polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2020, 26, 101633. [Google Scholar] [CrossRef]
- Jia, Z.W.; Du, L.Y.; Xie, Z.F.; Lv, S.C. Physiological Functions of n-6 Polyunsaturated Fatty Acids and Its Application in Beef Cattle Production. Chin. J. Anim. Nutr. 2023, 36, 1–11. [Google Scholar]
- Wang, Y.; Xue, F.G.; Jiang, L.S.; Xiong, B.H. Pro-Inflammatory and Indicative Effects of Prostaglandins and Their Receptors in Chronic Inflammation in Animals. Chin. J. Anim. Nutr. 2019, 31, 2495–2501. [Google Scholar]
- Araújo, B.C.; Rodriguez, M.; Honji, M.R.; Rombenso, A.N.; Rio-Zaragoza, O.B.; Cano, A.; Tinajero, A.; Mata-Sotres, J.A.; Viana, M.T. Arachidonic acid modulated lipid metabolism and improved productive performance of striped bass (Morone saxatilis) juvenile under sub-to optimal temperatures. Aquaculture 2020, 530, 735939. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Yu Wei, Y.; Wang, L.; Song, K.; Zhang, C.X.; Lu, K.L.; Rahimnejad, S. Dietary n-3/n-6 polyunsaturated fatty acid ratio modulates growth performance in spotted seabass (Lateolabrax maculatus) through regulating lipid metabolism, hepatic antioxidant capacity and intestinal health. Anim. Nutr. 2023, 14, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Gao, G.T.; Duan, A.L.; Gu, H.F.; Li, B.; Xu, S. Research progress in polyunsaturated fatty acids. Sci. Technol. Food Ind. 2012, 33, 418–423. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, F.; Wei, X.; Li, D.; Jin, X.; Wang, J.; Sun, Y. Analysis of Immunosuppression and Antioxidant Damage in Diploid and Triploid Crucian Carp (Carassius auratus) Induced by Saline-Alkaline Environmental Stress: From Metabolomic Insight. Metabolites 2024, 14, 721. https://doi.org/10.3390/metabo14120721
Yuan F, Wei X, Li D, Jin X, Wang J, Sun Y. Analysis of Immunosuppression and Antioxidant Damage in Diploid and Triploid Crucian Carp (Carassius auratus) Induced by Saline-Alkaline Environmental Stress: From Metabolomic Insight. Metabolites. 2024; 14(12):721. https://doi.org/10.3390/metabo14120721
Chicago/Turabian StyleYuan, Fangying, Xiaofeng Wei, Dongping Li, Xiaofeng Jin, Jing Wang, and Yanchun Sun. 2024. "Analysis of Immunosuppression and Antioxidant Damage in Diploid and Triploid Crucian Carp (Carassius auratus) Induced by Saline-Alkaline Environmental Stress: From Metabolomic Insight" Metabolites 14, no. 12: 721. https://doi.org/10.3390/metabo14120721
APA StyleYuan, F., Wei, X., Li, D., Jin, X., Wang, J., & Sun, Y. (2024). Analysis of Immunosuppression and Antioxidant Damage in Diploid and Triploid Crucian Carp (Carassius auratus) Induced by Saline-Alkaline Environmental Stress: From Metabolomic Insight. Metabolites, 14(12), 721. https://doi.org/10.3390/metabo14120721






